Abstract
Respiratory viral infections are known causes of mortality after allogeneic hematopoietic stem cell transplantation (HSCT). Here, we report a unique case of a child with viral pneumonia caused by coinfection with human metapneumovirus (MPV), respiratory syncytial virus (RSV), and SARS‐CoV‐2 after HSCT. A 9‐year‐old girl with acute lymphoblastic leukemia underwent allogeneic HSCT from a matched, unrelated donor. During the post‐transplant period, in profound leukopenia (below 10 leukocytes/µL), she was diagnosed with SARS‐CoV‐2, MPV, and RSV pneumonia and was treated with ribavirin and chloroquine. Before leukocyte recovery, the girl became asymptomatic, and SARS‐CoV‐2 and RSV clearance was achieved. The shedding of SARS‐CoV‐2 stopped before immune system recovery, and one may hypothesize that the lack of an inflammatory response might have been a contributing factor to the mild clinical course. Post‐transplant care in HSCT recipients with COVID‐19 infection is feasible in regular transplant units, provided the patient does not present with respiratory failure. Early and repeated testing for SARS‐CoV‐2 in post‐transplant patients with concomitant infection mitigation strategies should be considered in children after HSCT who develop fever, respiratory symptoms, and perhaps gastrointestinal symptoms to control the spread of COVID‐19 both in patients and in healthcare workers in hospital environments. Training of staff and the availability of personal protective equipment are crucial for containing SARS‐CoV‐2 infection.
Keywords: COVID19, metapneumovirus, pediatric hematopoietic stem cell transplantation, pneumonia, respiratory syncytial virus, SARS‐CoV‐2
Abbreviations
- ARDS
acute respiratory distress syndrome
- ATG
antithymocyte globulin
- COVID‐19
coronavirus disease 2019
- CRP
C‐reactive protein
- CRS
cytokine release syndrome
- CsA
cyclosporine A
- ECIL
European Conference on Infections in Leukaemia
- HSCT
allogeneic hematopoietic stem cell transplantation
- ICU
intensive care unit
- IL6
interleukin‐6
- MPV
human metapneumovirus
- RCT
randomized controlled trials
- RCTs
randomized clinical trials
- RdRp
RNA‐dependent RNA polymerase
- RSV
respiratory syncytial virus
- RVIs
respiratory viral infections
- SARS
severe acute respiratory syndrome
- TBI
total body irradiation
1. INTRODUCTION
RVIs are known causes of mortality after HSCT, and with the exception of human influenza virus, no antiviral therapies are available. According to ECIL guidelines, RVI is a contraindication for HSCT, and postponing the procedure is indicated, if possible. Systemic off‐label therapy with ribavirin is recommended for severe RNA RVIs after HSCT. 1 Here, we report a unique case of a child with viral pneumonia caused by coinfection of MPV, RSV, and new coronavirus SARS‐CoV‐2 diagnosed after HSCT. To date, there are no reports on the clinical course and outcome of SARS‐CoV‐2 infection after HSCT. To our knowledge, this is the first reported patient with SARS‐CoV‐2 infection during the period of bone marrow aplasia who successfully recovered from COVID‐19.
2. CASE REPORT
A 9‐year‐old girl was admitted to the Department of Bone Marrow Transplantation in March 2020 for HSCT. At the age of 7 years, she was diagnosed with pre‐B common acute lymphoblastic leukemia. Due to early bone marrow relapse, the child continued chemotherapy with bortezomib and was enrolled in one course of blinatumomab followed by HSCT from a 9/10 HLA‐matched unrelated donor. On admission, the patient was in good condition with normal vital signs, mild cough, a slightly sore throat, and normal auscultatory findings. A multitest using nasopharyngeal swabs (Viasure Respiratory Panel V Real Time PCR Detection Kit® CerTest Biotec for specific identification of influenza A and B, RSV, parainfluenza 1‐4, adenovirus, MPV, bocavirus, rhinovirus, enterovirus, coronavirus 229E, HKU1, NL63, and OC43, Legionella pneumophila, Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis) revealed MPV RNA. However, testing for SARS‐CoV‐2 was not available at the time of admission. Despite MPV replication, the decision was made to proceed with transplantation due to her good condition because the timing for HSCT was optimal and any delay would have increased the risk of leukemia relapse.
Pretransplant conditioning consisted of fractionated TBI at a total dose of 12 Gy, etoposide (at a dose of 60 mg/kg BW), and (ATG, Grafalon, Neovii®, total dose of 45 mg/kg BW). CsA from pretransplant day −1 and methotrexate on post‐transplant days +1, +3, and +6 were administered as graft‐versus‐host disease prophylaxis. On the first day of TBI, the patient developed a fever of 39.0°C, and empiric antibiotic therapy with piperacillin/tazobactam and amikacin was started.
On pretransplant day −1, the repeated PCR multitest revealed MPV with concomitant RSV infection. Fenoterol with ipratropium in nebulization and oral ribavirin (Copegus, Roche; 200 mg bid) were initiated. The early post‐HSCT period was uneventful, with persistent respiratory symptoms and signs and blood oxygen saturation >95% on room air. As of post‐transplant day +4, mucositis was observed, which is a standard complication after HSCT. Her respiratory symptoms progressed, and she exhibited diffuse wheezes and rhonchi with crepitant rales limited to basal areas of the lungs. Due to post‐transplant patient isolation, no imaging studies were performed. On post‐transplant day +6, the patient complained about weakness, chills, dysuria, and mucositis‐related oropharyngeal pain, with a temperature of 37.7°C, an increase in CRP to over 100 mg/L (normal range < 5 mg/L) and an increase in procalcitonin to 0.86 ng/mL (normal range < 0.05 ng/mL) (Figure 1). Because of swallowing difficulties, ribavirin was stopped after six days. On the +7 post‐transplant day, information about COVID‐19 was revealed in the radiotherapy center where the patient underwent the TBI procedure. A pharyngeal swab was positive for SARS‐CoV‐2 using a Vitassay qPCR test, which is based on qualitative assessment of two viral genes: ORF1ab and N. The COVID‐19 diagnosis was made on the +7 post‐transplant day, and treatment with chloroquine (10 mg/kg BW/24 hours; bid for 10 days) and azithromycin (10 mg/kg BW as the first dose followed by two daily doses of 5 mg/kg BW) was initiated on post‐transplant day +8 after COVID‐19 confirmation (as recommended for Polish pediatric patients in March 2020); ribavirin was restarted on post‐transplant day +10. The identification of COVID‐19 was followed by a decision to allow the patient to stay in the area of the pediatric transplantation unit. A part of the transplant unit was isolated for the patient, and she was isolated together with her mother in a single room with separate bathrooms and anteroom.
Figure 1.
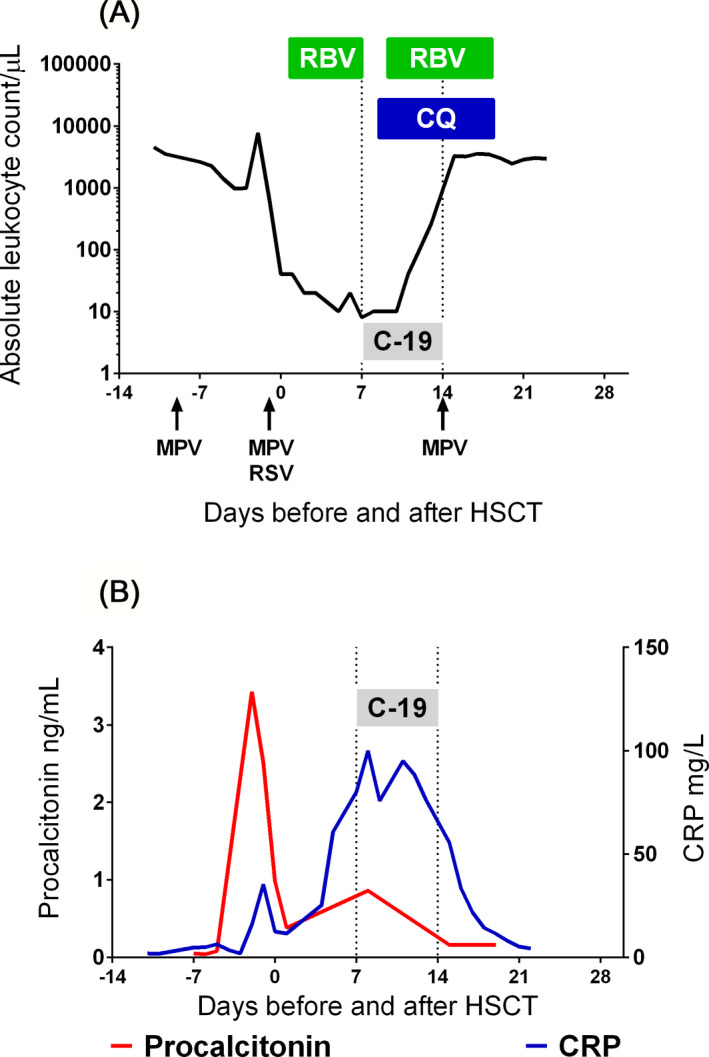
Graphic representation of the absolute leukocyte count (A.) and inflammation markers (B.), with diagnostic workup and antiviral treatment. Arrows show the positive results for MPV and RSV PCR. The dotted lines mark the period of documented COVID‐19 (C‐19). The green rectangles mark periods of ribavirin (RBV) and blue rectangle of chloroquine (CQ) therapy
The air conditioning in the patient's room was turned off to avoid spreading of infectious material due to positive pressure. Procedures typical for COVID‐19 were initiated (continuous remote vital sign monitoring, call contact, dedicated nursing staff, examination without auscultation). A broad and repeated screening for SARS‐CoV‐2 among the healthcare personnel was started, and new regulations on personal protective equipment (PPE) were issued. Visitations were limited to physicians and nurses trained to use class N95/FFP3 PPE and to enter the isolation area, and no SARS‐CoV‐2 infections were diagnosed among the dedicated staff. Of the remaining personnel, 3 cases of COVID‐19 were diagnosed within 10 days, which might have been transmitted by a patient. The patient continued CsA, acyclovir, cefepime, and micafungin, and filgrastim was started at a dose of 5 µg/kg/d. The girl's condition gradually improved, and her cough diminished. Bone marrow reconstitution was achieved on post‐transplant day +15. A repeated SARS‐CoV‐2 test on post‐transplant days +14, +15, and +21 did not reveal virus replication and secretion. At the time of this report, the patient was alive and well, with full donor engraftment. We were not able to identify the source of infection, but the patient's mother and other members of the family from imminent proximity repeatedly tested negative for SARS‐CoV‐2 by PCR, and the mother was negative for serum anti‐SARS‐CoV‐2 IgG/IgM antibodies (Rapid nCoV‐2019 IgG/IgM combo test card, Lotus NL).
3. DISCUSSION
Viral pneumonia caused by the novel β‐coronavirus SARS‐CoV‐2 was named COVID‐19 by the WHO on February 11, 2020, and currently constitutes the leading and overwhelming health problem worldwide. By the date of submission, 9 081 145 COVID‐19 cases and 471 316 related deaths have been confirmed worldwide. 2 Coronaviruses are positive‐sense single‐stranded RNA [(+)ssRNA] viruses with genetic material that can function both as a genome and as messenger RNA. Replication of the SARS‐CoV‐2 (+)ssRNA genome proceeds through double‐stranded RNA intermediates that are produced by RdRp, which catalyzes RNA replication from an RNA template and constitutes a promising therapeutic target. 3 COVID‐19 severity clearly depends on the age of the person infected: children present milder symptoms in comparison with the adult population, and the death rate is exceptionally low in the youngest age group. 4 , 5 Nevertheless, there are very few data concerning COVID‐19 outcomes in immunocompromised patients, and no data exist for HSCT recipients. 6 , 7 The situation of post‐HSCT is unique in terms of a lack of immunity due to extreme leukopenia and immunosuppressant administration, severe mucositis and a high risk of opportunistic infections. The transfer of patients during the peritransplant phase is generally not recommended unless not otherwise manageable life‐threatening conditions occur. In the reported case, the decision to keep the patient in the transplant unit was made due to the availability of spatially separated subunits with a room that provided isolation from other patients and allowed for maintaining specialistic nursing and physician care by the transplant team. Because the case was observed during the early phase of SARS‐CoV‐2 in Poland, epidemic mitigation strategies, such as adequate PPE distribution, compartmentalization of healthcare teams, and repeated testing of asymptomatic individuals, were instituted only after identification of the first patient but resulted in confinement of the infection; afterward, no further cases were identified. The patient's condition was affected by both COVID‐19 and viral coinfections; however, no data exist on the clinical course, treatment, and prognosis of SARS‐CoV‐2 alone or with RVI coinfections in HSCT recipients. According to Zhu et al, bacterial coinfections are dominant in all COVID‐19 patients, but Chinese data show that the coinfection pattern differs significantly depending on the geographic area. 8 , 9 Among viral COVID‐19 coinfections, influenza was reported as a cause of clinical deterioration, though the clinical course can be benign in children. 10 , 11 , 12
The influence of coinfections on severe viral respiratory disease is still unclear; however, RSV‐MPV coinfections in immunocompetent patients are generally comparable to single‐virus infections with regard to oxygen supplementation, ICU admission, or the need for mechanical ventilation. 13 , 14 , 15 Immunodeficiency in pediatric or adult HSCT recipients is a factor contributing to disease severity, and overall mortality in patients with post‐transplant RVI is reportedly 10%. 16 , 17 , 18
Due to a lack of proven therapies, initial COVID‐19 treatment was based on the recommendations issued in April 2020. Moreover, despite the large number of clinical trials started, no results of well‐designed prospective RCTs that support chloroquine/hydroxychloroquine or azithromycin efficacy in SARS‐CoV‐2 infection are available as of the date of publication. In the absence of clinical benefit, hydroxychloroquine toxicities have resulted in it being excluded from clinical recommendations. 3 , 19
Ribavirin was one of the first drugs belonging to the RdRp inhibitor family under investigation for COVID‐19 treatment and was included as a treatment option in the 7th edition of the Chinese Clinical Guidance for COVID‐19 Pneumonia Diagnosis and Treatment. 20 It must be stressed that these recommendations were not based on RCT results, and some experts even strongly recommend against the use of ribavirin for COVID‐19 treatment. The newer randomized study by Hung et al assessed the efficacy and safety of lopinavir‐ritonavir, with or without interferon beta‐1b, and ribavirin for treating patients with COVID‐19, showing a shorter duration of viral shedding and hospital stay in those treated with the combination therapy containing ribavirin and interferon. 21 In contrast to ribavirin, new RdRp inhibitors studied in clinical trials, such as remdesivir, have shown high affinity for SARS‐CoV‐2 RdRp and efficacy both in vitro and in animal models; at the time of publication, there is evidence for its efficacy in COVID‐19 patients. 22 , 23 , 24
Thus far, the role of the immune system in SARS‐CoV‐2 infection remains unclear, and conflicting evidence exists on the connection between virus replication, the inflammatory response, and tissue damage. Immunological recovery after HSCT is regarded as a primary mechanism required for infection control, and according to the immunodeficiency scoring index by Shah et al, the patient reported herein could be grouped in the high‐risk stratum, fulfilling the criteria of severe post‐transplant immunodeficiency as defined by Khanna et al. 25 , 26 The hypothesis of morbidity and mortality in COVID‐19 as a consequence of excessive tissue damage in the mechanism of CRS is supported by high plasma levels of inflammatory cytokines, especially IL6, in severely ill COVID‐19 patients. 23 , 27 Available data from small non‐randomized trials are conflicting, and to date, there is insufficient evidence to recommend for or against the use of IL6 blockade with tocilizumab or siltuximab. Suppression of an excessive inflammatory response in COVID‐19 has been studied, and in the RECOVERY trial, dexamethasone reduced the 28‐day mortality rate, showing the greatest benefit among patients requiring ventilation. 28 In our patient, profound pretransplant immunosuppression caused by in vivo lymphodepletion with ATG and myeloablation during the transplant conditioning regimen. Due to the early post‐transplant phase, stem cell donor–derived immunity (including leukocyte‐, lymphocyte‐, or antibody‐mediated mechanisms) could not have been involved in SARS‐CoV‐2 clearance, and at the moment of the first negative PCR result, the patient had only 10 lymphocytes per microliter. The shedding of SARS‐CoV‐2 stopped before immune system recovery, and one may hypothesize that the lack of an inflammatory response might have been a factor contributing to the mild clinical course. The role of ribavirin administration is difficult to evaluate in a single patient, but the elimination of multiple viral infections in the state of ultimate immunodeficiency suggests some efficacy of pharmacotherapy.
4. CONCLUSION
Post‐transplant care in HSCT recipients with COVID‐19 infection is feasible in regular transplant units, provided the patient does not present with respiratory failure. The present case emphasizes the need for testing for SARS‐CoV‐2 and other respiratory viruses prior to transplant, even in the absence of symptoms, which has been widely adopted by stem cell transplant centers as a countermeasure against RVI. Early and repeated testing for SARS‐CoV‐2 in post‐transplant patients with concomitant infection mitigation strategies should be considered in children after HSCT who develop fever, respiratory symptoms, and perhaps gastrointestinal symptoms to control the spread of COVID‐19 both in patients and in healthcare workers in hospital environments.
Finally, training of staff and the availability of personal protective equipment are crucial for containing SARS‐CoV‐2 infection.
CONFLICT OF INTEREST
The authors have no conflicts of interest relevant to this article to disclose.
AUTHOR CONTRIBUTIONS
Drs Jarmoliński and Matkowska‐Kocjan: Collected the data, drafted the initial manuscript, and reviewed and revised the manuscript; Drs Rosa, Olejnik, Gorczyńska, and Kałwak: Collected the data and reviewed and revised the manuscript; and Dr Ussowicz: Conceptualized and designed the study, coordinated and supervised the data collection, and critically reviewed and revised the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Jarmoliński T, Matkowska‐Kocjan A, Rosa M, et al. SARS‐CoV‐2 viral clearance during bone marrow aplasia after allogeneic hematopoietic stem cell transplantation—A case report. Pediatr. Transplant. 2021;25:e13875. 10.1111/petr.13875
Tomasz Jarmoliński and Agnieszka Matkowska‐Kocjan are contributed equally as cofirst authors.
Funding information
This work was supported by Wroclaw Medical University statutory grant ST.C200.18.013.
REFERENCES
- 1. Hirsch HH, Martino R, Ward KN, Boeckh M, Einsele H, Ljungman P. Fourth European Conference on Infections in Leukaemia (ECIL‐4): guidelines for diagnosis and treatment of human respiratory syncytial virus, parainfluenza virus, metapneumovirus, rhinovirus, and coronavirus. Clin Infect Dis. 2013;56(2):258‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Worldometer . COVID‐19 coronavirus pandemic. https://www.worldometers.info/coronavirus/. Accessed June 22, 2020.
- 3. Rios P, Radhakrishnan A, Antony J, et al. Effectiveness and safety of antiviral or antibody treatments for coronavirus. medRxiv. 2020. https://www.medrxiv.org/content/10.1101/2020.03.19.20039008v2 [Google Scholar]
- 4. Morand A, Fabre A, Minodier P, et al. COVID‐19 virus and children: what do we know? Arch Pédiatrie. 2020;27(3):117‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China. JAMA. 2020;323(13):1239. [DOI] [PubMed] [Google Scholar]
- 6. Guillen E, Pineiro GJ, Revuelta I, et al. Case report of COVID‐19 in a kidney transplant recipient: Does immunosuppression alter the clinical presentation? American Journal of Transplantation. 2020;20(7):1875–1878. 10.1111/ajt.15874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li F, Cai J, Dong N. First cases of COVID‐19 in heart transplantation from China. The Journal of Heart and Lung Transplantation. 2020;39(5):496–497. 10.1016/j.healun.2020.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhu X, Ge Y, Wu T, et al. Co‐infection with respiratory pathogens among COVID‐2019 cases. Virus Res. 2020;285:198005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xing Q, Li G, Xing Y, et al. Precautions Are Needed for COVID‐19 Patients with Coinfection of Common Respiratory Pathogens. SSRN Electronic Journal. 10.2139/ssrn.3550013 [DOI] [Google Scholar]
- 10. Cuadrado‐Payán E, Montagud‐Marrahi E, Torres‐Elorza M, et al. SARS‐CoV‐2 and influenza virus co‐infection. Lancet. 2020;395(10236):e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kakuya F, Okubo H, Fujiyasu H, Wakabayashi I, Syouji M, Kinebuchi T. The First Pediatric Patients with Coronavirus Disease 2019 (COVID‐19) in Japan: Risk of Co‐Infection with Other Respiratory Viruses. Japanese Journal of Infectious Diseases. 2020;73(5):377–380. 10.7883/yoken.jjid.2020.181 [DOI] [PubMed] [Google Scholar]
- 12. Yue H, Zhang M, Xing L, et al. The epidemiology and clinical characteristics of co‐infection of SARS‐CoV‐2 and influenza viruses in patients during COVID‐19 outbreak. Journal of Medical Virology. 2020. 10.1002/jmv.26163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goka EA, Vallely PJ, Mutton KJ, Klapper PE. Single and multiple respiratory virus infections and severity of respiratory disease: a systematic review. Paediatr Respir Rev. 2014;15(4):363‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martin ET, Kuypers J, Wald A, Englund JA. Multiple versus single virus respiratory infections: viral load and clinical disease severity in hospitalized children. Influenza Other Respi Viruses. 2012;6(1):71‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nolan VG, Arnold SR, Bramley AM, et al. Etiology and impact of coinfections in children hospitalized with community‐acquired pneumonia. J Infect Dis. 2018;218(2):179‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fisher BT, Danziger‐Isakov L, Sweet LR, et al. A multicenter consortium to define the epidemiology and outcomes of inpatient respiratory viral infections in pediatric hematopoietic stem cell transplant recipients. J Pediatric Infect Dis Soc. 2018;7(4):275‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hutspardol S, Essa M, Richardson S, et al. Significant transplantation‐related mortality from respiratory virus infections within the first one hundred days in children after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2015;21(10):1802‐1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Akhmedov M, Wais V, Sala E, et al. Respiratory syncytial virus and human metapneumovirus after allogeneic hematopoietic stem cell transplantation: Impact of the immunodeficiency scoring index, viral load, and ribavirin treatment on the outcomes. Transplant Infectious Disease. 2020;22(4): 10.1111/tid.13276 [DOI] [PubMed] [Google Scholar]
- 19. Cavalcanti AB, Zampieri FG, Rosa RG, et al. Hydroxychloroquine with or without Azithromycin in Mild‐to‐Moderate Covid‐19. New England Journal of Medicine. 2020. 10.1056/nejmoa2019014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. China National Health Commission . Chinese Clinical Guidance for COVID‐19 Pneumonia Diagnosis and Treatment (7th edn). China National Health Commission. http://kjfy.meetingchina.org/msite/news/show/cn/3337.htmlthere. Accessed April 18, 2020. [Google Scholar]
- 21. Hung IF‐N, Lung K‐C, Tso EY‐K, et al. Triple combination of interferon beta‐1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID‐19: an open‐label, randomised, phase 2 trial. Lancet. 2020;395(10238):1695‐1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Res. 2020;30(3):269‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the Treatment of Covid‐19 — Preliminary Report. New England Journal of Medicine. 2020. 10.1056/nejmoa2007764 [DOI] [PubMed] [Google Scholar]
- 25. Shah DP, Ghantoji SS, Ariza‐Heredia EJ, et al. Immunodeficiency scoring index to predict poor outcomes in hematopoietic cell transplant recipients with RSV infections. Blood. 2014;123(21):3263‐3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khanna N, Widmer AF, Decker M, et al. Respiratory syncytial virus infection in patients with hematological diseases: single‐center study and review of the literature. Clin Infect Dis. 2008;46(3):402‐412. [DOI] [PubMed] [Google Scholar]
- 27. Ruan Q, Yang K, Wang W, Jiang L, Song J. Correction to: Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Medicine. 2020;46(6):1294–1297. 10.1007/s00134-02-06028-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. The RECOVERY (Randomised Evaluation of COVid‐19 thERapY) trial . EudraCT 2020–001113‐21. NCT04381936. https://www.recoverytrial.net/news/low-cost-dexamethasone-reduces-death-by-up-to-one-third-in-hospitalised-patients-with-severe-respiratory-complications-of-covid-19. Accessed June 22, 2020.


