There is a worldwide discussion on the role of aerosols in the transmission of SARS‐CoV‐2. The WHO has recently highlighted 1 the possible role of aerosols in the transmission of SARS‐CoV‐2. Aerosols containing small respirable particles are increasingly recognized as a potentially important mode of transmission of SARS‐CoV‐2. 2 , 3 , 4 , 5 We investigate aerosol droplet persistence inside the confined space of hospital elevators and propose specific measures to reduce the associated risk of SARS‐CoV‐2 transmission. To investigate aerosol persistence in elevators, we mimic a single cough using a specially designed spray nozzle to disperse a controlled quantity of glycerol/ethanol droplets, that after evaporation of the ethanol have the same size distribution as evaporated respiratory droplets from a single cough. 5 A SprayScan® laser sheet is used to detect aerosols that were placed in the back of the elevator at half‐height. Experiments were performed inside elevator cabins during normal operation with about 10%‐20% open door time. We found that it typically takes 12‐18 minutes before the number of aerosol particles decreased 100‐fold during normal operation of both medium‐ and large‐sized elevator cabins. With elevator doors permanently open, this time is reduced to 2‐4 minutes (Figure 1). In all cases, the number of aerosols decreases exponentially in time.
Figure 1.
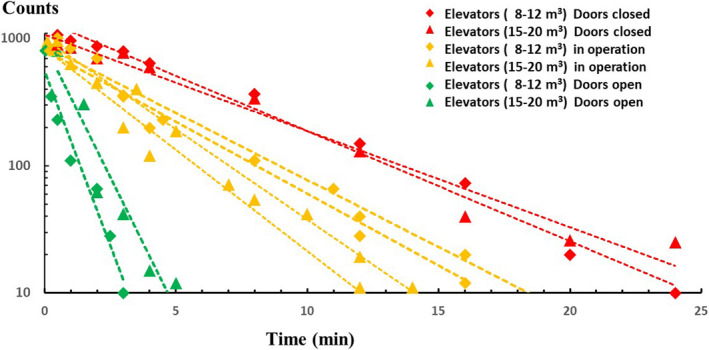
Aerosol droplets in elevators. Averaged number of aerosol droplets as a function of time since production as counted in large (15‐20 m3) elevator cabins [triangles] and medium‐sized (8‐12 m3) cabins [diamonds] during normal operation [orange], with permanently open doors [green], and permanently closed [red]. In all experiments, the ventilation was on with an average ACH = 10 value
SARS‐CoV‐2‐infected sputum droplets from hospitalized but generally mildly ill people may carry between 104 and 109 RNA copies per mL. 6 A value of 109 implies roughly one virus particle per aerosol droplet with a radius of 5 µm; loud speaking may produce up to a few hundred thousand droplets per minute, 7 , 8 , 9 whereas a single cough can already produce a few million droplets. 10 Breathing the air after speaking or coughing of an infected patient inside an elevator with a volume of 10‐15 m3 and inhaling about 8 L/min then implies a potential intake of tens up to many thousands SARS‐CoV‐2 RNA copies per minute, depending on the patient's infectivity. The minimum infectious dose for SARS‐CoV‐2 has not been reported; the severity of COVID‐19 is, however, believed to be proportional to the dose of the initial inoculum, implying that transmission by aerosols may lead to relatively milder symptoms.
Specific measures to reduce the risk of SARS‐CoV‐2 transmission may include leaving elevator doors open for a longer period, as is suggested by our data (Figure 1) on open doors. Also wearing an adequate face mask might possibly reduce this risk. A medical grade face mask comprising an electret layer (eg, N95 or N99) to capture aerosols with an efficacy of more than 95% is a good measure for more protection in the confined space of an elevator.
An increase of the elevator's mechanical ventilation capacity is also recommended. Current standards for the air change rate by mechanical ventilation in hospital elevators may vary between 6 and 20 air changes per hour. If we assume that aerosol particles will be continuously mixed with supply air, without taking into account particle deposition, re‐suspension, and stagnant flows, an air change rate of 10 times per hour implies a 100‐fold reduction in aerosol particles in about 28 minutes. From the experiments, we find a 100‐fold reduction in 24‐30 minutes (cf. ACH = 10) with closed doors, 12‐18 minutes during operation, and 3‐5 minutes with open doors (Figure 1). A remarkable finding was that the ventilation inside all studied elevators in idle position automatically shuts off after 1‐2 minutes, which of course can easily be prolonged by reprogramming the action control software. In most hospital elevators, the ventilator is present in the ceiling and exhausts air from the cabin toward the elevator shaft. A possible measure is reversing the flow direction of the ventilator, herewith creating a unidirectional downflow of fresh (eg, HEPA filtered) air from the ceiling towards the floor of the elevator cabin, a measure that is standard in most operating rooms to create and maintain an airborne microbial free environment.
CONFLICT OF INTEREST
None reported.
AUTHOR CONTRIBUTION
Cees van Rijn: Conceptualization (equal); Data curation (equal); Investigation (equal). G. Aernout Somsen: Conceptualization (equal); Investigation (equal). Leonard Hofstra: Conceptualization (equal); Data curation (equal). Ghassan Dahhan: Conceptualization (equal). Reinout A. Bem: Conceptualization (equal); Methodology (equal). Stefan Kooij: Data curation (equal); Investigation (equal). Daniel Bonn: Conceptualization (equal); Data curation (equal); Investigation (equal); Methodology (equal); Project administration (equal); Writing‐original draft (equal); Writing‐review & editing (equal).
Funding information
SARS‐CoV‐2 University of Amsterdam fund.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1111/ina.12744.
ACKNOWLEDGEMENTS
We thank Tess Heeremans for an elightening discussion.
DATA AVAILABILITY STATEMENT
All data reported here are available from the corresponding author upon reasonable request.
REFERENCES
- 1. World Health Organization . Transmission of SARS‐CoV‐2: implications for infection prevention precautions: scientific brief, 09 July 2020 (No. WHO/2019‐nCoV/Sci_Brief/Transmission_modes/2020.3). World Health Organization; 2020.
- 2. Nardell EA, Nathavitharana RR. Airborne spread of SARS‐CoV‐2 and a potential role for air disinfection. JAMA. 2020;324(2):141. [DOI] [PubMed] [Google Scholar]
- 3. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS‐CoV‐2 as compared with SARS‐CoV‐1. N Engl J Med. 2020;580:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yu ITS, Li Y, Wong TW, et al. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N Engl J Med. 2004;350:1731‐1739. [DOI] [PubMed] [Google Scholar]
- 5. Somsen GA, van Rijn C, Kooij S, et al. Small droplet aerosols in poorly ventilated spaces; the need for specific measures to prevent SARS‐CoV‐2 transmission. Lancet Respir Med. 2020;8(7):658‐659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID‐2019. Nature. 2020;581(7809):465‐469. [DOI] [PubMed] [Google Scholar]
- 7. Lee J, Yoo D, Ryo S, et al. Quantity, size distribution, and characteristics of cough‐generated aerosol produced by patients with an upper respiratory tract infection. Aerosol Air Qual Res. 2019;19:840‐853. [Google Scholar]
- 8. Stadnytskyi V, Bax EC, Bax A, Anfinrud P. The airborne lifetime of small speech droplets and their potential importance in SARS‐CoV‐2 transmission. Proc Natl Acad Sci. 2020;117:11875‐11877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Asadi S, Wexler AS, Cappa CD, Barreda S, Bouvier NM, Ristenpart WD. Aerosol emission and superemission during human speech increase with voice loudness. Sci Rep. 2019;9(1):2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Riediker M, Tsai D‐H. Estimation of viral aerosol emissions from simulated individuals with asymptomatic to moderate coronavirus disease 2019. JAMA Netw Open. 2020;3(7):e2013807. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data reported here are available from the corresponding author upon reasonable request.


