Abstract
SARS‐CoV‐2 infection and pregnancy has been the topic of hundreds of publications over the last several months; however, few studies have focused on the implications of infection in early pregnancy and reproductive tissues. Here, we analyzed available evidence pertaining to SARS‐CoV‐2 infection, in early pregnancy, and in reproductive tissues. We searched PubMed and Embase databases in accordance with guidelines of Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) for publications from inception to June 4, 2020. Four reviewers screened titles and abstracts and obtained full‐text articles for analysis. Sixty‐two studies were included in the review. Biological plausibility for infection with SARS‐CoV‐2 exists in testis, ovaries, and placenta as they express ACE2 receptor activity. In males, SARS‐CoV‐2 infection could lead to functional abnormalities leading to spermatogenic failure and male infertility. In females, an alteration of the ACE2 cascade via SARS‐CoV‐2 infection could lead to impairment in important follicular and luteal processes. There is also evidence of significant placental pathology in SARS‐CoV‐2 infection, but it is unclear what effects there may be for early pregnancy, though available data suggest less severe effects compared to other respiratory virus outbreaks. Further investigation is needed regarding SARS‐CoV‐2 in reproductive function and early pregnancy.
Keywords: COVID‐19, early pregnancy, ovaries, placenta, SARS‐CoV‐2, sperm, testes
1. INTRODUCTION
Pregnant women and their fetuses may be impacted disproportionally by emerging infections 1 as exemplified by the 2009 H1N1 influenza pandemic 2 and the severe fetal complications of Zika virus. 3 , 4 The novel coronavirus infection, severe acute respiratory coronavirus 2 (SARS‐CoV‐2), was first reported in Wuhan, China, on December 31, 2019, and was declared a pandemic by the WHO on March 11, 2020. 5 Since then, the number of worldwide reported cases of COVID‐19 has increased to 6.11 million and 370,000 deaths as of May 31, 2020. 6 To combat the rapidly spreading virus, nations have implemented strategies to suppress and mitigate the community‐acquired infections such as social distancing, availability of rapid testing, contact tracing, and vaccine development. 7 , 8
Coronaviruses are single‐stranded RNA, enveloped, non‐segmented viruses which cause complications ranging from common cold to pneumonia and death. 9 Common symptoms of SARS‐CoV‐2 infection include fever, cough, myalgia, headache, and diarrhea. 10 Highly infective, the virus is transmitted to 2‐3 people for every infected person, with a reproduction number (R0) of 2.2. 11 While an estimated 80% of COVID‐19 cases are mild and can be managed with home care, 15%‐20% require hospitalization, with 5% of cases severe enough to require mechanical ventilation. 6 The total mortality rate, including those from asymptomatic and mild cases, seems to be approximately 1%. 12 The primary mode of transmission occurs similarly to that of other respiratory infections; droplet particles are inhaled through close physical contact with an infected person when coughing or sneezing. 13 It does not appear that vertical transmission from pregnant women to fetuses occurs 14 ; however, this has not been determined conclusively. Other coronaviruses of the previous two decades, severe acute respiratory syndrome coronavirus (SARS‐CoV) and Middle East respiratory syndrome coronavirus (MERS‐CoV), have caused severe respiratory infections in humans. The novel SARS‐CoV‐2 shares 82% of the genome of the virus that causes SARS‐CoV. 15 Since its first identification, more cases of COVID‐19 have been counted than that of MERS and SARS combined. Limited studies on SARS‐CoV‐1 reported increased morbidity and mortality in pregnant women, while some recent studies have shown women with SARS‐CoV‐2 are less likely to have complications comparatively. 16 , 17 However, very little data are available regarding the effects of coronaviruses in early pregnancy.
Much of the available data focus solely on COVID‐19 infections in the third trimester and the possibility of vertical transmission. Though there is a lack of concrete data, SARS‐CoV‐2 does have the potential to cause pregnancy complications in the first trimester including miscarriage and congenital abnormalities. 18 In this paper, we aimed to summarize the available data related to the effects of SARS‐CoV‐2 infection on the ovaries, testicles, gametes, and early pregnancy. In addition to current SARS‐CoV‐2 literature, we also investigate the potential effects of COVID‐19 in early pregnancy using published data and outcomes from the SARS‐CoV and MERS‐CoV pandemics.
2. MATERIALS AND METHODS
A systematic search in the literature published in the PubMed and Embase databases was conducted in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA). Additional citations were identified from the reviewed literature. An academic data specialist developed the search strategy and searched the databases of PubMed and Embase from inception to June 4, 2020. We searched for articles that contained information related to SARS‐CoV‐2 and reproductive tissues (ovaries, testes), gametes, placentation, and early pregnancy in humans. Our search phrases included: “severe acute respiratory syndrome coronavirus 2”, “2019 ncov”, “sarscov 2”, “SARS‐Cov‐2”, “pregnancy”, “gravidity”, “abortion”, “germ cells”, “oocytes”, “gametes”, “embryonic structures”, “embryo”, “fertility”, “testes”, “miscarriage”(See Appendix 1 for completed list of databases search strategy and Figure 1 for PRISMA table). Due to limited number of publications available, we did not consider a sample size as an eligibility criterion. Majority of the literature composed of case reports, guidelines, and editorials. We did not use the Cochrane RoB 2.0 tool due to the absence of randomized clinical trials. Similarly, due to lack of cohort studies, we did not use the Newcastle‐Ottawa Scale to rate the studies.
FIGURE 1.
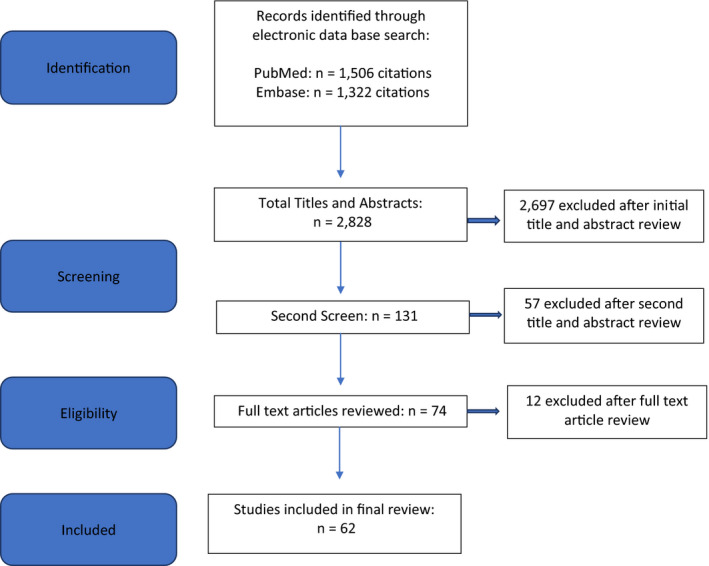
PRISMA flow chart of article identification, retrieval, review and inclusion
Using a standardized form, 4 reviewers (HS, MG, EK, and BS) screened titles, abstracts, and full‐text articles reporting potentially eligible studies. Any disagreements were resolved with a third reviewer (JS). We acknowledge the possibility of bias in this literature review due to inclusion of the limited research data available. SARS‐CoV‐2‐related reporting is increasing exponentially, and this review is limited to the date of the literature search was conducted. The search strategy described above identified 2,828 articles, of which 131 were considered for full review. Sixty‐two articles met the inclusion criteria and were used to support this review.
3. RESULTS
Table 1 summarizes case series and studies of SARS‐CoV‐2 infection within various reproductive tissues in patients who tested positive for SARS‐CoV‐2.
TABLE 1.
Summary of case series and studies of SARS‐CoV‐2 infection within various reproductive tissues in patients who tested positive for SARS‐CoV‐2
| Tissue assayed and study | PCR investigation | Pathologic investigation | Summary of results |
|---|---|---|---|
| Testis | |||
|
Li et al 24 |
Yes | No | Six of 38 patients with positive RT‐PCR for SARS‐CoV‐2 in semen |
|
Pan et al 25 |
Yes | No | One patient of 34 with positive qRT‐PCR for SARS‐CoV‐2 in semen |
| Ovaries and oocytes | |||
| None | |||
| Placenta and placental membranes | |||
| Chen et al 42 | Yes | No |
Nine placentas tested No positive results |
| Liu et al 53 | Yes | No |
Three placentas tested No positive results |
| Fan et al 41 | Yes | No |
Two placentas tested. No positive results. |
| Baergen et al 49 | No | Yes | Ten of 20 placentas showed evidence of fetal vascular malperfusion or fetal vascular thrombosis |
| Shanes et al 50 | No | Yes | Twelve of 15 placentas with evidence of maternal vascular malperfusion; 7 of 15 placentas with decidual arteriopathy |
| Penfield et al 51 | Yes | No | Three of 11 placental swabs positive |
| Patane et al 52 | Yes | Yes |
Two of two positive results for SARS‐CoV‐2 RNA on fetal side of placenta Both placentas with chronic intervillositis |
| Vaginal secretions | |||
| Qiu et al 56 | Yes | No |
Ten non‐pregnant women swabbed No positive results |
| Liu et al 53 | Yes | No |
Three vaginal swabs tested (peripartum). No positive results |
| Fan et al 41 | Yes | No |
Two vaginal swabs tested (peripartum) No positive results |
3.1. SARS‐CoV‐2 effects on testes and spermatocytes
Spermatogonia, Sertoli, and Leydig cells are predominantly enriched in ACE2 (19, 20). SARS‐CoV‐2 and MERS‐CoV use ACE2 receptors to gain entry into host cells. 21 Wang et al 19 performed uniform manifold approximation and projection (UMAP) and marker gene analyses to identify nine major cell cluster in testicular cells. ACE2 expression was determined by analyzing the RNA expression profile of ACE2 at single‐cell resolution. Spermatogonia and spermatids showed concentrated expression of transmembrane serine protease 2 (TMPRSS2) for viral spike (S) protein priming. Both Zhao and Wang 19 , 20 also reported almost 3‐fold higher percentage expression of ACE2 in Leydig and Sertoli cells compared to spermatogonia (4.25% vs. 1.40%). SARS‐CoV‐2 uses TMPRSS2 and SARS‐CoV‐2 ACE2 receptors to gain entry into host cells. 22 Therefore, there is a high potential for SARS‐CoV‐2 infection in human testes. In situ hybridization, morphological, and immunohistochemical analysis were performed on 6 testis obtained at autopsy from confirmed SARS‐CoV‐2‐infected males and from 4 non‐SARS autopsy control testis samples. 23 All control testis had normal morphology, whereas the SARS‐CoV‐2‐infected testis showed peritubular fibrosis and vascular congestion along with extensive germ cell destruction. There were significantly more CD3+ T lymphocytes and CD68+ macrophages (0.65% and 2.11% in control vs. 4.49% and 11.72%) infected. However, the virus did not affect the testis directly since in situ hybridization, using both sense and antisense RNA probes, showed no staining. 23 A cohort study by Li enrolled 38 SARS‐CoV‐2 patients for semen testing using RT‐PCR and found 6 patients had positive results for SARS‐CoV‐2 (4 in acute stage of the disease and 2 in recovery). However, no information is known about virus shedding, survival time, and concentration in semen. 24 A cross‐sectional observational study by Pan et al identified the presence of SARS‐CoV‐2 by qRT‐PCR in a single ejaculated sample of 34 confirmed cases of SARS‐CoV‐2 in China. SARS‐CoV‐2 was not detected in the semen 1 month after diagnosis. They also reported sparse expression of ACE2 and TMPRSS 2 using single‐cell transcriptome analysis, with almost no overlapping gene expression. 25
Both Jung and Carlsen reported that male fertility may be diminished due to fever associated with the SARS‐CoV‐2 infection for up to 72‐90 days following infection as fever can lead to decreased sperm concentration and motility. 26 , 27 In addition, SARS‐CoV‐2 patients may develop cytokines storm syndrome (hemophagocytic lymphohistiocytosis), which can impact testicular function and maintenance, as cytokines microenvironment is critical to testicular function and alterations may be tumorigenic. 28 , 29
3.2. SARS‐CoV‐2 effects on ovaries and oocytes
As stated earlier, SARS‐CoV‐2 uses TMPRSS2 and SARS‐CoV‐2 ACE2 receptor to gain entry into host cells. 21 Both reproductive‐age women and post‐menopausal women have ACE2 mRNA transcripts expressed abundantly in the ovary. 30 Therefore, the female reproductive system is potentially at a high risk of SARS‐CoV‐2 infection. There are no studies that evaluate SARS‐CoV‐2 infection inhuman ovarian tissue and cells.
3.3. SARS‐CoV‐2 and effects on placenta and placental membranes
The ACE2 receptor is widely expressed in the placenta. 31 Overall, there is limited evidence regarding SARS‐CoV‐2 and transplacental transmission, though most reviews and the American College of Obstetrics and Gynecology have stated there is no conclusive evidence of transplacental transfer of SARS‐CoV‐2 from infected mothers. 32 , 33 Comparable to other more well‐known transplacental infections, which are known to have hematogenous infectivity, SARS‐CoV‐2 has been shown to produce RNAemia and therefore plausibly could be transmitted via a transplacental route. 34 At present, the literature regarding vertical transmission is mixed; several recent studies have concluded there is no definitive evidence of vertical transmission, 35 , 36 , 37 while others have findings that suggest in utero transmission may occur, as evidenced by presence of neonatal IgM antibodies (which cannot otherwise be transferred transplacentally) 38 , 39 , 40 and neonatal nasopharyngeal and anal SARS‐CoV‐2 RNA isolation. 41 While this evidence of transplacental transmission is compelling, dedicated study of placental histopathology and PCR as it relates to SARS‐CoV‐2 is needed to better distinguish transplacental versus intrapartum versus post‐partum vertical transmission.
Our review identified two dedicated studies of placental histopathology in SARS‐CoV‐2. In one study investigating placentas of SARS‐CoV‐2‐positive mothers, 45% showed low‐grade fetal vascular malperfusion, though notably all infants tested negative. 42 Interestingly, Shanes et al 43 noted higher rates of arteriopathy and maternal vascular malperfusion than controls, whereas findings of fetal malperfusion were non‐significant compared to controls. Both studies noted significant increase in intervillous thrombi, which may be a direct correlate to the thromboembolic disorders believed to occur in SARS‐CoV‐2. Data regarding placental viral isolates in specifically SARS‐CoV‐2 are scarce and with mixed results. To date, there are few published reports of the presence of SARS‐CoV‐2 RNA in placenta or membrane samples. 44 , 45 In the study by Penfield et al, 44 3 of 11 placental or membrane swabs were positive within 30 minutes of delivery in women with severe to critical COVID‐19 at time of delivery. Notably, there were no signs of vertical transmission, and all infants were negative, raising the distinct possibility of contamination. A study by Patane et al 45 is the first to specifically describe SARS‐CoV‐2 RNA on the fetal side of the placenta. In two other published reports, viral PCR was performed on placental tissue and/or all products of conception in mothers diagnosed with symptomatic SARS‐CoV‐2 infection following third trimester delivery. All samples were negative. 36 , 46 To date, there appears to be a single study directed at investigating the intrauterine environment of early pregnancy and SARS‐CoV‐2 with several limitations. 47 Specifically, the study retrospectively reviewed records and laboratory results for two symptomatic women with COVID‐19 in the first trimester. The first woman, at 8‐week gestation, was given a clinical diagnosis given husband's diagnosis at home as well as radiologic findings. The second woman was diagnosed at 10‐week gestation via nasopharyngeal test. On the day of amniocentesis, both patients were negative for SARS‐CoV‐2 RNA via throat swabs, but positive for SARS‐CoV‐2 IgG antibodies in serum (the first woman also had IgM antibodies). SARS‐CoV‐2 RNA was not isolated in the amniotic fluid, though notably amniotic samples were collected over a month after symptom resolution and testing positive. However, according to Center for Disease Control, it is anticipated that SARS‐CoV‐2 infection will affect fetal development like other respiratory coronaviruses.
3.4. SARS‐CoV‐2 and effects on vaginal secretions
The status of SARS‐CoV‐2 within the vaginal environment is currently unknown. Many viruses have been previously isolated within vaginal fluid, such as hepatitis C virus and Zika virus, which are both RNA viruses. At present, it is not believed that SARS‐CoV‐2 is able to be sexually transmitted, which is consistent with other historical coronaviruses. However, inability for sexual transmission does not preclude SARS‐CoV‐2 from having a significant clinical impact. To our knowledge, there is one study investigating the presence of SARS‐CoV‐2 in the vaginal fluid of women with severe SARS‐CoV‐2. Other studies that tested vaginal swabs were tested in the peripartum period and were negative. 36 , 46 Specifically, 10 women with severe COVID‐19 were tested or SARS‐CoV‐2 in vaginal fluid, with all samples negative for virus. 48 Notably, these women were post‐menopausal and swabs were taken 17 or more days after onset of disease.
4. DISCUSSION
In summary, a systemic review of the literature was performed in accordance with PRISMA methodology regarding SARS‐CoV‐2 infection and its impact on reproductive tissues, gametes, and early pregnancy. In total, there were 62 articles identified. Overall, there are very limited data regarding SARS‐CoV‐2 infection and its clinical implications for many facets of reproduction. Biological plausibility exists for severe consequences to both male and female reproductive potential and at present, caution seems warranted.
4.1. Effects on testes and spermatocytes
Both Sertoli and Leydig cells have been demonstrated to have abundant ACE2 expression. Within the male reproductive system, it has been suggested that SARS‐CoV‐2 infection can lead to functional abnormalities leading to spermatogenic failure and male infertility by affecting the male germ cells and the supporting somatic cells. 23 The remaining questions are what percent of men have long‐term impaired testicular function following COVID‐19 and to what degree.
4.2. Effects on ovaries and oocytes
Animal studies have demonstrated that ACE2 is present within murine, leporine, bovine, and equine ovarian tissue (including but not limited to stromal cells, granulosa cells, and oocytes). Furthermore, in these studies, ACE2 is known to be an essential protein involved in multiple cascades responsible for steroid secretion, 49 , 50 oocyte maturation and ovulation, and resumption of meiosis in the oocyte 51 , 52 through its interplay with Ang‐II and Ang‐(1‐7). Most importantly, in regard to early pregnancy, Ang‐II and Ang‐I (and by extension ACE2) have been shown to be key proteins involved in formation, maintenance, and regression of corpus luteum. 53 Alterations in this cascade of proteins responsible for normal development of the corpus luteum may explain how SARS‐CoV‐2 can negatively impact early pregnancy. In the human ovary, there is evidence that folliculogenesis, oocyte maturation, and ovulation involve the renin‐angiotensin aldosterone (RAS) system, an important component which involves ACE2 enzyme expression. 49 Therefore, SARS‐CoV‐2 could theoretically damage ovarian tissue and decrease ovarian function and oocyte quality, resulting in female infertility or even miscarriage. The role of ACE2 with Ang‐II and Ang‐(1‐7), and their demonstrated importance in the formation and maintenance of corpus luteum, could explain how alterations in ACE2 may negatively impact early pregnancy. 24 , 53 Specifically, the impact of SARS‐CoV‐2 may be two‐fold: the effects of SARS‐CoV‐2 viral infection and local immune reaction itself, as well as the downstream consequences of altering the essential ACE2 and ACE2 receptor mechanism. Specifically, as the ACE2 and ACE2 receptor cascades are involved in multiple basic ovarian processes, it may be reasonable to infer that their normal function and availability could be altered due to SARS‐CoV‐2 use for entry.
4.3. Effects on placenta
Notably, placental pathology in the setting of respiratory illness is difficult to interpret, as protracted respiratory compromise and maternal hypoxia drive the release of potent vasoconstrictors resulting in placental hypoperfusion. Uterine blood flow might decrease with systemic vasodilatation due to shock. Possible resultant pathology findings as evidence of vertical transmission may be confounding and may not provide clarity regarding infection of placental tissue itself versus reactive changes from hematogenous spread originating upstream. An important though subtle consideration is the distinction between intrinsic placental cellular effects of SARS‐CoV‐2 versus reactive pathology as a consequence of upstream changes in maternal‐fetal physiology due to acute infection. Future studies may benefit from using placentas in other respiratory illnesses, such as influenza, as a control group to decipher if certain findings are pathognomonic for SARS‐CoV‐2 illness.
4.4. Effects on early pregnancy and early placental tissue
The effect of SARS‐CoV‐2 on early pregnancy is still unknown, and any potential effects are extrapolations from cell‐level plausibility and experiences from other recent viral respiratory syndromes, such as SARS and MERS. Very limited data may suggest that there are higher miscarriage rates with SARS and MERS versus COVID‐19. 35 Developmental consequences of continued pregnancies in setting of resolved early pregnancy SARS‐CoV‐2 infections have not been described, and dedicated studies are desperately needed. Indirect effects of the COVID‐19 pandemic, which include varying chemical exposures, treatments, and consequences of psychological stress, are also important considerations.
There is mixed evidence regarding transplacental transmission of SARS‐CoV‐2, though the biological plausibility via the ACE2 receptor and maternal‐fetal interface exists. The reports to date of a less significant effect of SARS‐CoV‐2 on early pregnancy, as reflected by a less severe miscarriage rate, should be regarded with caution. There are not yet enough published reports regarding early pregnancy and effects of SARS‐CoV‐2, perhaps owing to the overall proportionally decreased number of miscarriages seen associated with COVID‐19 compared to SARS/MERS epidemics. In the absence of robust data regarding viral isolation within placental samples and ensuing clinical consequences, other placental histologic markers of infection should be examined. However, this could be confounding as an important though subtle consideration is the distinction between systemic versus local effects of viral infection, as mentioned in the discussion section of “Effects on placenta.” Additionally, it should be noted that the aforementioned studies investigate placental consequences in symptomatic women. Given the current timeframe of pandemic, it is not yet known whether there could be in utero, thereby transplacental, sequelae from asymptomatic infection. Additionally, sufficient data may not yet even be available, as women whose estimated date of conception coincides with the WHO declaration of pandemic (March 11, 2020) are only just now completing their first trimester. Prior to the current pandemic, there has been limited histopathologic assessment of placenta or products of conception in women infected with coronaviruses. Additionally, the vast majority of presently published studies involve women who acquired the disease in the third trimester. The most robust data in placental pathology and coronavirus are born out of the 2002‐2003 SARS‐CoV coronavirus epidemic. Specifically, a study by Ng et al 54 demonstrated that placentas from third trimester pregnancies complicated by acute maternal SARS‐CoV infection showed extensive pathologic abnormalities with resultant significant fetal morbidity; specific pathologic placental findings included areas of avascular chorionic villi and increases in intervillous or subchorionic fibrin deposition. This study makes no mention of SARS‐CoV RNA isolation or specific viral cytopathic effects, however. Another study investigating vertical transmission of SARS‐CoV found that RT‐PCRs and viral cultures of placental tissues and amniotic fluid were all negative. 55
While the relationship between SARS‐CoV, SAR‐CoV‐2, and third trimester placental tissues has been investigated, it is unclear what the implications may be for infection of early placental tissue and early pregnancy and furthermore the specific consequences related to gestational age at time of infection. One study investigating ACE2 expression in human placental tissue, which is a known main protein for SARS‐CoV and SARS‐CoV‐2 viral transmission, 31 showed differential expression of ACE2 based on gestational age. For example, the expression of ACE2 was at very low levels in placental trophoblastic cells in the first trimester, with increased expression at 24‐week gestational age. This lower level ACE2 expression in the first trimester could suggest a decreased vulnerability to transplacental infection in early pregnancy. In comparison with Zika virus (ZIKV), which is known to be transmitted transplacentally and cause significant adverse pregnancy outcomes, AXL (an essential ZIKV entry cofactor) is more concentrated in the early maternal‐fetal interface. This may explain how ZIKV is more readily transmitted in early pregnancy. 56 Interestingly, although SARS‐CoV‐2 and SARS‐CoV seem to share this same pathogenic receptor and additionally have up to 85% sequence similarity, 57 , 58 by all published reports, the effect on early pregnancy appears very different between the two infections. While a study by Ng et al 54 described normal placental pathology findings in women convalescent from SARS‐CoV who had spontaneous miscarriage in the first trimester, the miscarriage rate overall with SARS‐CoV infection appears more severe. Specifically, data regarding miscarriage rate in SARS and MERS outbreaks may suggest a more severe effect on early pregnancy physiology, for example, higher spontaneous pregnancy loss, than in SARS‐CoV‐2 infection. One study demonstrated a 2% miscarriage/stillbirth rate for SARS‐CoV‐2% vs 18% and 25% for MERS‐CoV and SARS‐CoV, respectively. 35 Another study performed during the 2002‐2003 SARS pandemic showed that 4 of 7 (57%) pregnant women infected with SARS‐CoV had a spontaneous miscarriage in the first trimester of pregnancy, 55 though notably no viral inclusion bodies or particles were detected in the products of conception. Additional biochemical studies do not aid in further clinical understanding, as biophysical and structural evidence shows that SARS‐CoV‐2 actually binds ACE2 with higher affinity than SARS‐CoV and therefore could plausibly transmit to placenta to a greater degree than SARS‐CoV. 59 This does not appear to be consistent with the better outcomes seen with SARS‐CoV‐2 infection and pregnancy, and it is clear that further investigation is needed.
Notably, only transient positive results in amniocentesis have been reported for other RNA virus infections (Zika), and these patients were outside of the standard gestational age range for traditional amniocentesis.
4.5. Effects on vaginal secretions
In the event it is discovered that SARS‐CoV‐2 may be isolated in vaginal secretions, the implications, though unknown, could represent a mechanism for ascending infection. Interestingly, an animal study investigating rat coronavirus (RCV) found that in addition to infecting respiratory epithelium, it also infects the genital tract of females, reportedly causing perturbations of the hormonal cycle and miscarriage. 60 , 61 Isolation of virus within vaginal fluid could also theoretically represent a disruption of a microbiome or “virome,” of which the consequences of disruption may be reproductive failure, predisposition to other infection, and pregnancy complications. One study showed that higher viral diversity in the vagina was significantly associated with preterm birth. 62 Notably, viruses isolated in this study included adenoviruses, which are an important cause of respiratory illness globally. As such, it is important to understand the relationship of SARS‐CoV‐2 within vaginal fluid, as understanding implications of SARS‐CoV‐2 in early pregnancy may alter counseling, work up, and approach to pre‐pregnancy counseling and intervention.
5. CONCLUSIONS
The COVID‐19 outbreak continues to increase in the number of cases, deaths, and countries affected. Current data regarding reproductive tissues and early pregnancy in SARS‐CoV‐2 infection are limited. This may be a result of restricted capabilities of research institutions in time of pandemic, decreased patient interaction within hospitals and institutions due to social reasons and policy, and absolute gestational age relative to time and testing abilities within the pandemic, as many women are just now completing their first trimester.
In light of the currently available evidence, specific recommendations beyond standard personal protective equipment, social distancing accommodations, and hygienic practices can be made. Specifically, it can be suggested that clinicians practicing assisted reproductive technology should be particularly cautious, as SARS‐CoV‐2 infection and systemic effects may impact testicular tissues, ovarian tissue, and granulosa cells and therefore testicular and ovarian function, spermatozoa, oocyte quality, and pregnancy outcomes. 24
For men, there is evidence of direct testicular pathology as a result of SARS‐CoV infection. While long term testicular pathology has not yet been directly studied following SARS‐CoV‐2 infection, RNA has been isolated in semen in both acute and recovery phases of infection. It is unclear how SARS‐CoV‐2‐infected spermatic cells in combination with both local and systemic immune responses may impact male fertility, and furthermore, how long and to what degree impairment may exist. This plausible impairment and its consequences should be recognized by clinicians, and counseling should reflect these possibilities.
In females, limited data are available on pregnant women with COVID‐19 on which to base recommendations for pregnancy‐specific care and infertility care. However, early reports and lessons from SARS‐CoV, MERS‐CoV, and other respiratory infections suggest that there may be a lesser severe impact with SARS‐CoV‐2 on early pregnancy physiology, as suggested by a lesser comparative miscarriage rate. Considering this knowledge gap, it is important for clinicians to continue to have open conversations with their patients, as evolving understanding of SARS‐CoV‐2 in early pregnancy may alter counseling, work up, and approach to pre‐pregnancy counseling and intervention.
As standard reproductive health, pre‐pregnancy, and pregnancy patient care resumes, surveillance systems involving widespread testing of patients and tissues, as well as pathological evaluation, must be established to better investigate SARS‐CoV‐2 effects on reproductive health and early pregnancy.
APPENDIX 1.
SEARCH STRATEGIES
PubMed
(("severe acute respiratory syndrome coronavirus 2"[Supplementary Concept] OR "severe acute respiratory syndrome coronavirus 2"[All Fields] OR "2019 ncov"[All Fields]) OR ("severe acute respiratory syndrome coronavirus 2"[Supplementary Concept] OR "severe acute respiratory syndrome coronavirus 2"[All Fields] OR "sars cov 2"[All Fields]) OR (corona[All Fields] AND ("viruses"[MeSH Terms] OR "viruses"[All Fields] OR "virus"[All Fields])) OR ("coronavirus"[MeSH Terms] OR "coronavirus"[All Fields]) OR ("COVID‐19"[All Fields] OR "COVID‐2019"[All Fields] OR "severe acute respiratory syndrome coronavirus 2"[Supplementary Concept] OR "severe acute respiratory syndrome coronavirus 2"[All Fields] OR "2019‐nCoV"[All Fields] OR "SARS‐CoV‐2"[All Fields] OR "2019nCoV"[All Fields] OR (("Wuhan"[All Fields] AND ("coronavirus"[MeSH Terms] OR "coronavirus"[All Fields])) AND (2019/12[PDAT] OR 2020[PDAT]))) OR ("COVID‐19"[Supplementary Concept] OR "COVID‐19"[All Fields] OR "covid19"[All Fields])) AND (("gravidity"[MeSH Terms] OR "gravidity"[All Fields] OR "pregnant"[All Fields]) OR ("pregnancy"[MeSH Terms] OR "pregnancy"[All Fields]) OR ("live birth"[MeSH Terms] OR ("live"[All Fields] AND "birth"[All Fields]) OR "live birth"[All Fields]) OR ("abortion, spontaneous"[MeSH Terms] OR ("abortion"[All Fields] AND "spontaneous"[All Fields]) OR "spontaneous abortion"[All Fields] OR "miscarriage"[All Fields]) OR ("abortion, induced"[MeSH Terms] OR ("abortion"[All Fields] AND "induced"[All Fields]) OR "induced abortion"[All Fields] OR "abortion"[All Fields]) OR ("parturition"[MeSH Terms] OR "parturition"[All Fields] OR "birth"[All Fields]) OR ("J In Vitro Fert Embryo Transf"[Journal] OR "ivf"[All Fields]) OR ("IUI"[Journal] OR "iui"[All Fields]) OR ("sex"[MeSH Terms] OR "sex"[All Fields]) OR ("fertility"[MeSH Terms] OR "fertility"[All Fields]) OR ("infertility"[MeSH Terms] OR "infertility"[All Fields]) OR ("germ cells"[MeSH Terms] OR ("germ"[All Fields] AND "cells"[All Fields]) OR "germ cells"[All Fields] OR "gamete"[All Fields]) OR ("oocytes"[MeSH Terms] OR "oocytes"[All Fields] OR "oocyte"[All Fields]) OR ("embryonic structures"[MeSH Terms] OR ("embryonic"[All Fields] AND "structures"[All Fields]) OR "embryonic structures"[All Fields] OR "embryo"[All Fields]))
Embase
('2019 ncov' OR 'sars cov 2' OR 'corona virus'/exp OR 'corona virus' OR (corona AND ('virus'/exp OR virus)) OR 'coronavirus'/exp OR coronavirus OR 'covid 19' OR covid19) AND (pregnant OR 'pregnancy'/exp OR pregnancy OR 'live birth'/exp OR 'live birth' OR (live AND ('birth'/exp OR birth)) OR 'miscarriage'/exp OR miscarriage OR 'abortion'/exp OR abortion OR 'birth'/exp OR birth OR 'ivf'/exp OR ivf OR iui OR 'sex'/exp OR sex OR 'fertility'/exp OR fertility OR 'infertility'/exp OR infertility OR 'gamete'/exp OR gamete OR 'oocyte'/exp OR oocyte OR 'embryo'/exp OR embryo)
Singh B, Gornet M, Sims H, Kisanga E, Knight Z, Segars J. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) and its effect on gametogenesis and early pregnancy. Am J Reprod Immunol. 2020;84:e13351. 10.1111/aji.13351
REFERENCES
- 1. Rasmussen SA, Hayes EB. Public health approach to emerging infections among pregnant women. Am J Public Health. 2005;95:1942‐1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siston AM, Rasmussen SA, Honein MA, et al. Pandemic 2009 influenza A (H1N1) virus illness among pregnant women in the United States. JAMA. 2010;303:1517‐1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moore CA, Staples JE, Dobyns WB, et al. Characterizing the pattern of anomalies in congenital Zika syndrome for pediatric clinicians. JAMA Pediatr. 2017;171:288‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med. 2016;374:1981‐1987. [DOI] [PubMed] [Google Scholar]
- 5. Cucinotta D, Vanelli M. WHO Declares COVID‐19 a Pandemic. Acta Biomed. 2020;91(1):157‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization . Coronavirus Disease (COVID‐2019 Situation Reports. https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/situation‐reports. Accessed 31 May 2020.
- 7. Prem K, Liu Y, Russell TW, et al. The effect of control strategies to reduce social mixing on outcomes of the COVID‐19 epidemic in Wuhan, China: a modelling study [published correction appears in Lancet Public Health 2020 May; 5(5):e260]. Lancet Public Health. 2020;5(5):e261‐e270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weiss SR, Navas‐Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol Mol Biol Rev. 2005;69:635‐664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Su S, Wong G, Shi W, et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24:490‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rasmussen SA, Smulian JC, Lednicky JA, Wen TS, Jamieson DJ. pregnancy : what obstetricians need to know. Am J Obstet Gynecol. 2020;222(5):415‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of Novel Coronavirus‐Infected Pneumonia. N Engl J Med. 2020;382(13):1199‐1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dorigatti I, Okell L, Cori A, et al. Severity of 2019‐novel coronavirus (nCoV). Imperial CollegeLondon (10‐02‐2020), 10.25561/77154 [DOI]
- 13. Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382(10):929‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Poon LC, Yang H, Kapur A, et al. Global interim guidance on coronavirus disease 2019 (COVID‐19) during pregnancy and puerperium from FIGO and allied partners: information for healthcare professionals. Int J Gynaecol Obstet. 2020;149(3):273‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chan JF, Kok KH, Zhu Z, et al. Genomic characterization of the 2019 novel human‐pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan [published correction appears in Emerg Microbes Infect 2020 Dec; 9(1):540]. Emerg Microbes Infect. 2020;9(1):221‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schwartz DA, Graham AL. Potential maternal and infant outcomes from (Wuhan) Coronavirus 2019‐nCoV infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses. 2020;12(2):194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Qiao J. What are the risks of COVID‐19 infection in pregnant women? Lancet. 2020;395(10226):760‐762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shah PS, Diambomba Y, Acharya G, Morris SK, Bitnun A. Classification system and case definition for SARS‐CoV‐2 infection in pregnant women, fetuses, and neonates. Acta Obstet Gynecol Scand. 2020;99(5):565‐568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang Z, Xu X. scRNA‐seq Profiling of Human Testes Reveals the Presence of the ACE2 Receptor, A Target for SARS‐CoV‐2 Infection in Spermatogonia, Leydig and Sertoli Cells. Cells. 2020;9(4):920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W. Single‐Cell Rna Expression Profiling of Ace2, the Receptor of SARS‐CoV‐2. [published online ahead of print, 2020 April 09]. Am J Respir Crit Care Med. 2020.202(5): 756–759. 10.1164/rccm.202001-0179LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Verma S, Saksena S, Sadri‐Ardekani H. CE2 Receptor Expression in Testes: Implications in COVID‐19 Pathogenesis [published online ahead of print, 2020 May 19]. Biol Reprod. 2020;na(na):1–3. 10.1093/biolre/ioaa080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271‐280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu J, Qi L, Chi X, et al. Orchitis: a complication of severe acute respiratory syndrome (SARS). Biol Reprod. 2006;74(2):410‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li D, Jin M, Bao P, Zhao W, Zhang S. Clinical characteristics and results of semen tests among men with Coronavirus Disease 2019. JAMA Netw Open. 2020;3(5):e208292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pan F, Xiao X, Guo J, et al. No evidence of severe acute respiratory syndrome‐coronavirus 2 in semen of males recovering from coronavirus disease 2019. Fertil Steril. 2020;113(6):1135‐1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jung A, Schuppe HC. Influence of genital heat stress on semen quality in humans. Andrologia. 2007;39:203‐215. [DOI] [PubMed] [Google Scholar]
- 27. Carlsen E, Andersson AM, Petersen JH, Skakkebaek NE. History of febrile illness and variation in semen quality. Hum Reprod. 2003;18:2089‐2092. [DOI] [PubMed] [Google Scholar]
- 28. Loveland KL, Klein B, Pueschl D, et al. Cytokines in Male Fertility and Reproductive Pathologies: Immunoregulation and Beyond. Front Endocrinol (Lausanne). 2017;8:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mehta P, McAuley DF, Brown M, et al. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jing Y, Run‐Qian L, Hao‐Ran W, Ya‐Bin L, Yang G, Fei C. Potential influence of COVID‐19/ACE2 on the female reproductive system. Mol Hum Reprod. 2020; 26(6):367–373. 10.1093/molehr/gaaa030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ge XY, Li JL, Yang XL, et al. Isolation and characterization of a bat SARS‐like coronavirus that uses the ACE2 receptor. Nature. 2013;503(7477):535‐538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rasmussen SA, Jamieson DJ. Coronavirus Disease 2019 (COVID‐19) and Pregnancy: Responding to a Rapidly Evolving Situation. Obstet Gynecol. 2020;135(5):999‐1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. American College of Obstetricians and Gynecologists, Practice Advisory . Novel Coronavirus 2019 (COVID‐19). April 2020. https://www.acog.org/clinical/clinical‐guidance/practice‐advisory/articles/2020/03/novel‐coronavirus‐2019. Accessed 24 May 2020
- 34. Muldoon KM, Fowler KB, Pesch MH, Schleiss MR. SARS‐CoV‐2: is it the newest spark in the TORCH? J Clin Virol. 2020;127:104372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dashraath P, Wong JLJ, Lim MXK, et al. Coronavirus disease 2019 (COVID‐19) pandemic and pregnancy. Am J Obstet Gynecol. 2020;222(6):521‐531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fan C, Lei D, Fang C, Li C, Wang M, Liu Y et al. Perinatal Transmission of COVID‐19 Associated SARS‐CoV‐2: Should We Worry?. Clin Infect Dis. 2020. Mar 17:ciaa226. 10.1093/cid/ciaa226. Epub ahead of print. [DOI] [Google Scholar]
- 37. Chen Y, Peng H, Wang L, et al. Infants Born to Mothers With a New Coronavirus (COVID‐19). Front Pediatr. 2020;8:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zeng H, Xu C, Fan J, et al. Antibodies in infants born to mothers with COVID‐19 pneumonia. JAMA. 2020;323(18):1848‐1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dong L, Tian J, He S, et al. Possible vertical transmission of SARS‐CoV‐2 from an infected mother to her newborn. JAMA. 2020;323(18):1846‐1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Egloff C, Vauloup‐Fellous C, Picone O, Mandelbrot L, Roques P. Evidence and possible mechanisms of rare maternal‐fetal transmission of SARS‐CoV‐2 [published online ahead of print, 2020 May 18]. J Clin Virol. 2020;128:104447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zeng L, Xia S, Yuan W, et al. Neonatal Early‐Onset Infection With SARS‐CoV‐2 in 33 Neonates Born to Mothers With COVID‐19 in Wuhan, China. [published online ahead of print, 2020 Mar 26]. JAMA Pediatr. 2020. https://jamanetwork.com/journals/jamapediatrics/fullarticle/2763787?utm_campaign=articlePDF&utm_medium=articlePDFlink&utm_source=articlePDF&utm_content=jamapediatrics.2020.0878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Baergen RN, Heller DS. Placental pathology in Covid‐19 positive mothers: preliminary findings. Pediatr Dev Pathol. 2020;23(3):177‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shanes ED, Mithal LB, Otero S, Azad HA, Miller ES, Goldstein JA. Placental Pathology in COVID‐19 [published online ahead of print, 2020 May 22]. Am J Clin Pathol. 2020.154(1):23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Penfield CA, Brubaker SG, Limaye MA, et al. Detection of severe acute respiratory syndrome coronavirus 2 in placental and fetal membrane samples. Am J Obstet Gynecol MFM. 2020. 2(3):10013. 10.1016/j.ajogmf.2020.100133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Patanè L, Morotti D, Giunta MR, et al. Vertical transmission of coronavirus disease 2019: severe acute respiratory syndrome coronavirus 2 RNA on the fetal side of the placenta in pregnancies with coronavirus disease 2019‐positive mothers and neonates at birth. Am J Obstet Gynecol MFM. 2020; 2(3): 100145. 10.1016/j.ajogmf.2020.100145. Epub 2020 May 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu W, Wang Q, Zhang Q, et al. Coronavirus Disease 2019 (COVID‐19) During Pregnancy: A Case Series. Preprints. 2019;2020:2020020373.https://www.preprints.org/manuscript/202002.0373/v1
- 47. Yu N, Li W, Kang Q, Zeng W, Feng L, Wu J. No SARS‐CoV‐2 detected in amniotic fluid in mid‐pregnancy. Lancet Infect Dis. Apr 22: S1473‐3099(20)30320‐0 2020. 10.1016/S1473-3099(20)30320-0. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Qiu L, Liu X, Xiao M, et al. SARS‐CoV‐2 is not detectable in the vaginal fluid of women with severe COVID‐19 infection. Clin Infect Dis. 2020. 71(15):813‐817. 10.1093/cid/ciaa375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Reis FM, Bouissou DR, Pereira VM, Camargos AF, dos Reis AM, Santos RA. Angiotensin‐(1–7), its receptor Mas, and the angiotensin‐converting enzyme type 2 are expressed in the human ovary. Fertil Steril. 2011;95:176‐181. [DOI] [PubMed] [Google Scholar]
- 50. Shuttleworth G, Broughton Pipkin F, Hunter MG. In vitro development of pig preantral follicles cultured in a serum‐free medium and the effect of angiotensin II. Reproduction. 2002;123:807‐818. [PubMed] [Google Scholar]
- 51. Ferreira R, Gasperin B, Rovani M, et al. Angiotensin II signaling promotes follicle growth and dominance in cattle. Endocrinology. 2011;152:4957‐4965. [DOI] [PubMed] [Google Scholar]
- 52. Viana GE, Pereira VM, Honorato‐Sampaio K, Oliveira CA, Santos RA, Reis AM. Angiotensin‐(1–7) induces ovulation and steroidogenesis in perfused rabbit ovaries. Exp Physiol. 2011;96:957‐965. [DOI] [PubMed] [Google Scholar]
- 53. Sugino N, Suzuki T, Sakata A, et al. Angiogenesis in the human corpus luteum: changes in expression of angiopoietins in the corpus luteum throughout the menstrual cycle and in early pregnancy. J Clin Endocrinol Metab. 2005;90(11):6141‐6148. [DOI] [PubMed] [Google Scholar]
- 54. Ng WF, Wong SF, Lam A, et al. The placentas of patients with severe acute respiratory syndrome: a pathophysiological evaluation. Pathology. 2006;38(3):210‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wong SF, Chow KM, Leung TN, et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol. 2004;191(1):292‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zheng QL, Duan T, Jin LP. Single‐cell RNA expression profiling of ACE2 and AXL in the human maternal–Fetal interface. Reprod Dev Med. 2020;4:7‐10. [Google Scholar]
- 57. Zhang YZ.Novel 2019 coronavirus genome. Virological. http://virological.org/t/novel‐2019‐coronavirus‐genome/319. Accessed 24 May 2020
- 58. Chan JF, Kok KH, Zhu Z, et al. Genomic characterization of the 2019 novel human‐pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9(1):221‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wrapp D, Wang N, Corbett KS, et al. Cryo‐EM structure of the 2019‐nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260‐1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Holmes KV. Coronaviruses. In: Webster RG, Granoff A, eds. Encyclopedia of Virology. London; San Diego: Academic Press; 1994:255‐260. [Google Scholar]
- 61. Macy JD, Weir EC, Barthold SW. Reproductive abnormalities associated with a coronavirus infection in rats. Lab Anim Sci. 1996;46(1):129‐132. [PubMed] [Google Scholar]
- 62. Wylie KM, Wylie TN, Cahill AG, Macones GA, Tuuli MG, Stout MJ. The vaginal eukaryotic DNA virome and preterm birth. Am J Obstet Gynecol. 2018;219(2):189.e1‐189.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]


