Abstract
There have been multiple descriptions of seizures during the acute infectious period in patients with COVID‐19. However, there have been no reports of status epilepticus after recovery from COVID‐19 infection. Herein, we discuss a patient with refractory status epilepticus 6 weeks after initial infection with COVID‐19. Extensive workup demonstrated elevated inflammatory markers, recurrence of a positive nasopharyngeal SARS‐CoV‐2 polymerase chain reaction, and hippocampal atrophy. Postinfectious inflammation may have triggered refractory status epilepticus in a manner similar to the multisystemic inflammatory syndrome observed in children after COVID‐19.
Keywords: COVID‐19, inflammatory response, postinfectious, refractory status epilepticus, SARS‐CoV‐2, seizures
1. INTRODUCTION
Although the prevalence and pathophysiology have yet to be fully elucidated, there have been multiple reports of seizures in the setting of acute COVID‐19 infection. 1 , 2 , 3 This report describes a case of refractory status epilepticus (RSE) after recovery from acute COVID‐19, likely secondary to a postinfectious inflammatory response.
2. CASE REPORT
A 69‐year‐old African American woman was initially admitted to our medical center with confusion, diarrhea, and cough in March 2020. She had diabetes with a hemoglobin A1c of 9.4%, which had been complicated by nephropathy necessitating a renal transplant and was on tacrolimus, mycophenolate, and prednisone. Although she had no history of seizures, she had a routine electroencephalogram (EEG) and magnetic resonance imaging (MRI) 3 months prior to presentation as part of the workup for forgetfulness. The EEG captured awake and drowsy states, and revealed mild generalized slowing. The MRI was notable for mild hippocampal atrophy, right greater than left (Figure 1A).
FIGURE 1.
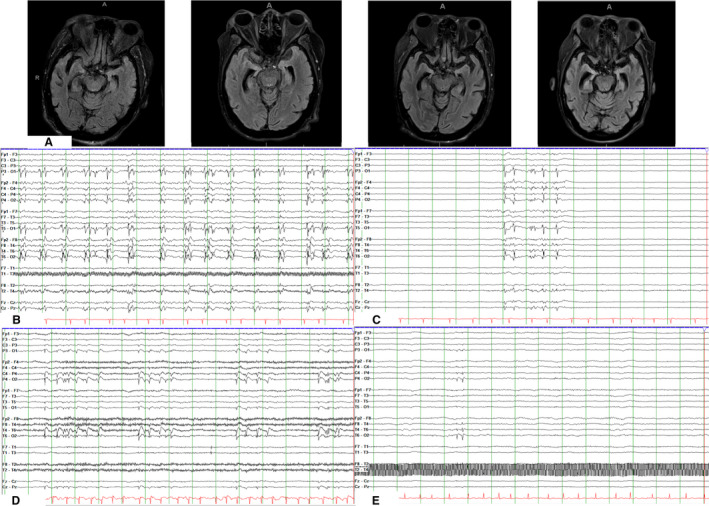
A, Magnetic resonance imaging brain axial fluid‐attenuated inversion recovery sequences, from left to right, obtained 3 months prior to diagnosis of COVID‐19, on hospital day (HD) #8, HD #15, and HD #29 of hospital course #2, demonstrating global cerebral atrophy and progressive right > left hippocampal atrophy. B, Electroencephalogram (EEG) from HD #8 of hospital course #2, demonstrating bilateral synchronous periodic discharges maximal over the right posterior quadrant and the left occipital pole. C, EEG from HD #9 of hospital course #2, demonstrating a burst suppression pattern with 20% of the page showing bursts, following initiation of propofol, midazolam, and ketamine drips. Bursts consist of synchronous polyspike and wave discharges. D, EEG from HD #29 of hospital course #2, demonstrating increased burden of brief runs of rhythmic discharges that are again maximal biposteriorly. E, EEG from HD #11 of hospital course #3 demonstrating right posterior quadrant discharges with moderate to severe generalized background slowing. All EEG studies in Figure 2 are shown in time base of 30 mm/s, read at 7 µV/mm, with notch filter of 60 Hz applied, in double banana montage with subtemporal leads
On admission in March 2020, she had a positive severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) nasopharyngeal polymerase chain reaction (PCR) and was intubated for hypoxia. She was empirically given a 5‐day course of azithromycin and hydroxychloroquine, and a dose of tocilizumab. On hospital day (HD) #2, she had a 2‐minute episode of spontaneous, symmetric, tonic movements of her arms and left gaze deviation without reported head turn. The episode resolved with lorazepam 2 mg and did not recur. She was hemodynamically stable at the time of event. On neurologic assessment, while on sedation for ventilator dyssynchrony, she had no response to voice or pain and no movement of extremities to noxious stimuli, but had intact brain stem reflexes with no evidence of focality. As the event was transient and isolated, both EEG and neuroimaging were deferred due to constraints of the pandemic. Antiseizure medications were not initiated.
During her intensive care unit admission, she required continuous sedation with a combination of fentanyl (max infusion rate = 200 µg/kg/h), dexmedetomidine (max infusion rate = 1.5 µg/kg/h), and 4 days of ketamine (max infusion rate = 2 mg/kg/h); an insulin drip for hyperglycemia; norepinephrine for hypotension; a furosemide drip for diuresis; and a heparin drip for stroke prophylaxis. Tacrolimus and prednisone were continued throughout her admission, but mycophenolate was held for 3 weeks.
Her inflammatory markers fluctuated over the course of her admission: C‐reactive protein (CRP; normal < 3 mg/L) was 99.6 mg/L initially, peaked at 171.1 mg/L, normalized, then was 16.6 mg/L prior to discharge; erythrocyte sedimentation rate (ESR; normal = 0‐20 mm/h) was initially 111 mm/h, then improved to 108 mm/h midway through her admission; interleukin‐6 (normal ≤ 5 pg/mL) was initially 18 pg/mL, peaked at 136 pg/mL, then improved to 81 pg/mL midway through her admission.
By HD #39, SARS‐CoV‐2 nasopharyngeal PCR was negative, and she was discharged to subacute rehabilitation (SAR). She was oriented to herself, able to follow commands, had no focal deficits, and was on room air. She was discharged on her home immunosuppressant regimen of tacrolimus, mycophenolate, and prednisone. See Figure 2 for a timeline of her hospitalization.
FIGURE 2.
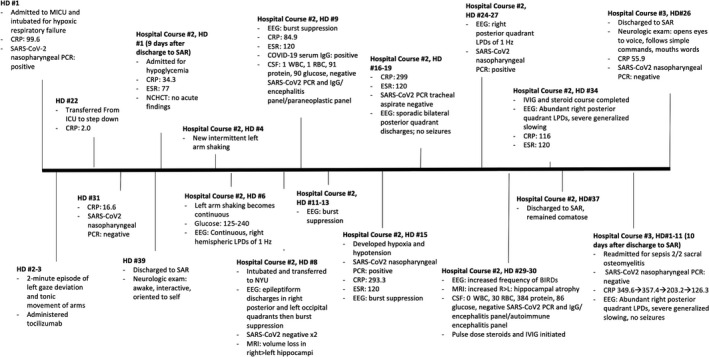
Timeline of the 114 days from admission for COVID‐19 to discharge from her third hospitalization. BIRD, brief potentially ictal rhythmic discharge; CRP, C‐reactive protein; CSF, cerebrospinal fluid; EEG, electroencephalography; ESR, erythrocyte sedimentation rate; HD, hospital day; ICU, intensive care unit; IVIG, intravenous immunoglobulin; L, left; LPD, lateralized periodic discharge; MICU, medical ICU; MRI, magnetic resonance imaging; NCHCT, noncontrast head computed tomography; NYU, New York University Hospital; PCR, polymerase chain reaction; R, right; RBC, red blood cells; SAR, subacute rehabilitation; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; WBC, white blood cells.
Nine days after admission to rehabilitation, she was transferred to a local hospital for lethargy. She was afebrile and hemodynamically stable. She was found to be hypoglycemic at 16 mg/dL, but her blood sugar and mental status improved after glucagon. On HD #4, she developed intermittent, nonsuppressible left arm shaking. During these episodes, she was normoglycemic and able to respond to questions. She was started on levetiracetam. By HD #6, she developed continuous left arm shaking, prompting addition of clonazepam and lacosamide, but the shaking persisted. A routine EEG showed continuous right hemispheric lateralized periodic discharges at 1 Hz. She was initially alert despite the continuous shaking, but she gradually become more lethargic, prompting a request for transfer back to our hospital for continuous EEG monitoring on HD #8. At this time, due to concern that she was not protecting her airway, we requested intubation and initiation of a propofol drip to facilitate a safe transfer.
Upon arrival, on propofol 40 µg/kg/min, she grimaced to pain, had minimal spontaneous movement of her right arm and leg, and withdrew her left arm and leg to pain. She was started on norepinephrine for hypotension. Brain MRI without contrast on HD #8 showed worsened right > left hippocampal atrophy compared to the MRI obtained 3 months prior to her initial hospitalization, diffuse parenchymal volume loss, and no evidence of diffusion restriction (Figure 1A). EEG showed continuous lateralized periodic polyspike and wave discharges, often with sporadic superimposed fast activity, in the right posterior quadrant and left occipital pole (Figure 1B). A midazolam infusion was initiated and titrated up to 3 mg/kg/h. A ketamine drip was subsequently added to achieve burst suppression (Figure 1C).
Studies obtained on HD #8 were remarkable for negative SARS‐CoV‐2 nasopharyngeal PCR ×2, ESR of 120 mm/h, and CRP of 55.2 mg/L. Additional workup including routine laboratory tests, a cortisol stimulation test, free T4, thiamine, and vitamin B12 was unrevealing. Sputum culture grew Escherichia coli.
Cerebrospinal fluid (CSF) sampling on HD #9 revealed elevated protein (91 mg/dL), but only 1 white blood cell. Infectious studies, including bacterial and fungal culture, cryptococcal antigen, meningitis‐encephalitis pathogen panel, and SARS‐CoV‐2 PCR, were negative in CSF. The Mayo Clinic autoimmune encephalitis panel, which tests for 20 autoantibodies including anti–N‐methyl‐D‐aspartate, was also negative. Although serum SARS‐CoV‐2 IgG was positive at 4.5 S/C (reference range < 1.4 S/C), CSF SARS‐CoV‐2 IgG was negative. She had elevated CSF IgG (16.8 mg/dL; normal = 0‐6 mg/dL), and IgG synthesis rate (23.3 mg/dL; normal ≤ 8 mg/dL), and borderline high CSF IgG index (0.64; normal = 0.28‐0.66). However, CSF/serum SARS‐CoV‐2 IgG index was <1, indicating lack of intrathecal SARS‐CoV‐2 antibody production. CSF albumin was elevated to 52 mg/dL (normal = 0‐35 mg/dL), as was albumin index (25.6; normal = 0‐9).
All drips were discontinued by HD #13, but she remained comatose. She developed septic shock on HD #15; SARS‐CoV‐2 nasopharyngeal PCR was repeated, and found to be positive, although tracheal PCR was negative. Hemodynamic status subsequently improved, but neurologic status remained poor. Although she did not have any seizures, she continued to have abundant lateralized discharges in the right posterior quadrant on her EEG (Figure 1D). Repeat lumbar puncture on HD #29 revealed protein of 384 mg/dL. MRI brain with and without contrast on HD #29 showed no evidence of diffusion restriction or enhancement, but bilateral hippocampal atrophy had progressed (Figure 1A). Given her active EEG, a 5‐day course of pulse dose steroids and intravenous immunoglobulin (IVIG) was initiated on HD #30. However, the abundant right posterior quadrant periodic discharges, which had been present throughout the 25 days she was on EEG, persisted and her ESR and CRP remained elevated (120 mm/h and 116 mg/L, respectively). On HD #37, she remained comatose and was discharged to SAR on clobazam 10 mg twice daily (BID), levetiracetam 2000 mg BID, lacosamide 200 BID, and phenytoin 100/100/200 mg.
Ten days later, she was readmitted for sepsis secondary to osteomyelitis and was still comatose. Continuous EEG on HD #6‐11 revealed abundant right posterior quadrant discharges (less frequent than on her prior EEG) with moderate to severe background slowing (Figure 1E). She was weaned off clobazam, and levetiracetam was decreased to 1000 mg BID, without change in EEG. By HD #26, when she was discharged, her CRP had improved to 55.9 mg/L and her examination had markedly improved; she opened her eyes to voice, stuck out her tongue and wiggled her toes to command, and mouthed words in response to questions.
3. DISCUSSION
Since the onset of the COVID‐19 pandemic, there have been multiple reports of seizures in the setting of active COVID‐19 infection. 1 , 2 , 3 This has been hypothesized to be the result of cytokine storming and viral invasion of the central nervous system (CNS) via cranial nerves or hematogenous spread. 1 , 3 This population is also at risk for seizures due to hypoxia, electrolyte derangements, hypo‐ or hyperglycemia, acute kidney injury, shock, systemic infections, medications, and stroke. 4 In most reports, seizures have been focal. 2 , 3 Patients with acute COVID‐19 can also have seizure mimics due to electrolyte derangements or anxiety. 4 Although our patient had a transient event early in her first hospitalization for acute COVID‐19 infection, we are unsure whether this was a seizure or a seizure mimic; however, the left gaze deviation is consistent with a right hemispheric seizure focus. Nonetheless, despite lack of treatment, she had no additional events concerning for seizure for 50 days after this initial event, until she developed focal RSE. We believe this is the first report of RSE in a patient who recovered from COVID‐19.
Postviral refractory seizures have been reported in a child with leukoencephalopathy and microhemorrhages on MRI and pathology evidence of necrotizing white matter with superimposed hypoxic‐ischemic injury. 5 Although some patients with COVID‐19 also have leukoencephalopathy and microhemorrhages, this was not the case for our patient. 6 However, her mild cognitive impairment and hippocampal atrophy placed her at increased risk for seizures. 7 This alone does not explain RSE, so we sought to identify other etiologies for her presentation. She was profoundly hypoglycemic on the day of her second admission, but this resolved quickly, her mental status returned to baseline, and 3 days passed before she started having seizures. At the onset of RSE, in addition to having a normal blood sugar level, she was normonatremic and had normal kidney function. Because of her recent critical illness and prolonged hospitalization, we ruled out thiamine deficiency and adrenal insufficiency as possible explanations for RSE. Additionally, we evaluated for infection of the CNS by COVID‐19 or other organisms. As there have been reports of postviral autoimmune encephalitis, we also sent an autoimmune encephalitis panel in the CSF, which was negative. 8 Thus, her only notable laboratory findings at the time of RSE onset were the elevated serum inflammatory markers and CSF protein, IgG, IgG synthesis rate, IgG index, albumin, and albumin index.
As we continue to follow survivors of severe COVID‐19 infection, we have learned about multiple presentations of postinfectious inflammatory responses. Neurologic examples of a post–COVID‐19 inflammatory response include Guillain‐Barré syndrome and acute disseminated encephalomyelitis. 9 , 10 The most prominent presentation of an inflammatory response after COVID‐19 has been reported in the pediatric population; children with asymptomatic SARS‐CoV‐2 infection have developed multisystem inflammatory syndrome in children (MIS‐C), characterized by fever, elevated inflammatory markers, and single or multiorgan failure, in the absence of active infection. 11 Seizures have been observed in several children with this syndrome at our institution.
We suspect that our patient's RSE was the result of a postinfectious inflammatory response as evidenced by her elevated systemic inflammatory markers. Although she did not have multisystem organ failure or fever, 11 MIS‐C encompasses a broad phenotypic spectrum, and her lack of fever may be explained by her immunosuppressed state. She did not have evidence of inflammation on her brain MRI or intrathecal production of SARS‐CoV‐2 antibodies, as has been described in some COVID‐19 patients with severe encephalopathy and encephalitis, 12 but she did have elevated CSF albumin index, reflecting breakdown of the blood‐brain barrier, 13 and elevated CSF IgG synthesis, which may suggest inflammation in the CNS. 14 The elevated protein in her repeat CSF studies on HD #30 was hypothesized to be secondary to longstanding diabetes mellitus as well as the aforementioned CNS inflammation and further breakdown of the blood‐brain barrier. We were unable to test cytokine levels in the CSF to compare them to serum levels, as was done in a case report in a patient who had COVID‐19 by Pilotto et al, 15 but this would have been interesting, as it has been suggested that cytokine levels may differ in various bodily fluids in patients with COVID‐19 such that local cytokine storm in some organs may be more extreme than systemic cytokine storm. 16
Although our patient demonstrated recurrence of a positive SARS‐CoV‐2 nasopharyngeal PCR and developed recrudescence of hypoxia and hypotension (albeit also in the setting of E. coli and Klebsiella in her sputum), intermittent positive PCR has been described in patients with COVID‐19 up to 12 weeks after initial infection. 17 This is attributed to prolonged viral shedding, which may have autoimmune repercussions. As of July 22, 2020, the Centers for Disease Control and Prevention reported that there have been no confirmed cases of COVID‐19 reinfection. 17
Patients with MIS‐C have been shown to respond to IVIG and pulse dose steroids. 11 Given our patient's lack of clinical improvement and persistently active EEG through HD #29, the decision was made to administer steroids and IVIG. This did not change her EEG or clinical examination, but she ultimately improved 1 month later when she was tapered off clobazam.
4. CONCLUSION
The epidemiology, range of presentations, pathophysiology, and ideal treatment of postinfectious inflammatory response to COVID‐19 remains unknown, but our case adds to the growing literature on this topic. We believe this is the first report of a patient with post–COVID‐19 inflammatory syndrome manifesting as RSE.
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose.
ETHICAL PUBLICATION STATEMENT
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Carroll E, Neumann H, Aguero‐Rosenfeld ME, et al. Post–COVID‐19 inflammatory syndrome manifesting as refractory status epilepticus. Epilepsia. 2020;61:e135–e139. 10.1111/epi.16683
REFERENCES
- 1. Sohal S, Mansur M.COVID‐19 presenting with seizures. IDCases. 2020;20:e00782. [DOI] [PMC free article] [PubMed]
- 2. Somani S, Pati S, Gaston T, Chitlangia A, Agnihotri S. De novo status epilepticus in patients with COVID‐19. Ann Clin Transl Neurol. 2020;7(7):1240–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Balloy G, Mahe P‐J, Leclair‐Visonneau L, Pereon Y, Derkinderen P, Magot A, et al. Non‐lesional status epilepticus in a patient with coronavirus disease 2019. Clin Neurophysiol. 2020;131(8):2059–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lu L, Xiong W, Liu D, Liu J, Yang D, Li N, et al. New onset acute symptomatic seizure and risk factors in coronavirus disease 2019: a retrospective multicenter study. Epilepsia. 2020;61(6):e49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Achiriloaie A, Michelson D, Lei L, Denham L, Oberg K, Raghavan R. Acute postviral encephalopathy. Child Neurol Open. 2016;3:2329048X1665884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Radmanesh A, Derman A, Lui YW, Raz E, Loh JP, Hagiwara M, et al. COVID‐19–associated diffuse leukoencephalopathy and microhemorrhages [published online ahead of print May 21, 2020]. Radiology. doi: 10.1148/radiol.2020202040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dhikav V, Anand K. Hippocampal atrophy may be a predictor of seizures in Alzheimer’s disease. Med Hypotheses. 2007;69:234–5. [DOI] [PubMed] [Google Scholar]
- 8. Lynch DR, Rattelle A, Dong YN, Roslin K, Gleichman AJ, Panzer JA. Anti‐NMDA receptor encephalitis: clinical features and basic mechanisms. In: Gavril WP, Joseph TC editors Advances in Pharmacology. London, UK: Academic Press; 2018:235–60. [DOI] [PubMed] [Google Scholar]
- 9. Chan M, Han SC, Kelly S, Tamimi M, Giglio B, Lewis A. A case series of Guillain‐Barré syndrome following Covid‐19 infection in New York [published online ahead of print]. Neurol Clin Pract. 10.1212/CPJ.0000000000000880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Novi G, Rossi T, Pedemonte E, Saitta L, Rolla C, Roccatagliata L, et al. Acute disseminated encephalomyelitis after SARS‐CoV‐2 infection. Neurol Neuroimmunol Neuroinflamm. 2020;7(5):e797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS‐CoV‐2. JAMA. 2020;324(3):259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Benameur K, Agarwal A, Auld SC, Butters MP, Webster AS, Ozturk T, et al. Encephalopathy and encephalitis associated with cerebrospinal fluid cytokine alterations and coronavirus disease, Atlanta, Georgia, USA, 2020. Emerg Infect Dis. 2020;26(9):2016–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood‐brain barrier. Nat Med. 2013;19(12):1584–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eeg‐Olofsson O, Wigertz A, Link H. Immunoglobulin abnormalities in cerebrospinal fluid and blood in children with febrile seizures. Neuropediatrics. 1982;13(1):39–41. [DOI] [PubMed] [Google Scholar]
- 15. Pilotto A, Odolini S, Masciocchi S, Comelli A, Volonghi I, Gazzina S, et al. Steroid‐responsive encephalitis in coronavirus disease 2019 [published online ahead of print May 17, 2020]. Ann Neurol. 2020;88: 423–427. 10.1002/ana.25783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang C, Kang K, Gao Y, Ye M, Lan X, Li X, et al. Cytokine levels in the body fluids of a patient with COVID‐19 and acute respiratory distress syndrome: a case report [published online ahead of print May 12, 2020]. Ann Intern Med. doi: 10.7326/L20-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention . Duration of isolation and precautions for adults with COVID‐19. [Accessed 2020 Aug 16]. Available at: https://www.cdc.gov/coronavirus/2019‐ncov/hcp/duration‐isolation.html


