1.
Dear Editors,
Coronavirus disease (COVID‐19) is caused by SARS‐CoV‐2, 1 whose genetic structure is similar to SARS. 2 Majority of patients have mild and nonspecific symptoms, or some are even asymptomatic. 3 Several laboratory tests, such as lymphocyte counts, neutrophil/lymphocyte ratio, C‐reactive protein, chest imaging studies, and nuclear acid testing, have been described and used to aid to diagnose the disease. 4 As we know, circulating mononuclear cells, such as monocytes and lymphocytes, play important roles in immune surveillance and pathogen clearance. They are among the first to respond to invading intracellular pathogenic organisms, such as SARS‐CoV‐2. The immune defense against a viral infection includes monocyte and natural killer cell activation, subsequent lymphocyte stimulation, proliferation, differentiation and cytokine or antibody secretion. It is imaginable that these functionally activated immune cells may undergo morphologic changes as compared to their normal “resting” counterparts. A classic example is that the numbers of atypical lymphocytes are significantly increased in infectious mononucleosis caused by Epstein‐Barr virus infection. Recent studies also demonstrated that alteration in peripheral lymphocyte subsets was associated with the clinical characteristics of COVID‐19 5 and the lymphocyte morphologic parameters were also altered in other viral infections. 6 , 7 , 8
VCS technology uses Coulter Principle of direct current impedance for the cell volume (V) of all cell types, radio frequency opacity to evaluate cellular conductivity (C) for cytoplasmic chemical composition and nuclear volume, and the coherent light of a laser beam to measure multiple angled light scatters (S) for cell surface topography, cytoplasmic granularity, and nuclear structure. These morphometric measurements of peripheral blood leukocytes are known as cell population data (CPD). With this powerful VCS technology, we hypothesized that the changes in volumetric parameters of circulating activated mononuclear cells could be quantitatively determined and would serve as potential viral biomarkers for detecting SARS‐CoV‐2 infection.
In this retrospective case‐control study, clinical data of 155 patients including contact history, initial symptoms, routine hematology measurement, chest computed tomography (CT), and RT‐PCR analysis were reviewed and collected from February 14 to February 29, 2020, in The Wuhan Union Hospital, Wuhan China. There were 93 COVID‐19 hospitalized patients including 39 males (mean age: 50.92 ± 17.87) and 54 females (mean age; 43.65 ± 15.06). The main symptoms included fever (68%), dry cough (47%), shortness of breath (41%), fatigue (29%), myalgias (20%), and diarrhea (6%). Approximately 65% (60/93) of patients had CT lung image resembling viral infection. All COVID‐19 patients were confirmed by positive RT‐PCR testing. There were 62 control patients including 37 males (mean age: 42.28 ± 14.67) and 25 females (mean age; 45.4 ± 18.06) without known contact history, viral symptoms, and radiological evidence of viral infection. The patients with known hematological disorders or evidence of severe bacterial infection at time of admission were excluded from study. The study protocol was approved by the Ethics Committee of Union Hospital, Tongji Medical College. All blood samples were collected at the time of admission and analyzed on a UniCel DxH 800 hematology analyzer (Beckman Coulter, Brea, CA) within 4 hours of collection. This instrument measures CBC with differentials and cell morphologic parameters, such as mean neutrophil volume (MNV), neutrophil distribution width (NDW), mean monocyte volume (MMV), monocyte distribution width (MDW), mean lymphocyte volume (LV), and lymphocyte distribution width (LV‐SD). Lymph index was calculated as LV* LV‐SD/LC (lymphocyte conductivity) as previously described. 6 , 8 Currently, these cell morphologic parameters are research use parameters on DxH 800, and their clinical utility has not been established. All analyses including receiver operating characteristics were performed with SAS software, version 9.4. A P value less than 0.05 was considered significant.
As illustrated in Table 1 and Figure 1, the mean monocyte volume (MMV), monocyte distribution width (MDW), mean lymphocyte volume (LV), lymphocyte distribution width (LV‐SD), and lymph index were significantly increased in COVID‐19 patients compared to those in the controls. No significant differences in neutrophil volumetric parameters or WBC between the patients and the controls were observed. The sensitivity and the specificity of CBC and CPD parameters were then calculated at the designated cutoff values for their best ability to discriminate COVID‐19 patients from controls (Table 2). Lymph index (cutoff value, 11.7) and the MDW (cutoff value, 20.1) showed the superior sensitivities of 77.42% and 82.26% and the specificities of 75.81% and 72.58%, respectively, to detect SARS‐CoV‐2 infection compared to those of conventional hematologic parameters (NE%, LY%, MO% and NLR). ROC curves analysis revealed that lymph index and the MDW had the largest areas under the curve (AUC) of 0.84 and 0.86, respectively. Lymph index in combination with the MDW demonstrates excellent diagnostic performance with AUC increasing to 0.89.
Table 1.
CBC and CPD parameters
| Parameters | Controls (N = 62) | Patients (N = 93) | P Values |
|---|---|---|---|
| MDW | 18.86 ± 2.02 | 22.09 ± 2.32 | <.0001 |
| MMV | 169.65 ± 6.79 | 174.05 ± 8.59 | .0004 |
| MNV | 145.50 ± 6.01 | 144.13 ± 5.78 | .4406 |
| NDW | 17.57 ± 2.66 | 17.29 ± 1.71 | .9151 |
| LV | 87.18 ± 3.57 | 89.01 ± 4.26 | .0058 |
| LV‐SD | 14.01 ± 1.52 | 16.21 ± 1.64 | <.0001 |
| LC | 111.06 ± 5.76 | 111.17 ± 3.25 | .1739 |
| Lymph Index | 11.01 ± 1.18 | 13.01 ± 1.64 | <.0001 |
| WBC (×109/L) | 6.32 ± 2.16 | 5.75 ± 2.09 | .0675 |
| NE (%) | 59.27 ± 9.75 | 63.29 ± 15.44 | .0222 |
| LY (%) | 28.89 ± 9.36 | 23.91 ± 11.48 | .0051 |
| MO (%) | 8.46 ± 2.30 | 10.80 ± 8.45 | .0005 |
| NLR | 2.66 ± 2.48 | 4.24 ± 4.79 | .0091 |
Data are expressed as the mean ± SD.
Abbreviations: CBC, complete blood count; CPD, cell population data; LC, lymphocyte conductivity; LV, mean lymphocyte volume; LV‐SD, lymphocyte distribution width; LY, lymphocyte; Lymph Index = (LV × LV‐SD)/LC; MDW, monocyte distribution width; MMV, mean monocyte volume; MNV, mean neutrophils volume; MO, monocyte; NDW, neutrophil distribution width; NE, neutrophil; NLR, neutrophils/lymphocyte ratio; WBC, white blood cell.
Figure 1.
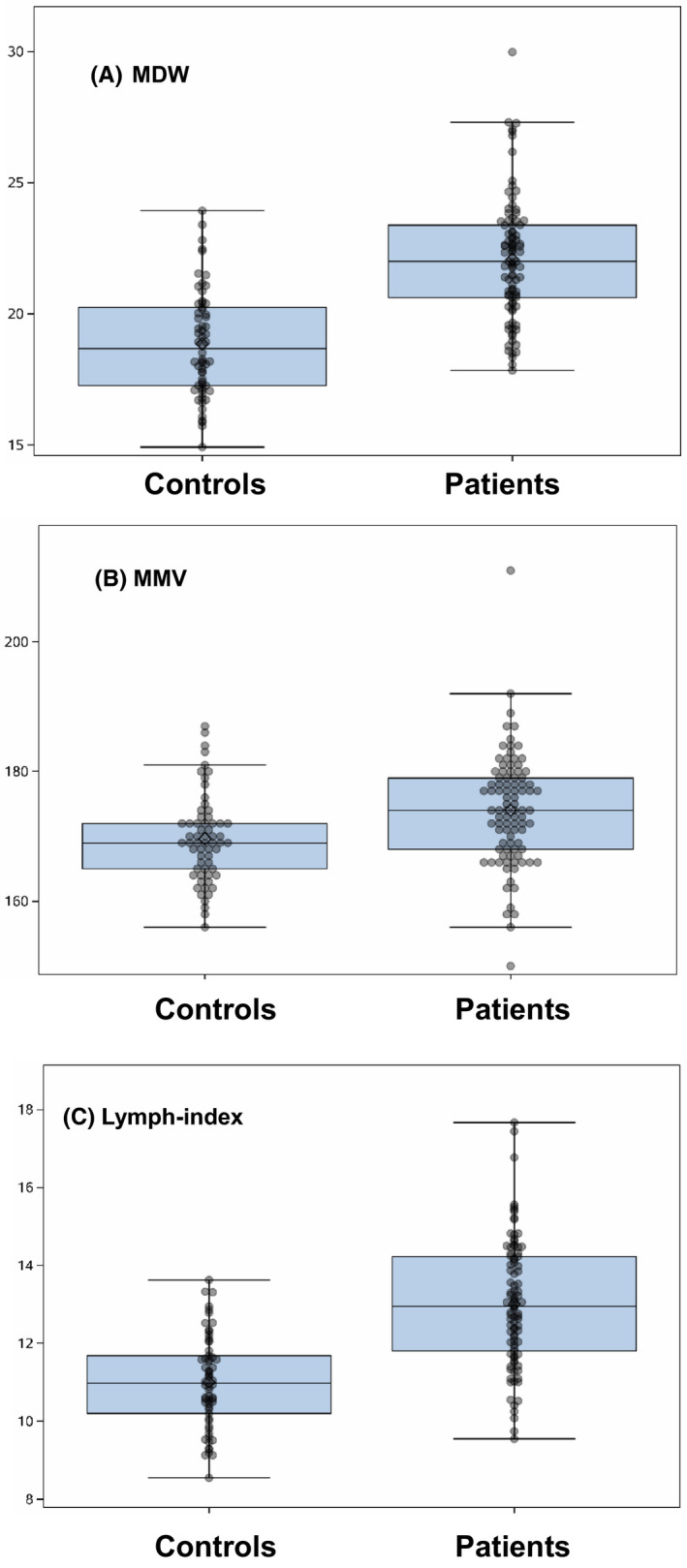
The monocyte volumetric parameters and lymph index in COVID‐19 patients. MDW (A), MMV (B), and lymph index (C) were significantly increased in COVID‐19 patients compared to those in controls
Table 2.
CBC and CPD parameters in detecting SARS‐CoV‐2 infection
| Variables | AUC | 95% CI for AUC | Cutoff Point | Sensitivity | Specificity |
|---|---|---|---|---|---|
| MDW | 0.8550 | 0.7950, 0.9152 | 20.11 | 82.26% | 72.58% |
| MMV | 0.6710 | 0.5842, 0.7580 | 172.000 | 58.06% | 75.81% |
| Lymph Index | 0.8370 | 0.7755, 0.8985 | 11.680 | 77.42% | 75.81% |
| MNV | 0.5370 | 0.4447, 0.6290 | 143.000 | 54.84% | 38.71% |
| NDW | 0.5050 | 0.4083, 0.6021 | 18.140 | 19.35% | 69.35% |
| LV | 0.6380 | 0.5496, 0.7260 | 87.000 | 65.59% | 61.29% |
| LV‐SD | 0.8590 | 0.7979, 0.9194 | 15.150 | 73.12% | 85.48% |
| LC | 0.5650 | 0.4697, 0.6596 | 110.000 | 61.29% | 56.45% |
| WBC (×109/L) | 0.5870 | 0.4947, 0.6791 | 5.017 | 53.76% | 29.03% |
| NE (%) | 0.6100 | 0.5214, 0.6981 | 64.900 | 48.39% | 80.65% |
| LY (%) | 0.6470 | 0.5602, 0.7340 | 22.700 | 49.46% | 22.58% |
| MO (%) | 0.6700 | 0.5822, 0.7570 | 8.900 | 69.89% | 67.74% |
| NLR | 0.6260 | 0.5378, 0.7133 | 2.820 | 49.46% | 77.42% |
| MDW + MMV | 0.8560 | 0.7973, 0.9155 | 0.618 | 76.34% | 79.03% |
| MDW + lymph index | 0.8940 | 0.8448, 0.9426 | 0.615 | 80.65% | 79.03% |
| MMV + lymph index | 0.8390 | 0.7771, 0.9000 | 0.491 | 82.8% | 70.97% |
| MDW + MMV +lymph index | 0.8990 | 0.8511, 0.9471 | 0.716 | 77.42% | 93.55% |
Data are expressed as the mean ± SD.
Abbreviations: CBC, complete blood count; CPD, cell population data; LC, lymphocyte conductivity; LV, mean lymphocyte volume; LV‐SD, lymphocyte distribution width; LY, lymphocyte; Lymph Index = (LV × LV‐SD)/LC; MDW, monocyte distribution width; MMV, mean monocyte volume; MNV, mean neutrophils volume; MO, monocyte; NDW, neutrophil distribution width; NE, neutrophil; NLR, neutrophils/lymphocyte ratio; WBC, white blood cell.
Circulating monocytes and lymphocytes play a decisive role in maintaining immune homeostasis and inflammatory response throughout the body. These immune cells are among the first to respond to viral infection. 9 The previous studies have shown that some morphologic parameters of lymphocytes are altered during viral infections. 6 , 7 , 8 Therefore, the changes of these volumetric parameters in the mononuclear cells may have potential as viral biomarkers. In this proof of concept study, we have demonstrated that both lymph index and the MDW were significantly increased in COVID‐19 patients. These observations were supported by a recent study that showed also elevated MDW in COVID‐19 patients. 10 Elevated lymph index and the monocyte volumetric parameters indicate increased overall cell sizes with greater cell size variations among circulating lymphocyte and monocyte populations. With designated cutoff values, lymph index and the MDW outperformed other hematologic parameters in diagnosing SARS‐CoV‐2 infection. In addition, lymph index in combination with the MDW shows excellent diagnostic performance with AUC increasing to 0.89. We did not see any significant changes in neutrophil volumetric parameters, the MNV and the NDW. This is consistent with the results of recent human studies that neutrophils mainly function as first responders during the innate immune response to acute bacterial infection or sepsis. 11 , 12 Although a recent report demonstrated that the MDW was significantly increased in septic patients, these patients had also significantly elevated neutrophil volumetric parameters (MNV and NDW). 13 Therefore, combination of both elevated lymph index and the MDW without significant increases in MNV and NDW as well as normal or mildly decreased WBC or lymphocyte count appear to be unique leukocyte profiles for viral infection.
The complete blood cell analysis plays an important role in healthcare decision making from diagnosis through therapy and prognosis. Currently, the automated hematology analyzers are able to provide accurate numerical cell counts. However, information provided by conventional leukocyte parameters, such as total leukocyte count, tends to be extremely variable and nonspecific. Therefore, our observations of altered leukocyte volumetric parameters in SARS‐CoV‐2 infection may have some potential clinical application. These parameters are generated during automated leukocyte differential analysis and offer more robust turn around time. Although a larger prospective study is needed to confirm that our findings can be standardized across multiple laboratories using similar or other instruments and to determine whether these results perform better when monitored over time, we believe that the mononuclear cell volumetric parameters, lymph index and the MDW, may have potential to be used as viral biomarkers in hematology laboratory and would provide clinicians with valuable information during initial phase of patient evaluation.
CONFLICT OF INTEREST
The authors have no competing interests.
AUTHOR CONTRIBUTIONS
Xiaoqian Zeng supervised the project, collected and analyzed data. Hui Xing, Yan Wei, Zhaoming Tang, Xiao Lu, Zhao Wang, Yuying Liu, and Liang Xu collected and analyzed data. Lihua Hu and Lin Wang supervised the project and revised the manuscript. Dongsheng Xu designed the project and wrote the manuscript.
REFERENCES
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pan Y, Guan H, Zhou S, et al. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019‐nCoV): a study of 63 patients in Wuhan, China. Eur Radiol. 2020;30: 3306‐3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person to person transmission: a study of a family cluster. Lancet. 2020;395(10223):514‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tan W, Zhao X, Ma X, et al. A novel coronavirus genome identified in a cluster of pneumonia cases‐Wuhan, China 2019–2020. N Engl J Med. 2020;382(8):727‐733.31978945 [Google Scholar]
- 5. Wang F, Nie J, Wang H, et al. Characteristics of peripheral lymphocyte subset alteration in COVID‐19 pneumonia. J infect Dis. 2020;221(11):1762‐1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhu Y, Cao X, Xu D. Detection of morphologic changes in peripheral mononuclear cells in Hepatitis B Virus infection using the Beckman Coulter LH750. Lab Hematol. 2011;17:22‐26. [DOI] [PubMed] [Google Scholar]
- 7. Jung YJ, Kim JH, Park IR, et al. Evaluation of cell population data on the UniCel DxH 800 Coulter Cellular Analysis system as a screening for viral infection in children. Int J Lab Hematol. 2012;34:283‐289. [DOI] [PubMed] [Google Scholar]
- 8. Zhu Y, Cao X, Tao G, et al. The lymph index: a potential hematological parameter for viral infection. Int J Infect Dis. 2013;17:e490‐e493. [DOI] [PubMed] [Google Scholar]
- 9. Chan PKS, Chen GG. Mechanisms of lymphocyte loss in SARS coronavirus infection. Hong Kong Med J. 2008;14(suppl 4):21‐26. [PubMed] [Google Scholar]
- 10. Ognibene A, Lorubbio M, Magliocca P, et al. Elevated monocyte distribution width in COVID‐19 patients: The contribution of the novel sepsis indicator. Clin Chim Acta. 2020;509:22‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chaves F, Tierno B, Xu D. Quantitative determination of neutrophil VCS parameters by the Coulter automated hematology analyzer: new and reliable indicators for acute bacterial infection. Am Clin Pathol. 2005;124:440‐444. [DOI] [PubMed] [Google Scholar]
- 12. Chaves F, Tierno B, Xu D. Neutrophil volume distribution width: a new automated hematologic parameter for acute infection. Arch Pathol Lab Med. 2006;130:378‐380. [DOI] [PubMed] [Google Scholar]
- 13. Crouser ED, Parrillo JE, Seymour C, et al. Improved early detection of sepsis in the ED with a novel monocyte distribution width biomarker. Chest. 2017;152:518‐526. [DOI] [PMC free article] [PubMed] [Google Scholar]
Contributor Information
Lihua Hu, Email: dxu@cblpath.com.
Lin Wang, Email: lin_wang@hust.edu.cn.
Dongsheng Xu, Email: hulh@126.com.


