A 69‐year‐old woman with a 2‐day history of fever, dry cough, sore throat, fatigue and diarrhoea was admitted in the Infectious Disease Unit in University Hospital of Ioannina in Greece after being confirmed positive for SARS‐CoV‐2. Previous medical history was unremarkable. On physical examination, temperature was 38.5°C, blood pressure 135/80 mmHg, pulse rate 99 b.p.m., respiratory rate 20/min and oxygen saturation 85% while the patient was breathing ambient air. Bilateral crackles were evident on auscultation; chest X‐ray showed basilar streaky opacities in both lungs. The patient was supplied with oxygen at a flow rate of 10 L/min with a Venturi face mask (FiO2 = 50%) and treated with ceftriaxone (2 g once a day (q.d.)), azithromycin (500 mg q.d.) and hydroxychloroquine (400 mg twice a day (b.i.d.) for the first day and 200 mg b.i.d. afterwards), as proposed by Hellenic National Public Health Organisation.
Laboratory evaluation on day 1 showed increased inflammatory markers and persisting hypokalaemia (Table 1). Arterial blood gas test was consistent of both metabolic and respiratory alkalosis. Further assessment indicated increased renal excretion of potassium: urine potassium 60 mmol/L and urine potassium‐to‐creatinine ratio 38 mmol/g. For the treatment of hypokalaemia, potassium was administered intravenously via a peripheral line at a daily dosage of 80 mEq.
Table 1.
Clinical laboratory results
| Reference range | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | |
|---|---|---|---|---|---|---|
| Haematocrit (%) | 41–53 | 38.7 | 37.9 | 38.6 | 37.9 | 42 |
| Haemoglobin (g/dL) | 13.5–17.5 | 13.2 | 13 | 13.3 | 12.7 | 13.9 |
| White cell count (per μL) | 4500–11 000 | 5890 | 6250 | 6920 | 8180 | 8850 |
| Absolute neutrophil count (per μL) | 1500–8000 | 4320 | 5060 | 5467 | 6500 | 6640 |
| Absolute lymphocyte count (per μL) | 1000–4800 | 1180 | 820† | 969† | 1010 | 1450 |
| Platelet count (per μL) | 150 000–400 000 | 262 000 | 299 000 | 312 000 | 375 000 | 465 000‡ |
| International normalised ratio | ≤1.1 | 1.1 | — | — | — | 1.1 |
| D‐dimers (μg/mL) | <0.5 | 1.37‡ | — | — | — | 3.21‡ |
| Fibrinogen (mg/dL) | 200–400 | 684‡ | — | — | — | 786‡ |
| Fasting plasma glucose (mg/dL) | 70–125 | 92 | 96 | 85 | 95 | 85 |
| Creatinine (mg/dL) | 0.6–1.2 | 0.79 | 0.76 | 0.73 | 0.79 | 0.81 |
| Urea (mg/dL) | 11–54 | 19 | 16 | 19 | 19 | 19 |
| Potassium (mmol/L) | 3.5–5.3 | 2.9† | 3.0† | 3.4 | 3.9 | 3.8 |
| Sodium (mmol/L) | 136–146 | 137 | 138 | 136 | 137 | 137 |
| Chloride (mmol/L) | 98–106 | 103 | 105 | 103 | 107 | 108 |
| Magnesium (mmol/L) | 1.3–2.1 | 2.01 | 1.88 | — | — | 1.88 |
| Alanine aminotransferase (U/L) | 10–35 | 23 | — | — | 31 | 34 |
| Aspartate aminotransferase (U/L) | 10–35 | 29 | — | 31 | 32 | 34 |
| Alkaline phosphatase (U/L) | 30–125 | 52 | — | — | — | — |
| Lactate dehydrogenase (U/L) | 115–230 | 341‡ | 368‡ | 344‡ | 431‡ | 400‡ |
| Total bilirubin (mg/dL) | 0.1–1.1 | 0.7 | — | 0.8 | 0.8 | — |
| Direct bilirubin (mg/dL) | 0.01–0.2 | 0.2 | — | 0.31 | — | — |
| Creatinine kinase (U/L) | 25–160 | 36 | 45 | 193 | 51 | 48 |
| High‐sensitivity troponin (pg/mL) | 0–11.6 | 5.8 | 5 | 5.3 | 4.3 | 4.2 |
| Ferritin (mg/dL) | 11–306.8 | 372‡ | — | — | — | 339‡ |
| C‐reactive protein (mg/L) | <6 | 80 | 75 | 78 | 81 | 91 |
| pH | 7.36–7.44 | 7.55‡ | 7.52‡ | 7.52‡ | 7.52‡ | 7.50‡ |
| PO2 (mmHg)§ | ≥60 | 56.7† | 58.5† | 61.8 | 55.1† | 70.4 |
| PCO2 (mmHg) | 36–44 | 32† | 32† | 30.4† | 25.4† | 27.4† |
| HCO3 (mEq/L) | 21–27 | 30.4‡ | 28.1‡ | 26.8 | 25.4 | 24.8 |
| Anion gap (mmol/L) | 3–9 | 3.6 | 4.9 | 6.2 | 4.6 | 4.2 |
| Lactate (mmol/L) | 0.4–2 | 1 | 0.7 | 1 | 0.8 | 0.9 |
The value in the patient was below normal.
The value in the patient was above normal.
Oxygen was supplied by Venturi mask at a flow rate of 10 L/min (FiO2 = 50%).
The following days, the patient remained febrile and stable without any signs of respiratory improvement, whereas she reported 1–2 diarrhoeas daily. After administering 400 mg tocilizumab on day 5, symptoms and laboratory improvement were noticed.
We were prompted to present this case by the results of a pre‐printed retrospective study including 175 COVID‐19 patients showing that 55% were diagnosed with hypokalaemia during their hospitalisation. 1 However, these findings were not confirmed by another study reporting mean potassium levels of 3.8 mmol/L (interquartile range: 3.5–4.2). 2 Considering the life‐threatening risk of electrolyte abnormalities and especially hypokalaemia, it is useful to consider potential factors contributing to hypokalaemia in COVID‐19 patients (Fig. 1). Delays regarding the handling of blood specimens might result in pseudo‐hypokalaemia, especially in warm environments. 3 Lengthy hospitalisations (~10 days) of SARS‐CoV‐2 patients might adversely affect potassium intake and lead to a negative imbalance.2, 3 COVID‐19 symptoms, such as cough, dyspnoea and tachypnoea, could lead to respiratory alkalosis which lowers serum potassium by its intracellular shift, whereas diarrhoeas could increase potassium losses.2, 3 Cardiovascular complications of COVID‐19, such as myocardial infarction or myocarditis, along with the infection‐induced stress could increase intracellular potassium shift due to beta2‐adrenergic stimulation.2, 3, 4 Although septic shock is infrequent among patients with COVID‐19 (~1%), it could be associated with extracellular volume depletion and metabolic alkalosis which increase both intracellular shift of potassium and renal loss. 3 Drug‐induced hypokalaemia should always be considered in SARS‐CoV‐2 patients. 3 Inhaled beta2‐adrenergic agonists and vasopressors, usually administered in those with respiratory infections and septic shock, increase beta2‐adrenergic stimulation. 3 Chloroquine, used in treatment protocols against SARS‐CoV‐2, may be associated with severe hypokalaemia in case of intoxication.3, 5 Hypokalaemia induced by diuretics should be considered in hypertensive patients, while renal losses of potassium may be increased due to osmotic diuresis in those with poorly controlled diabetes. 3 Antibiotics, in particular piperacillin and ticarcillin, and nucleoside analogues may increase renal potassium losses. 3 However, remdesivir, a novel nucleotide analogue with in vitro activity against SARS‐CoV‐2 used as compassionate therapy, has not been connected with the development of hypokalaemia. 5 Finally, it has been proposed that after the initial engagement of SARS‐CoV‐2 spike protein, there is subsequent down‐regulation of ACE2 abundance on cell surfaces, leading to angiotensin II accumulation. 6 The latter could induce the secretion of aldosterone by the adrenal cortex, resulting in sodium reabsorption and potassium excretion from the collecting duct in kidney.
Figure 1.
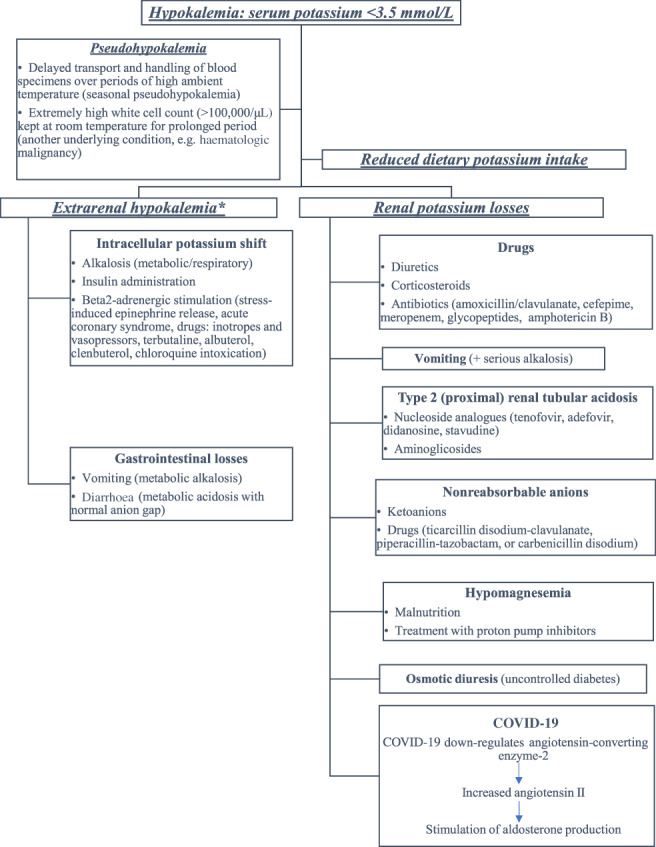
Diagnostic approach of hypokalaemia in patients with coronavirus disease 2019 (COVID‐19). *Urinary potassium excretion <25 mmol per day or spot urine concentration <15 mEq/L or urine potassium‐to‐creatinine ratio <13 mmol/g indicate extrarenal potassium losses.
Although our patient presented with alkalosis and diarrhoea upon her admission, the analysis of her urine sample strongly indicated ‘inappropriate’ renal potassium losses. According to the diagnostic approach described above, the ACE2 theory supported her persistent hypokalaemia. It has been proposed that hypokalaemia could be a prognostic marker of the viral load and COVID‐19 severity. 1 Of note, the variations of our patient's partial oxygen pressure paralleled those of potassium (Table 1). A keen eye and further investigation may confirm this relationship.
All things considered, hypokalaemia is of multifactorial origin in SARS‐CoV‐2 patients and may adversely affect outcome especially in those with pre‐existing cardiovascular disease. Therefore, potassium levels should be monitored and restored to normal, especially in severely affected SARS‐CoV‐2 patients.
References
- 1. Chen D, Li X, Song Q, Hu C, Su F, Dai J, Ye Y, Huang J, Zhang X. Assessment of hypokalemia and clinical characteristics in patients with coronavirus disease 2019 in Wenzhou, China. JAMA Netw. Open 2020; 3: e2011122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Palmer BF, Clegg DJ. Physiology and pathophysiology of potassium homeostasis: core curriculum 2019. Am J Kidney Dis 2019; 74: 682–95. [DOI] [PubMed] [Google Scholar]
- 4. Zheng YY, Ma YT, Zhang JY, Xie X. COVID‐19 and the cardiovascular system. Nat Rev Cardiol 2020; 17: 259–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID‐19): a review. JAMA 2020; 323: 1824–36. [DOI] [PubMed] [Google Scholar]
- 6. Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin–angiotensin–aldosterone system inhibitors in patients with Covid‐19. N Engl J Med 2020; 382: 1653–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


