Coronavirus disease‐19 (COVID‐19) is a new disease caused by SARS‐CoV‐2. Since the beginning of 2020, it has become one of the main challenges of our times, causing a high incidence of severe pneumonia, acute respiratory distress syndrome (ARDS), multiorgan failure, and death. 1 At the root of COVID‐19 lies the sudden development of “cytokine storms,” hyperinflammatory responses involving the release of pro‐inflammatory cytokines (eg, TNF, IL‐6, IL‐1, IL‐8, and MCP‐1) that impair the gas exchange function of the lung and lead in select patients, mostly with underlying comorbidities, to multiorgan failure, and death. 1 , 2 Additional complications triggered by “cytokine storms” include endothelial dysfunction and hypercoagulation, increasing the risk of thromboembolytic events, and life‐threatening cardiovascular complications. Anti‐inflammatory therapies are thus being considered for alleviating the damaging side effects of hyperinflammation with many trials including anti‐cytokine biologicals, disease‐modifying antirheumatic drugs (DMARDs), and corticosteroids being ongoing. 3 Surprisingly, among them dexamethasone has taken center stage as initial results from the RECOVERY trial, a large multicenter randomized open‐label trial, for hospitalized patients run in the United Kingdom, revealed notable efficacy in the treatment of critically ill COVID‐19 patients. 4
Dexamethasone is one of the oldest synthetic glucocorticoid agonists synthesized in 1957 and introduced into the clinic in 1961. When administered at 6 mg daily, either orally or intravenously for 10 days, dexamethasone was shown in the RECOVERY trial to reduce deaths in both patients receiving invasive mechanical ventilation (29.3% vs. 41.4%, rate ratio, 0.64; 95% CI, 0.51 to 0.81) and patients getting oxygen without invasive mechanical ventilation (23.3% vs. 26.2%; rate ratio, 0.82; 95% CI, 0.72 to 0.94. 4 Following age adjustment, this corresponded to a remarkable reduction in mortality of one‐third in mechanically ventilated patients and one‐fifth in patients with oxygen. Benefit was restricted to patients requiring respiratory support; as in milder cases, this was not significant. This notable efficacy of dexamethasone treatment goes against the current view of corticosteroid use in respiratory viral infections which remains contradictory. Although corticosteroids improve ventilator weaning and can lower the intensity of the host response to the virus, tempering the “cytokine storm” and limiting immunopathology, they can also reduce viral clearance and lead to more severe disease. Understanding therefore how dexamethasone mediates its effects is of paramount importance.
Dexamethasone, as other corticosteroids, is held to mediate its anti‐inflammatory and immunosuppressive effects via the glucocorticoid receptor (GR), a ligand activated transcription factor. Upon ligand binding, the cytoplasmic GR dimerizes and translocates to the nucleus where it can enhance transcription by binding cooperatively as a homodimer to glucocorticoid response elements (GREs). 5 On the contrary, the monomeric GR can repress transcription by binding directly to negative glucocorticoid response elements (nGREs) or GRE half‐sites or by tethering to DNA‐bound transcription factors such as NF‐κB or AP‐1, regulating the expression of diverse sets of genes. This results in the inhibition of inflammatory cell activity, including neutrophils, macrophages, and lymphocytes, and the suppression of pro‐inflammatory cytokines such as TNF and interleukins and other genes such as cyclooxygenase‐2 and inducible nitric oxide synthase. 6 However, we have recently uncovered that dexamethasone can also induce the D‐series proresolving lipid mediator pathway leading to the production of 17S‐dihydroxydocosahexaenoic acid (17S‐HDHA) and the protectins D1 and DX. 7 These are potent major players of the molecular machinery driving the resolution of inflammation, that is, the proper regulated termination of pro‐inflammatory responses involving the catabolism of pro‐inflammatory mediators, the removal of inflammatory cells, and the restoration of the tissue in a timely and highly coordinated manner. 8 Although resolution of inflammation has long been considered to occur spontaneously as a result of the waning of pro‐inflammatory responses, this is now known to be an ordered and highly regulated process involving the timely production of enzymatically oxygenated lipid‐derived mediators such as protectins, D‐series resolvins and maresins derived from the omega‐3 fatty acid docosahexaenoic acid (DHA), E‐series resolvins derived from eicosapentaenoic acid (EPA), and lipoxins biosynthesized from omega‐6 fatty acids following eicosanoid class switching. 8 Interestingly, certain lipid mediators have been shown to exert additional nonconventional functions; resolvin D4 can attenuate pathologic thrombosis, reduce NETosis, and promote clot removal 9 which is now recognized as a key pathology of COVID‐19 infection, while resolvin E4 (RvE4) stimulates efferocytosis of senescent erythrocytes in hemorrhagic exudates especially under hypoxic conditions that characterize COVID‐19. 10 Moreover, corticosteroids have been reported to reduce fibrinogen and procoagulant factors under pro‐inflammatory conditions and increase anticoagulant factors. 11
The ability of viral infections to induce proresolving lipid mediators has been reported earlier. Toll‐like receptor 7 (TLR7), a major pattern recognition receptor of viral RNA, activates PD1 and PDX production. 12 Moreover, influenza virus infection has been demonstrated to drive proresolving lipid mediator networks including the production of PD1 which limits influenza pathogenicity by directly interacting with the RNA replication machinery to inhibit viral RNA nuclear export. 13 , 14 Notably, in particularly virulent strains of influenza virus such as the H5N1 avian strain, PD1 formation is not sufficiently upregulated, leading to more efficient viral replication and host demise. 13 It is therefore plausible that the efficacy of dexamethasone in COVID‐19 is due at least in part to its ability to induce proresolving lipid mediators. These possess multiple anti‐inflammatory and proresolving actions tempering down inflammation and promoting its resolution, preventing coagulation, and enhancing viral and bacterial clearance (Figure 1), yet they are not immunosuppressive. Whether other corticosteroids beyond dexamethasone can also mediate such effects, and to what extent, is not known. Whether inhaled corticosteroids, such as those given to asthmatic patients, can also induce proresolving lipid mediator networks locally and thus prevent the development of severe SARS‐CoV‐2 infection remains to be determined. There is evidence suggesting that asthmatic patients may exhibit reduced incidence of severe and/or critical COVID‐19. 15
FIGURE 1.
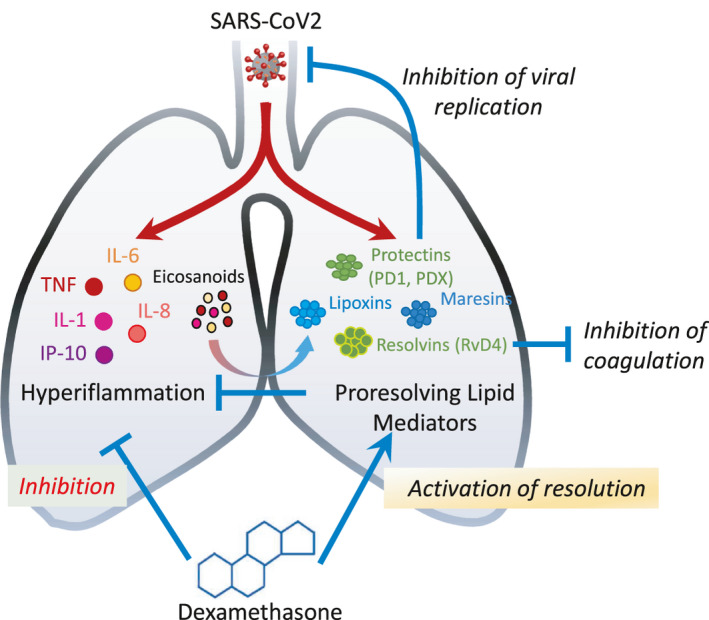
Hypothetical model of dexamethasone mode of action involving inhibition of the cytokine storm and induction of proresolving lipids such as Protectin D1 (PD1) and Resolvin D4. SARS‐CoV‐2 infection triggers hyperinflammation characterized by a “cytokine storm” involving TNF, IL‐1, IL‐6, IL‐8, and MCP‐1 production and release of eicosanoids. Proresolving meditators including protectins, resolvins, and maresins are also induced as an effort of our organism to counterbalance the immune response. These act to reduce inflammation and promote its resolution but may also help resolve coagulation and block viral replication. Strengthening this response through the temporal administration of dexamethasone, triggering conventional anti‐inflammatory effects as well as production of D‐series protectins, could result in notable benefit in patients
Recently, severe COVID‐19 patients showed a stronger association with serum arachidonate‐derived pro‐inflammatory lipid mediators, for example prostaglandins, than certain proresolving mediators such as resolvin E3 which were preferentially upregulated in the moderate COVID‐19 group. This suggested that an imbalance in lipid mediators with a swift toward pro‐inflammatory mediators in severe disease may contribute to COVID‐19 severity. 16 Although the involvement of proresolving lipid mediator pathways in COVID‐19 is an attractive hypothesis, further evidence from human trials is needed as there are no studies at present reporting the induction or modulation of such networks in the context of the various disease stages and treatments. It is thus of uttermost priority to investigate proresolving lipid mediators in COVID‐19, in a temporal and longitudinal manner, as modulating these networks either through drug treatment or direct administration of resolvins and protectins as agonists of resolution has the potential to affect this highly lethal and devastating disease in a way other approaches cannot. Such studies are therefore eagerly awaited.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
EA is supported by research grants from the European Commission (IMMUNAID, No 779295 and CURE, No. 767015) and the Hellenic Foundation for Research and Innovation (INTERFLU, No 1574). CNS contributions are supported by the NIH grant P01GM095467.
REFERENCES
- 1. Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 Pneumonia in Wuhan, China. JAMA Int Med. 2020;180(7):934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID‐19): A Review. JAMA 2020;323(18):1824‐1836. [DOI] [PubMed] [Google Scholar]
- 4. Horby P, Lim WS, Emberson JR, et al. Dexamethasone in Hospitalized Patients with Covid‐19 ‐ Preliminary Report. N Engl J Med. 2020. 10.1056/NEJMoa2021436. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weikum ER, Knuesel MT, Ortlund EA, Yamamoto KR. Glucocorticoid receptor control of transcription: precision and plasticity via allostery. Nat Rev Mol Cell Biol. 2017;18(3):159‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids–new mechanisms for old drugs. N Engl J Med. 2005;353(16):1711‐1723. [DOI] [PubMed] [Google Scholar]
- 7. Pyrillou K, Chairakaki AD, Tamvakopoulos C, Andreakos E. Dexamethasone induces omega3‐derived immunoresolvents driving resolution of allergic airway inflammation. J Allergy Clin Immunol. 2018;142(2):691‐695. [DOI] [PubMed] [Google Scholar]
- 8. Serhan CN. Pro‐resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cherpokova D, Jouvene CC, Libreros S, et al. Resolvin D4 attenuates the severity of pathological thrombosis in mice. Blood. 2019;134(17):1458‐1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Norris PC, Libreros S, Serhan CN. Resolution metabolomes activated by hypoxic environment. Sci Adv. 2019;5(10):eaax4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Zaane B, Nur E, Squizzato A, et al. Systematic review on the effect of glucocorticoid use on procoagulant, anti‐coagulant and fibrinolytic factors. J Thromb Haemost. 2010;8(11):2483‐2493. [DOI] [PubMed] [Google Scholar]
- 12. Koltsida O, Karamnov S, Pyrillou K, et al. Toll‐like receptor 7 stimulates production of specialized pro‐resolving lipid mediators and promotes resolution of airway inflammation. EMBO Mol Med. 2013;5(5):762‐775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morita M, Kuba K, Ichikawa A, et al. The lipid mediator protectin D1 inhibits influenza virus replication and improves severe influenza. Cell. 2013;153(1):112‐125. [DOI] [PubMed] [Google Scholar]
- 14. Tam VC, Quehenberger O, Oshansky CM, et al. Lipidomic profiling of influenza infection identifies mediators that induce and resolve inflammation. Cell. 2013;154(1):213‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Avdeev S, Moiseev S, Brovko M, et al. Low prevalence of bronchial asthma and chronic obstructive lung disease among intensive care unit patients with COVID‐19. Allergy. 2020;75:2703‐2704. 10.1111/all.14420. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schwarz B, Sharma L, Roberts L, et al. Severe SARS‐CoV‐2 infection in humans is defined by a shift in the serum lipidome resulting in dysregulation of eicosanoid immune mediators. Res Sq. 2020. 10.21203/rs.3.rs-42999/v1. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]


