Summary
We identified types of immune cells that contribute to clearing COVID‐19 during the acute phase of the infection in mouse model and human. Our results suggest that both innate and adaptive immune responses are essential for controlling COVID‐19 infection. Mild infection report of children by COVID‐19 comparing adults' infection causes conclusion of higher resistance of immune system of children comparing adults. Our results show innate immune system including phagocytes contribute severely to the elimination of COVID‐19 in both mouse model and human. Our results also show the elimination of COVID‐19 required the activation of B cells by CD4+ T cells. CD4+ T cells play an important role in elimination of COVID‐19 in primary effection. We measured IgM and IgG in all patients including adults and kids (human) and found IgM and IgG in kids patients are much higher than other adults patients. It causes production of much more natural antibodies in kids' bodies to protect them against COVID‐19 that shows reason of mild effection of kids comparing adults. Our observations have important ramifications for the development of novel vaccination and medicine strategies to alleviate COVID‐19. The most important result is for producing any vaccine for COVID‐19, increasing and producing these factors must be included: (a) Phagocytes (IgM and IgG), (b) T Cells, and (c) White Cells.
1. INTRODUCTION
Coronaviruses are important human and animal pathogens. At the end of 2019, a novel coronavirus, was identified as the cause of a cluster of pneumonia cases in Wuhan, China. It rapidly spread, resulting in an epidemic throughout China, with sporadic cases reported globally. From December 2019 till now, the disease, which resulted in several millions cases with many deaths, was caused by a novel type of coronavirus, termed COVID‐19. Patients with COVID‐19 usually developed a high fever followed by clinical symptoms of cough and shortness of breath. Full‐genome sequencing and phylogenic analysis indicated that the coronavirus that causes COVID‐19 is a betacoronavirus in the same subgenus as the severe acute respiratory syndrome (SARS‐CoV) virus (as well as several bat coronaviruses), but in a different clade. 1 The apparent structure of the receptor‐binding gene region in COVID‐19 is very similar to that of the SARS coronavirus, and there is speculation that it will be shown to use the same receptor for cell entry. Full‐length genome sequences were obtained from five patients at the early stage of the outbreak show they are almost identical to each other and share 79.5% sequence identify to SARS‐CoV. 2 Furthermore, it was found that COVID‐19 is 96% identical at the whole‐genome level to a bat coronavirus. 2 However, there has been no subsequent consensus regarding which treatment, if any, benefited COVID‐19 patients during the outbreak.
The development of an effective treatment and vaccination strategy for COVID‐19 cases will require clarifying the precise mechanisms by which host immune responses control COVID‐19 infection. Cumulative evidence suggests that patients who recovered from COVID‐19 possessed specific acquired immunity based on both T and B cells. 3 However, the effector cells or molecules that act to eliminate COVID‐19 during the acute phase of the infection remain unclear. The mechanisms by which SARS‐CoV infection causes the pathogenesis and host immune responses has been investigated using adequate animal infectious models. 4 , 5 , 6 , 7 , 8 Lethal disease in BALB/c mice infected with a mouse‐adapted strain of SARS‐CoV, MA15, showed a lack of activation of innate immune response, resulting in a barely detectable antivirus T cell response. 8 On the other hand, aged BALB/c mice that were infected with a human clinical isolate of SARS‐CoV (Urbani strain) successfully eliminated the invasive virus within 1 week post‐infection; these mice exhibited high and prolonged levels of viral replication, signs consistent with clinical symptoms, and pathologic changes in the lung resembling those seen in elderly SARS patients. 4 Therefore, the infection of these aged mice is considered a model for the successful elimination of SARS‐CoV by host immune responses. A study reported that CD4+ T cells play an important role in the control of SARS‐CoV infection. 9 These researchers also reported an important role for innate defense mechanisms in controlling SARS‐CoV infection, as demonstrated by the clearance of SARS‐CoV over 9 days post‐infection (dpi) in BALB/c mice depleted of both CD4+ and CD8+ T cells. 9 These results suggest that both innate and adaptive immune responses are essential for controlling SARS‐CoV infection. The results in Reference 10 show also that the cooperation of anti‐SARS‐CoV antibodies and phagocytic cells plays an important role in the elimination of SARS‐CoV.
We studied the identity and role of effector cells and molecules participating in the elimination of COVID‐19 during the acute phase of COVID‐19 in our work. In this study, we attempted to identify the types of immune cells that contribute to clearing COVID‐19 during the acute phase of the infection in mice models plus human. We demonstrate that phagocytic cells play an important role in the elimination of COVID‐19 in both mouse and human models of infection.
2. RESULTS
The contributions of cellular vs humoral immune responses in the resolution of acute COVID‐19 infection have not yet exactly been experimentally addressed. Therefore, using the infection of viral titers in the lungs of aged (>8 months old) BALB/c mice, young (<4 weeks old) BALB/c mice, and (8 weeks old) SCID mice following infection with acute COVID‐19 at 42 years old male patient's throat lavage, we aimed to determine the role of T cell vs B cell responses during acute COVID‐19 infection. Prior to intrabronchial COVID‐19 inoculation, 32 aged BALB/c mice, and 32 young BALB/c mice, and 32 SCID mice were divided into four groups of animals each: (a) control; (b) CD8+ T cell depleted; (c) CD4+ T cell depleted; and (d) CD20+ B cell depleted. T and B cell depletion regimens were initiated 7 days prior to infection to ensure that the targeted lymphocyte population was not present on the day of infection (0 dpi).
2.1. Importance of adaptive immune responses for the pulmonary‐infected COVID‐19
The intranasal (i.n.) infection of aged BALB/c mice with COVID‐19 resulted in over 108 median tissue culture infectious dose (TCID 50)/g lung tissue at 2 dpi, 6 dbi, 9 dbi, 10 dbi, and 20 dbi. The infected mice at 2 dbi exhibited histological signs of severe pneumonia, including interstitial cell thickening, immune cell infiltration, and epithelial damage in the bronchus, at 10 dpi and 20 dpi. The observed titers in the aged‐mouse model were considerably higher than those seen in infected young BALB/c mice and very young SCID mice, which did not show apparent histological signs of pneumonia. Titers in the aged mice remained high (~107 TCID 50/g lung tissue) at 6 dpi. However, the titers decreased to levels below the lower limit of detection (LLOD; <1000 TCID 50/g lung tissue) in the lungs of aged BALB/c mice and young BALB/c mice at 9 dpi. In contrast, very young SCID mice, which lack functional T and B cells, were persistently infected with COVID‐19 during the experimental period (through 20 dpi). At this time, SCID mice did not exhibit histological signs of severe pneumonia at 9 and 20 dpi, although a high viral titer was detected in the lungs of these animals during the experimental period. Zhao and Perlman 7 and also Yasui and et al 10 obtained similar results in Sars‐Cov suggesting that animals lack T and B cell populations. To investigate the effect of adaptive immune responses on clearance of pulmonary‐infected COVID‐19, either naïve splenocytes or sensitized splenocytes were adoptively transplanted into naïve very young SCID mice 1 day before COVID‐19 infection. The very young SCID mice and the BALB/c mice that received naïve splenocytes eliminated COVID‐19 from their lung early. The very young SCID mice transplanted with COVID‐19‐sensitized splenocytes of BALB/c mice eliminated COVID‐19 more rapidly than mice transplanted with naïve splenocytes, although the initial pulmonary viral titers were effectively the same in all groups of very young SCID mice at 2 dpi, with or without the transfer of splenocytes. Only three of the very young SCID mice that received sensitized splenocytes showed detectable lung pulmonary viral titer (2.75 × 104 TCID 50/g lung) at 5 dpi; titers in the remaining 29 animals of this group were below the LLOD. The very young SCID mice transplanted with naïve‐ or sensitized‐splenocytes derived from aged BALB/c mice did not show histological signs of severe pneumonia at 10 dpi.
These results indicated that induction of adaptive immune responses is essential for the clearance of pulmonary‐infected COVID‐19 and young mice immune responses are higher than old mice.
2.2. Important Role of CD4+ T cells on COVID‐19‐infected pulmonary cells
To determine host protection(s) involved in the elimination of COVID‐19 in the lungs, we depleted CD4+ cells and/or CD8+ cells before and after COVID‐19 infection in BALB/c mice. Depletion of CD4+ cells or CD8+ cells was done by intravenous (i.v.) injection of monoclonal antibody; control experiments showed that the injection of these mAbs caused an almost complete depletion of the corresponding cell populations. Unlike peripheral blood, the incidence of CD20 B cells was very low in BALB/c mice. We examined (using one of the two models) that CD4+ T cells can directly eliminate COVID‐19 by secretion of IFN‐y. The IFN‐γ‐deficient mice and the very young SCID mice that received splenocytes from IFN‐γ deficient mice controlled the COVID‐19 infection as well as the wild‐type mice did. In summary, CD20 and CD8 T cell depletions were more profound (achieving ~100% loss by day 0) and lasted longer than CD4+ T cell depletion. Following depletion, recovery was very slow in all three lymphocyte subsets and the frequencies did not return to baseline by the end of the study. We found that loss of CD8+ T cells led to a very low peak viral load and the loss of CD4+ T cells led to significantly higher viral loads and disseminated COVID‐19. We found also the elimination of COVID‐19 required the activation of B cells by CD4+ T cells.
These results demonstrated the cellular immunity and more specifically CD4+ T cells are an essential cell type for the control of COVID‐19 infection, and that the effect of this cell fraction is indirect.
These observations have important ramifications for the development of novel vaccination strategies to alleviate COVID‐19 associated diseases.
2.3. Innate immunity and phagocytic cells are essential for the elimination of the COVID‐19‐infected pulmonary cells
Phagocytosis in mammals serves as an important first line defense mechanism against invading pathogens. It is also essential for continuous clearance of dying cells, tissue remodeling, and acquisition of nutrients for some cells.
To identify the effectors involved in the elimination of COVID‐19‐infected pulmonary cells in our mouse models, we tested the contribution of several candidate effectors. COVID‐19‐infected BALB/c mice were depleted of the first candidate. These results demonstrated that the complement‐antibody complex is not required for the control of COVID‐19 infection in this model.
We next investigated the contribution of NK cells to the elimination of by administering anti‐IL‐2Rβ mAb (TM‐β1), a treatment known to result in long‐term depletion of NK cells. 11 We injected i.p. TM‐β1 (100 μL of ascites) into both SCID and BALB/c mice. Three days after TM‐β1 treatment, NK cell‐depleted BALB/c mice splenocytes were transferred adoptively into NK cell‐depleted SCID mice. The SCID mice were infected i.n. with COVID‐19 at 1 day after the splenocyte transfer. NK cells were considerably depleted in both the donor BALB/c mice (spleen) and the recipient SCID mice. However, virus still was eradicated in this NK‐depleted model; thus, Ab‐dependent cell‐mediated cytotoxicity, which requires NK cells, was excluded as a mechanism for the elimination of COVID‐19.
Other blood cells also may serve as effectors for the control of COVID‐19 Specifically, elevated levels of alveolar macrophages, monocyte‐derived infiltrating macrophages, and neutrophils that were observed also in many SARS patients. 12 To investigate the role of these myeloid cells in the clearance of COVID‐19‐infected pulmonary tissue, each subset of these myeloid cells was depleted by administration of a specific mAb or reagent. Consistent with other reports in SARS, 13 alveolar macrophages were depleted for more than 5 days following i.n. administration of 100 μL of 33% clodronate liposome. Neutrophils (CD11b + and Ly‐6G hi) and Gr‐1 int monocytes in blood and lung were significantly depleted for at least 3 days after i.p. treatment with 250 μg of anti‐Gr‐1 mAb, whereas neutrophils alone were depleted upon administration of anti‐Ly‐6G mAb (1A8). The Gr‐1+ cell‐ and/or alveolar macrophage‐depleted groups failed to eliminate the pulmonary COVID‐19 infection, whereas the alveolar macrophage‐depleted group showed partial elimination of the virus. In contrast, the neutrophil‐depleted BALB/c animals (anti‐Ly‐6G mAb‐treated mice) eliminated COVID‐19 by 9 dpi. Importantly, the neutralizing Ab titer of these cell‐depleted mice was comparable to that of untreated BALB/c mice. These results suggest that phagocytic cells, especially monocyte‐derived infiltrating macrophages, cooperate with anti‐COVID‐19 Abs to provide control of COVID‐19 infection in these mouse models while Abs individually have small role in control of COVID‐19 infection.
2.4. Innate Immunity and phagocytes cells are essential in human immunity
The majority of COVID‐19 cases (about 80%) is asymptomatic or exhibits mild to moderate symptoms, but approximately the 15% progresses to severe pneumonia and about 5% eventually develops acute respiratory distress syndrome (ARDS), septic shock and/or multiple organ failure.
Surprisingly there is rare investigation about phagocytes cells role in COVID‐19 patients while mild effection of children to this virus gives great idea about critical role of phagocytes cells in COVID‐19.
Finding a human (especially child) in early stage of COVID‐19 is extremely difficult but we found 13 children in ages between 4 and 10 who were in the first days of infection. We also investigated 33 adults human with severe infection of COVID‐19.
We investigated both innate and adaptive immune systems in all patients. Seroconversion curves of reference [14] were constructed from the data and showed that total antibodies and IgM isotype antibodies were 100% detectable approximately 1 month after symptom onset. However, in the first week after developing signs of illness, antibodies were present in less than 40% of patients tested. The seroconversion rate and antibody levels rose quickly during the fortnight after symptom onset, and the cumulative seropositive rate was 50% on day 11 and 100% on day 39.
Our purpose was to compare IgM isotype antibodies between children and adults. As we expected their bodies first line of defense, the innate immune response, starts right after infection, like an infantry going after a foreign invader, killing the virus and any cells damaged by it. The second line of defense, the adaptive immune response, kicks in days later if any virus remains, employing what it has learned about the virus to mobilize a variety of special forces such as T cells and B cells.
We observed, in the early phases of infection in children, natural antibodies play a most important role. Natural antibodies, mostly of IgM isotype and generated independently of previous antigen encounters, have a broad reactivity and a variable affinity. They contain the infection during the 2 weeks necessary for production of high‐affinity antibodies and MBCs that will clear the virus and prevent reinfection. In humans, natural antibodies are produced by innate or IgM MBCs, a population of MBCs that is generated independently of the germinal centers and is most abundant in children (Figures 1 and 2 and Table 1).
FIGURE 1.
CORONA VIRUS (reference from the internet)
FIGURE 2.
White blood cell immune phagocytosis coronavirus covid‐19 disease cells infection 3d render illustration Premium Photo
TABLE 1.
Role of Adaptive and Innate Immunities in COVID‐19
| Adaptive Immunity | Innate Immunity | ||||
|---|---|---|---|---|---|
| B Cells make antibodies that block the virus. B cells play an important role in elimination of COVID‐19 in primary effection. | T Cells play a role in attacking the coronavirus. T cells activate B cells. | Activation of B cells by CD4+ T cells are very important for elimination of COVID‐19 in primary step of effection. | Neutralizing Ab plays a lesser role but better role together phagocytes. It shows important role of phagocytes in elimination of COVID‐19 | CD8+ T cells are cytotoxic and kill virus‐infected cells | Essential Role of Phagocytes, IgM, and IgG in Elimination of COVID‐19. This is main reason for good immunity of children against COVID‐19 and extremely important for producing vaccine for that virus. |
We measured IgM in all patients including adults and kids. As seen in Figure 3 one may see IgM and IgG in kids patinets are much higher than adults patients in beginning days of infection but it becomes equal almost after 30 days. Higher IgM and IgG in beginning days cause production of much more natural antibodies in kids' bodies to protect them against COVID‐19. This clearly shows why kids are more immune against COVID‐19. It gives also idea about novel vaccination and medicine strategies to alleviate COVID‐19.
FIGURE 3.
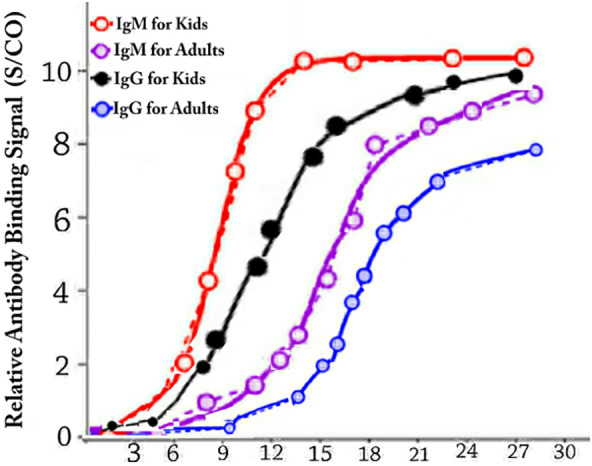
Average IgM and IgG in kids (13 kids in ages between 4 and 10) and adults patients (33 adults between ages 18 and 67) blood per days after infection to COVID‐19. One may see IgM and IgG in kids patients are much higher than adult's patients in beginning days of infection but it becomes equal almost after 30 days. Higher IgM and IgG in beginning days cause production of much more natural antibodies in kids' bodies to protect them against COVID‐19. This clearly shows why kids are more immune against COVID‐19. It gives also idea about novel vaccination and medicine strategies to alleviate COVID‐19
We observed adult patients with severe COVID‐19 showed lower percentage and count in CD4+ and CD8+ lymphocytes populations, strong predictive values for in‐hospital mortality, organ injury, and severe pneumonia. We also observed significantly lower number of total T cells, both helper T cells and suppressor T cells.
Cytotoxic T‐cells (CTLs) and Natural Killer (NK) cells are required to generate an effective immune response against viruses in a human body. Our studies in human found that increased cytokine levels (eg, IL‐6, IL‐10, and TNFα) and lymphopenia (significantly reduced CD4+ and CD8+ T cells) correlate with disease severity of COVID‐19. In addition to reduced T cell counts, the surviving T cells appear dysfunctional. Patients with severe COVID‐19 present significantly lower lymphocyte, and higher neutrophil, counts in blood. Specifically, CD8+ lymphocytes and NK cells were significantly reduced in cases of severe infection compared to patients with mild infection and healthy individuals. 15
3. DISCUSSION
The outbreak of COVID‐19 from December 2019 till now resulted in over several million cases, with about 3% mortality. The worst symptoms might correlate with age‐dependent defects of immune response, given that mortality exceeded 50% in patients over 65 years of age. Retrospective analyzes of recovered COVID‐19 patients suggests that patients who recovered from COVID‐19 possessed specific acquired immunity based on both T and B cells. Notably, most patients who recover from COVID‐19 had elevated and sustained levels of neutralizing Abs, and patients with longer illnesses exhibited lower levels of neutralizing Abs than did patients with shorter durations of illness.
Cytotoxic T‐cells (CTLs) and Natural Killer (NK) cells are required to generate an effective immune response against viruses, 16 functional exhaustion of which results in disease progression. 17 Indeed, patients with COVID‐19 presented with significantly lower lymphocyte and higher neutrophil counts in blood compared to healthy controls. 14 Specifically, CD8+ lymphocytes and NK cells were significantly reduced in severe infection compared to patients with mild infection and healthy controls. 14
We observed and hypothesize that patients with severe COVID‐19 have a severely compromised innate immune response and are therefore more prone to co‐infections and opportunistic infections of the lung.
In this study, we found the lower control of the virus infection by mouse anti‐COVID‐19 antiserum when neutralization titers against COVID‐19 were transferred into recipient mice. Therefore, it appears that the neutralization activity of Ab against COVID‐19 does not correlate so much with the clearance efficacy of the virus from infected murine lung. On the other hand, some other antibody property (such as avidity) may differ, given that a polyclonal species derived from COVID‐19‐infected mice. This result suggests that the anti‐infective activity of a neutralizing Ab is mediated primarily via prevention of COVID‐19 invasion; the neutralizing Ab plays a lesser role in eliminating the virus after establishment of infection.
Therefore, we focused on the cooperation between anti‐COVID‐19 Abs and other effectors in the control of COVID‐19 infection. Candidate effectors include complement (eg, C3 and other members of the complement‐antibody complex pathway), NK cells (mediators of the Ab‐dependent cell‐marriage cytotoxicity pathway), and Fc gamma receptor (FcγR)‐bearing cells (notably alveolar macrophages, monocytes [monocytes‐derived infiltrating macrophages], and neutrophils). We used both anti‐Gr‐1 mAb and neutrophil‐specific mAb (anti‐Ly‐6G mAb) to discriminate monocytes and neutrophils. We tested the role of these candidates by selective depletion of a mouse infection model for various factors using CVF (complement depletion), anti‐IL‐2Rβ mAb (TM‐β1; NK cell depletion), clodronate liposomes (alveolar macrophage depletion), anti‐Gr‐1 mAb (monocytes/neutrophil depletion), or anti‐Ly6G mAb (neutrophil depletion) before or after COVID‐19 infection. Notably, the groups administered with clodronate liposome or anti‐Gr‐1 mAb, but not those treated with anti‐Ly‐6G mAb, failed to eliminate COVID‐19 from their lungs by 9 dpi. Our results indicated that phagocytic cells such as monocyte‐derived infiltrating macrophages and partially alveolar macrophages, but not neutrophils, play a crucial role in the elimination of COVID‐19‐infected pulmonary cells in mice. Our results are critical for COVUD‐19, although further studies are necessary to clarify the mechanism(s) of elimination of COVID‐19 via cooperation of phagocytic cells and COVID‐19‐specific antibodies.
We here demonstrated that both monocyte‐derived macrophages (infiltrating‐type) and partially alveolar macrophages (resident‐type) contribute to the elimination of COVID‐19‐infected pulmonary cells in the presence of anti‐COVID‐19 Abs.
In contrast, Gr‐1+ monocyte‐derived cells are involved in the clearance of COVID‐19 infection, although the role of Gr‐1+ monocyte‐derived cells in the development of pathogenesis during COVID‐19 infection remains unknown.
We investigated also both innate and adaptive immune systems in all human patients. We observed, in the early phases of infection in human, natural antibodies play a most important role. Natural antibodies, mostly of IgM and IgG isotypes and generated independently of previous antigen encounters, have a broad reactivity and a variable affinity. In humans, natural antibodies are produced by innate or IgM, IgG MBCs, a population of MBCs that is generated independently of the germinal centers and is most abundant in children. It shows why children are more immune against COVID‐19. 18
In conclusion, we demonstrate a crucial role for cooperation of antigen‐specific antibodies and phagocytic cells (monocyte‐derived infiltrating macrophages and partially alveolar macrophages) in the elimination of CIVID‐19 in mouse models and human of infection. Our findings provide a better understanding of the mechanism(s) by which host defenses control COVID‐19 infection. Ideally, this information can contribute to the development of novel therapeutic protocols or treatments for COVID‐19. 19
Main effort of all researchers on COVID‐19 in future should be on innate immune system especially phagocytes.
ACKNOWLEDGEMENTS
We express our gratitude to Dr. Kasmai of the Institute of Medical Science for technical assistance in the evaluation of lung histopathology.
This study was supported financially 90% by Dr. Haghro owner of Peace and Health Organization, and other 10% by Prof. Emami from Teratron Inc.
Farshi E, Kasmapur B, Arad A. Investigation of immune cells on elimination of pulmonary‐Infected COVID‐19 and important role of innate immunity, phagocytes. Rev Med Virol. 2021;31:e2158. 10.1002/rmv.2158
Funding information Peace and Health Organization, Grant/Award Number: 134613
DATA AVAILABILITY STATEMENT
Data available on request from the authors The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Gorbalenya AE, Baker SC, Baric RS, et al. The species severe acute respiratory syndrome‐related coronavirus: classifying 2019‐nCoV and naming it SARS‐CoV‐2. Nat Microbiol. 2020;5:536‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou P, Yang X, Wang X, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID‐19) in Wuhan. Clin Infect Dis. 2020;71:762‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roberts A, Paddock C, Vogel L, Butler E, Zaki S, Subbarao K. Aged BALB/c mice as a model for increased severity of severe acute respiratory syndrome in elderly humans. J Virol. 2005;79:5833‐5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roberts A, Deming D, Paddock CD, et al. A mouse‐adapted SARS‐coronavirus causes disease and mortality in BALB/c mice. PLoS Pathog. 2007;3:e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nagata N, Iwata N, Hasegawa H, et al. Mouse‐passaged severe acute respiratory syndrome‐associated coronavirus leads to lethal pulmonary edema and diffuse alveolar damage in adult but not young mice. Am J Pathol. 2008;172:1625‐1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhao J, Perlman S. T cell responses are required for protection from clinical disease and for virus clearance in severe acute respiratory syndrome coronavirus‐infected mice. J Virol. 2010;84:9318‐9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhao J, Van Rooijen N, Perlman S. Evasion by stealth: inefficient immune activation underlies poor T cell response and severe disease in SARS‐CoV‐infected mice. PLoS Pathog. 2009;5:e1000636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen J, Lau YF, Lamirande EW, et al. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS‐CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS‐CoV infection. J Virol. 2010;84:1289‐1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yasui F, Kohara M, Kitakabe M, et al. Phagocytic cells contribute to the antibody‐mediated elimination of pulmonary‐infected SARS coronavirus. Virology. 2014;454‐455:157‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tanaka T, Kitamura F, Nagasaka Y, Kuida K, Suwa H, Miyasaka M. Selective long‐term elimination of natural killer cells in vivo by an anti‐interleukin 2 receptor beta chain monoclonal antibody in mice. J Exp Med. 1993;178:1103‐1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nicholls JM, Poon LL, Lee KC, et al. Lung pathology of fatal severe acute respiratory syndrome. Lancet. 2003;361(9371):1773‐1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pribul PK, Harker J, Wang B, et al. Alveolar macrophages are a major determinant of early responses to viral lung infection but do not influence subsequent disease development. Virology. 2008;82:4441‐4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS‐CoV‐2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020;ciaa344. 10.1093/cid/ciaa344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID‐19 patients. Cell Mol Immunol. 2020;17:533‐535. 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Long Q‐X, Liu B‐Z, Deng H‐J, et al. Antibody responses to SARS‐CoV‐2 in patients with COVID‐19. Nat Med. 2020;26:845‐848. [DOI] [PubMed] [Google Scholar]
- 19. Sun B, Feng Y, Mo X, et al. Kinetics of SARS‐CoV‐2 specific IgM and IgG responses in COVID‐19 patients. Emerg Microbes Infect. 2020;9(1):940‐948. 10.1080/22221751.2020.1762515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the authors The data that support the findings of this study are available from the corresponding author upon reasonable request.


