Abstract
Knowledge on the mechanisms of viral spread, of time‐related changes, and age‐specific factors of severe acute respiratory syndrome coronavirus 2 infections is important to develop recommendations aimed at controlling the pandemic. In this context, longitudinal data on proportions of positive results in different age groups are rare. Data on total positive counts and on shares of positive counts deriving from a private (MVZ) and a University (RWTH) laboratory were analyzed retrospectively and compared with public data on total positive counts of the Robert Koch Institute (RKI). Data were covered for Weeks 9–24 of the year 2020 and all patient ages. Total positive counts were lower in children compared to adults. Proportions of children and adults tested positive were 3%–5% and 5%–7%, respectively. RKI and MVZ data showed similar time‐related patterns. Patients of 20–60 years of age did account for the initial virus spread (maximum infection rates at Weeks 9–11). Thereafter, infection rates decreased in older patients whereas children did not show a comparable time‐related decrease. Pediatric data generated in outpatient settings and hospitals differed markedly which should be considered in further studies. In summary, compared with adults children are less affected by severe acute respiratory syndrome coronavirus 2 infections and are unlikely to account for the initial viral spread. However, children show sustained viral activity and may serve as a viral reservoir.
Keywords: children, COVID‐2019, epidemiology, SARS‐CoV‐2, viral reservoir, virus spread
Highlights
Adolescents and adults seem to account for initial spreading of SARS‐CoV‐2.
SARS‐CoV‐2 positivity rates are lower in children compared to adults.
Children may show a retarded decline of SARS‐CoV‐2 positivity rates.
Reservoir exploration studies of SARS‐CoV‐2 should investigate if children serve as a viral reservoir.
1. INTRODUCTION
Children play a particular role in the concepts aiming at controlling severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infections. They are less affected clinically than adults regarding numbers of infected individuals and disease severity. Prevention concepts are more difficult to enforce: the younger, the less they will tolerate masks or respect physical distancing; the most important tools to prevent SARS‐CoV‐2 transmission. Similar to adults, typical symptoms in children are fever, respiratory problems, and, less frequently, enteritis. Children may show prolonged respiratory and fecal viral shedding as viral RNA has been detected for more than 4 weeks in respiratory and stool specimens. 1 , 2 , 3 , 4 , 5 , 6 , 7 Transmission of SARS‐CoV‐2 by children, however, has been reported to be considerably less frequent than by adults and most pediatric infections could so far be attributed to intrafamilial transmission. 8 , 9 , 10 , 11 These data determine pediatric infection control recommendations including controversially discussed decisions on the closure of schools and nurseries. 12 , 13 , 14 , 15
One important tool to elucidate the role of children in the SARS‐CoV‐2 pandemic are longitudinal investigations of total counts of SARS‐CoV‐2 positive patients and of the share of positive test results among all patients tested. In this context, the course of the pandemic in the west of the German state of North Rhine‐Westphalia was analyzed retrospectively, based on SARS‐CoV‐2 polymerase chain reaction (PCR) data from a commercial laboratory (MVZ Dr. Stein und Kollegen in Mönchengladbach, Germany; MVZ) and the Laboratory Diagnostics Center of RWTH Aachen University Hospital (RWTH). These data were compared to data from the German nationwide infection surveillance of the Robert Koch Institute (RKI; 188,133 datasets), a federal office, providing data on the number of patients infected with SARS‐CoV‐2 throughout Germany which derive from public health offices of all counties. These data are publicly accessible via the “surfstat” database (https://survstat.rki.de) and crucial for decisions of the German government aimed at controlling the COVID‐19 pandemic.
2. METHODS
Data obtained from the MVZ and RWTH laboratories and the RKI database comprised age at diagnose, SARS‐CoV‐2 PCR result from different respiratory specimens, and week at investigation. MVZ data further differentiated between the source of the specimen (hospital vs. not hospital) and included the postal codes of the patients. Datasets did comprise analyses performed between February 24 and June 6, for example, the Weeks 9–24 of the year 2020. The vast majority of MVZ samples derived from patients living in the west of North Rhine‐Westphalia, Germany (10,000,000 inhabitants). The specimens were collected from patients with respiratory symptoms as well as from asymptomatic individuals during screening campaigns in different outpatient settings and hospitals. RWTH samples were derived from patients with respiratory symptoms and from routinely screened patients without respiratory symptoms from the region of Aachen (550,000 inhabitants, part of the western area of North Rhine‐Westphalia). Data on the age distribution of citizens in North Rhine‐Westphalia derived from a public database provided by the state of North Rhine‐Westphalia (www.lgz.nrw.de).
MVZ PCR data were generated with two different methods. The SARS‐CoV2 IVD CE Test from Roche Diagnostics, Penzberg, Germany, was used on the fully automated Cobas 6800 analyzers according to the manufacturer's instructions. The second method was an in‐house PCR with primer pairs, published by Corman et al., 16 used on the Light‐Cycler 480 or on the Cobas 6800 after extraction of the virus RNA by Magna Pure Nucleic Acid Extraction System from Roche Diagnostics. RWTH PCR studies were performed using the Realstar SARS‐CoV‐2 RT PCR Kit RNA (Altona) following RNA extraction with the QIAamp Viral RNA Mini Kit (Qiagen) as recommended by the manufacturers. The study was approved by the Ethics Committee of the Medical Faculty of RWTH Aachen University (EK‐244‐20). For descriptive analyses, EXCEL and SPSS softwares were used.
3. RESULTS
3.1. Absolute numbers of SARS‐CoV‐2 positive adults peak before absolute numbers of SARS‐CoV‐2 positive children
For analysis of the kinetics of SARS‐CoV‐2 infections, MVZ data from 102,626 PCR analyses with 5,579 positive results were available. In total, 16,650 of the tests had been performed in the Heinsberg region which experienced the first general lockdown in Germany during the pandemic in Week 9.
Testing rates among patients of different ages within the different weeks are provided in Figure 1A,B. Figure 1A depicts the mean numbers of patients tested between weeks 9 and 24, differentiating between patients of 0 to 14 years and patients from 15 years of age. Figure 1B summarizes the mean numbers of patients weekly tested at different ages. With exception of Weeks 9 and 10, SARS‐CoV‐2 testing was performed less frequently among children than among adults. Independently of the respective week, SARS‐CoV‐2 testing was performed less frequently among children and among patients over 90 years of age. In contrast to total counts, the share of persons tested among elderly persons was high. Persons below 20 years of age not only showed low total test counts but also a low share of persons tested (Figure S1).
Figure 1.
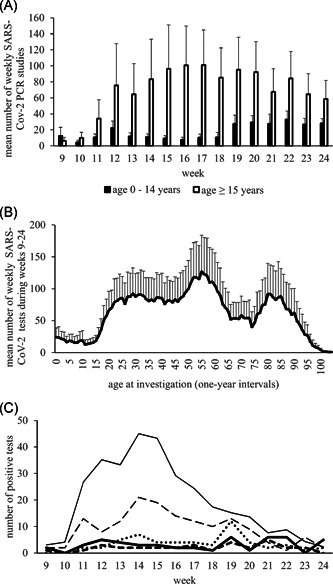
(A) (MVZ data) Mean absolute numbers of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) tests among pediatric age groups (age 0–14 years) and older age groups (age ≥15 years) studied in 1‐year intervals. With exception from Weeks 9 and 10, SARS‐CoV‐2 testing was performed less frequently in younger age groups. From Week 19, the testing rates increased in children and decreased among older patients without reaching similar levels. (B) (MVZ data) Mean numbers of weekly SARS‐CoV‐2 studies. Investigations at different ages between Weeks 9 and 24. Lowest rates of SARS‐CoV‐2 tests were observed among children and very old patients with a maximum of tests in patients of about 50–60 years of age. (C) (MVZ data) Absolute counts of positive test results between Weeks 9–24 for different age groups. Adults (≥20 years): thin solid line, mean of absolute counts of positive tests calculated over all 5‐year‐age groups. Children: absolute counts of positive tests for age group 0–4 years (thick solid line), age group 5–9 years (thick dashed line), age group 10–14 years (thick dotted line), and age group 15–19 years (thin dashed line). The number of children tested positive was considerably lower with highest numbers after Week 18 in age groups 0–14 years
To further study the number of patients tested positive at different ages, data were further analyzed focusing on 5‐year age groups, which mirrors the RKI data (Figure 1C). According to MVZ data, absolute numbers of SARS‐CoV‐2 positive adults peak around Weeks 12–15. Absolute numbers of SARS‐CoV‐2 positive children below 15 years of age peak after Week 18. Hereby, absolute numbers of children tested positive were low.
The low number of children tested positive might suggest a low share of positive results among all children tested. The positive rate among children between age 0 and 9 years; however, was only moderately lower than among adults (Figure S2). Children of 0–4 years of age were found positive in 3% (49/1678), of 5–9 years of age in 2.3% (32/1366), of 10–14 years in 4.7% (55/1167), and of 15–19 years in 4.9% (148/3033), respectively. Highest shares of positive results were found among patients between 100 and 109 years of age (10%), however, comprising a small age group of only 71 patients. For comparison, 7.2% of all patients studied in the Heinsberg region tested positive (1,300/16,650).
3.2. Data of the MVZ laboratory and the RKI database show similar time courses of the SARS‐CoV‐2 pandemic
To study to which extent regional data from North Rhine‐Westphalia might mirror the nationwide RKI data, the following method of data normalization was applied: At first, the absolute number of patients tested positive during Weeks 9–24 was calculated for one age group. The highest absolute count of positive tests within one particular week attributed a value of 100. The absolute numbers of SARS‐CoV‐2‐positive specimens of the remaining weeks of this age group were normalized by the week with the maximum number. This was performed for all 5‐year age groups of the MVZ data (Figure 2A) and of the RKI data (Figure 2B, 188,133 datasets on positive test results).
Figure 2.
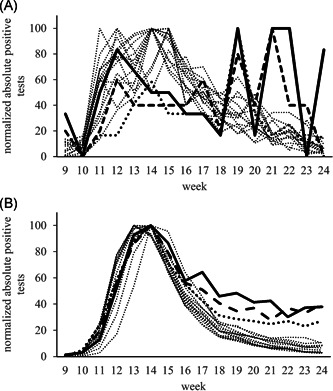
(A) (MVZ data) Normalized absolute counts of positive test results of MVZ data. Analyses performed between Weeks 9 and 24 for different age groups (n = 5,579) (MVZ cohort). Adult patients in 5‐year intervals: thin dotted lines. Pediatric age groups: age 0–4: thick solid line; age 5–9: thick dashed line; age 10–14: thick dotted line. Maximum counts of patients newly diagnosed positive for SARS‐CoV‐2 infection (100%) were recorded in Weeks 12–15 for all adult age groups. During the same time, a first peak was also found among children aged 0–14 years who not only failed to show decreasing numbers during the following time but also experienced their maximum peak during Weeks 19–21. (B) (RKI data) Normalized absolute counts of positive test results of RKI data. Data collected during the Weeks 9–24 for different age groups (n = 188,133). Similar to the MVZ data, all age groups of the RKI data showed the highest absolute numbers of positive findings (100%) in Weeks 13–14. Thereafter, in all adult groups (thin pointed lines; 5‐year intervals) numbers of newly infected patients decreased to below 20% of the maximum numbers until Week 24. In children up to 14 years of age, in contrast, no comparable decrease of newly diagnosed patients was recorded (age 0–4: thick solid line; age 5–9: thick dashed line; age 10–14: thick dotted line). SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
Data from both sources showed a similar distribution pattern indicating a good agreement: An initial increase of absolute positive results was followed by a marked decrease among adults and older children. No comparable decrease was observed for children below 15 years of age.
3.3. Proportions of adults tested positive for SARS‐CoV‐2 peak earlier than of children between 5 and 14 years of age
To evaluate whether the retarded decline of children positive for SARS‐CoV‐2 observed in Figure 2B is still visible when analyzing shares of positive tests, we used percentages of positive test results from MVZ data on 5‐year age groups and again applied the above‐described method of normalization (Figure 3A). Younger adults and adolescents (age 15–44 years) were found to show maximum positivity rates at Week 9. They were followed by older adults (age from 45 years) who mostly peaked in Week 11. Children of 5–14 years peaked later, namely at Weeks 13 and 16, respectively. This indicates that adolescents and younger adults might first have been affected by the pandemic.
Figure 3.

(A) (MVZ data) Normalized shares of positive results from MVZ data. Calculations performed for different 5‐year age intervals, distinguishing between different weeks. Normalization performed for the week with the highest share of positive findings (100%). Most middle‐aged age groups (age 15–44 years) showed their highest rates of positive findings at Week 9 (thin dotted lines), followed by most of the remaining adults (thin solid lines). Children between 5 and 14 years, in contrast, showed their peaks later and also showed a delayed decrease. Children: age 0–4: thick solid line; age 5–9: thick dashed line; age 10–14: thick dotted line. (B) (MVZ data) Patient age versus week of maximum share of positive SARS‐CoV‐2 findings. Calculations performed for 1‐year intervals. Closed circle: peak observed in one distinct week. Open circle: two peaks in two different weeks observed for the respective age group. Patients between 20 and 60 years of age showed maximum percentages of positive tests early during the pandemic (Weeks 9–11). In older patients maximum percentages of positive tests occurred between Weeks 11 and 15, whereas most children showed their peaks from Week 13 on. (C) (MVZ data) Cumulative change of share of positive test results in comparison to the preceding week depending on age. Whereas infection rates continuously decrease from 10 years of age, this is not the case between 0 and 9 years of age. SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
These findings were specified by refocusing on 1‐year age groups. For every 1‐year age group, the week with the highest share of positive tests was determined. Afterward, the respective week was plotted against the respective 1‐year age group. This resulted in three clusters (Figure 3B): Adults between 20 and 60 years of age (Cluster 1) were affected early during the pandemic (Weeks 9–11), followed by older persons (Cluster 2; Weeks 11–15). Children below 15 years of age were the latest to show highest infection rates (up to week 19; Cluster 3). The major process underlying this delayed peak of infection was constant low‐level infectious activity. This is depicted in Figure 3C showing the cumulative weekly changes of infection rates in different age groups. In contrast to other age groups that show a constant decrease of positivity rates after a peak of positivity was reached, children up to 9 years of age show, after an initial increase of the positivity rate, an oscillation of positivity rates around a value of ±5%.
3.4. In children test results obtained at hospitals differ from those obtained in ambulatory settings
Tests performed in ambulatory versus hospital settings might differ with regard to the prevalence of positive findings. Therefore, pediatric (age <15 years) and older (age ≥15 years) patient groups were compared using MVZ data on ambulatory patients (3,779 of 67,423 positive; 5.6%), MVZ data on patients treated in hospitals (1,800/34,994 positive; 5.1%) and data of patients treated at RWTH Aachen University Hospital (513/10,187 positive; 5%). Hereby, the abovementioned method of data normalization was applied again. As for adults, similar patterns were observed for all three settings (Figure 4A). Data from children tested in ambulatory settings showed a similar pattern as known from the overall analysis (Figure 4B). Children tested in hospitals, in contrast, only rarely tested positive which confirms that SARS‐CoV‐2 infections are less severe in children and that pediatric data generated in hospitals might not mirror the true epidemiological situation.
Figure 4.
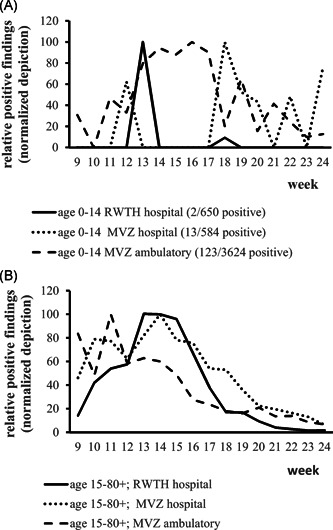
(A) (MVZ + RWTH data) Comparison of test results in different settings among children of 0–14 years of age. Comparison of the share of positive findings in ambulatory (MVZ) and hospital (MVZ/RWTH) settings (normalized depiction). Only studies among children performed in ambulatory settings showed a similar result pattern as on overall analysis (dashed line) whereas only sporadic positive cases were detected in hospitals. (B) (MVZ + RWTH data) Comparison of test results in different settings among patients from 15 years of age. Comparison of the share of positive findings in ambulatory (MVZ) and hospital (MVZ, RWTH) settings (normalized depiction). Irrespective from the site of analysis similar result patterns over time were observed. RWTH, RWTH Aachen University Hospital
4. DISCUSSION
This is one of the rare studies providing data on serial investigations on SARS‐CoV‐2 in respiratory specimens, also including proportions of patients tested positive in different age groups. The findings on total numbers of SARS‐CoV‐2 positive patients corroborate data of the nationwide German SARS‐CoV‐2 database of the RKI, which confirms that MVZ data can be used to analyze the situation in Germany (Figure 2A,B). As RKI data do not include general testing rates, the MVZ data additionally facilitate the interpretation of RKI data. For example, children were tested less frequently, which limits knowledge of the pandemic in this patient group (Figure 1B). As the MVZ data contain data of the Heinsberg region, the first German region that experienced a SARS‐CoV‐2‐related lockdown from Week 9, these data also include the early phase of the pandemic in Germany.
Studying these age‐ and time‐related data, it was found that first infection peaks occurred in adolescents and adults up to 60 years of age which might therefore account for initial viral spreading (Figure 3A,B). This observation should not have been biased by different testing rates as similar numbers of tests were found for all age groups during the initial Weeks 9 and 10 (Figure 1A). At this time, the general lockdown was already introduced in Heinsberg (from Week 9), before strong nationwide measures. This initial spread of the infection was followed by infections in older adults (Weeks 11–14), the closure of schools and nurseries (Week 12), and rules on contact restriction in Germany (Week 13). These lockdown measures resulted in a constant reduction of positivity rates in all age groups apart from young children not showing a time‐related decrease of rates of positive SARS‐CoV‐2 tests (Figures 1C and 3C). Whereas the number of tests performed increased with time, infection rates in children did not decrease, they constantly remained positive on a low level with peak positive numbers observed until Week 19. As wearing of mouth and nose masks was introduced in Week 18, the effect of these measures on children cannot conclusively be assessed by the here reported data.
Testing rates among children were low compared to adults and slightly increased from Week 19 (Figure 1A). This might indicate that children in general are less affected clinically by the pandemic. Positivity rates among children (3%–5%) were also lower than in adults which is in line with the literature (Figure S2). 17 , 18 These positivity rates, however, were considerably higher than the share of children tested positive among all patients tested positive for SARS‐CoV‐2 (0.9%–1.7%). 19 , 20 , 21 , 22 , 23 This shows that relying on absolute positive findings might be misleading and recommends to include positivity rates of different age groups into epidemiological studies.
In the literature, data on serial SARS‐CoV‐2 investigations in children are rare. Pan et al. 24 reported on serial studies on adults and pediatric patients age 0–19 years between December 8, 2019 and March 8, 2020 in Wuhan, China. In contrast to all adult patient groups showing a peak in January 2020, a delayed increase of absolute pediatric infections was observed. They concluded that “vigorous efforts should be made to protect and reduce transmission and symptom progression in vulnerable populations including both elderly people and young children.” 24 In line with this observation, Australian data on infections among children age 15–17 years who were newly diagnosed between 14 January 2020 and 7 June 2020 showed a peak in the 3rd and 5th weeks of March, followed by an only partial decline. 25
The findings reported here comply with the observation that the infection is not initially spread by children who show lower attack rates and are commonly infected by adults. 19 , 26 , 27 , 28 They also should not be misunderstood as an argument to reintroduce closures of schools and nurseries. There are alternative effective approaches as for instance shown by Taiwan, which seems to have developed a model on how to combine infection control with fulfilling the social and educative needs of children. 29 Nevertheless, the data reported here may indicate that children might be affected by the SARS‐CoV‐2 pandemic in a particular fashion, characterized by prolonged and sustained virus circulation. Together with low clinical involvement, they could form natural reservoirs for maintaining the infectious virus in a population in a phase of fainting numbers of COVID‐19 patients and serve as a link in the transmission chain. 30 , 31 , 32 Notably, although transmission from children to adults seems to be rare, it has repeatedly been reported. 33 , 34 , 35 For example, in a study summarizing experience on hospitalized children in Chicago Mannheim et al. 35 described child‐to‐adult transmission in 2 out of 15 transmissions where children were involved. Lam et al. 36 reported an increase of the share of pediatric patients (0–14 years of age) tested SARS‐CoV‐2 positive in Hong Kong from 0% to 20.3% between December 2019 and May 2020. The vast majority of these children were not detected because of clinical symptoms or known familial cases but during border controls introduced for infection control purposes. This underlines the impact of random testing for epidemiological purposes. According to the here reported data, the results of these studies may be affected by the study site as pediatric data generated in ambulatory and hospital settings differ markedly.
4.1. Limitations
This study is limited by the fact that data were not collected prospectively and that the number of pediatric samples is low compared to the share of children of the total population. Selection bias may derive from the fact that symptomatic patients and individuals with contact to symptomatic patients were tested preferentially. Moreover, the data presented here do not reflect the fact that different counties in the west of North Rhine‐Westphalia were not affected simultaneously but sequentially and with varying severity.
5. CONCLUSIONS
Compared with adults, children are clinically less affected by SARS‐CoV‐2 infections and they are unlikely to account for the initial viral spread. However, children show sustained viral activity and may serve as a viral reservoir which recommends to perform reservoir exploration studies of SARS‐CoV‐2 in children.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
All authors contributed to the conceptualization of the study, data curation, data acquisition, revision and editing of the manuscript, and approved the final version of the manuscript. Martin Häusler and Michael Kleines were responsible for the formal data analysis, data validation, data visualization, and writing of the original draft.
Supporting information
Supporting information.
Supporting information.
Supporting information.
ACKNOWLEDGMENT
Open access funding enabled and organized by Projekt DEAL.
Häusler M, van Helden J, Kleines M. Retarded decline of the share of SARS‐CoV‐2‐positive children in North Rhine‐Westphalia, Germany. J Med Virol. 2021;93:2039–2045. 10.1002/jmv.26564
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. De Ioris MA, Scarselli A, Ciofi Degli Atti ML, et al. Dynamic viral SARS‐CoV‐2 RNA shedding in children: preliminary data and clinical consideration of Italian regional center. J Pediatric Infect Dis Soc. 2020;9:366‐369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kam KQ, Yung CF, Cui L, et al. A well infant with coronavirus disease 2019 (COVID‐19) with high viral load. Clin Infect Dis. 2020:847‐849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Song R, Han B, Song M, et al. Clinical and epidemiological features of COVID‐19 family clusters in Beijing, China. J Infect. 2020;81:e26‐e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xing YH, Ni W, Wu Q, et al. Prolonged viral shedding in feces of pediatric patients with coronavirus disease 2019. J Microbiol, Immunol, Infect. 2020;53:473‐480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lu Y, Li Y, Deng W, et al. Symptomatic infection is associated with prolonged duration of viral shedding in mild coronavirus disease 2019: a retrospective study of 110 children in Wuhan. Pediatr Infect Dis J. 2020;39:e95‐e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hua CZ, Miao ZP, Zheng JS, et al. Epidemiological features and viral shedding in children with SARS‐CoV‐2 infection. J Med Virol. 2020;92:2804‐2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. She J, Liu L, Liu W. COVID‐19 epidemic: disease characteristics in children. J Med Virol. 2020;92:747‐754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chang TH, Wu JL, Chang LY. Clinical characteristics and diagnostic challenges of pediatric COVID‐19: a systematic review and meta‐analysis. J Formosan Med Assoc. 2020;119:982‐989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen J, Wang XF, Zhang PF. Asymptomatic SARS‐CoV‐2 infection in children: a clinical analysis of 20 cases. Chin J Contemp Pediatr. 2020;22:414‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID‐19 among children in China. Pediatrics. 2020;145:e20200702. [DOI] [PubMed] [Google Scholar]
- 11. Lavezzo E, Franchin E, Ciavarella C, et al. Suppression of a SARS‐CoV‐2 outbreak in the Italian municipality of Vo'. Nature. 2020;584:425‐429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Armitage R, Nellums LB. Considering inequalities in the school closure response to COVID‐19. The Lancet Global Health. 2020;8:e644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Esposito S, Principi N. School closure during the coronavirus disease 2019 (COVID‐19) pandemic: an effective intervention at the global level? [published online ahead of print May 13, 2020]. JAMA Pediatr. 10.1001/jamapediatrics.2020.1892 [DOI] [PubMed] [Google Scholar]
- 14. García‐Salido A. SARS‐COV‐2 children transmission: the evidence is that today we do not have enough evidence. Acta Paediatr. 2020;109:1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ludvigsson JF. Children are unlikely to be the main drivers of the COVID‐19 pandemic ‐ A systematic review. Acta Paediatr. 2020;109:1525‐1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019‐nCoV) by real‐time RT‐PCR. Euro Surveill. 2020;25:2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gudbjartsson DF, Helgason A, Jonsson H, et al. Spread of SARS‐CoV‐2 in the Icelandic population. N Engl J Med. 2020;382:2302‐2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Otto WR, Geoghegan S, Posch LC, et al. The epidemiology of SARS‐CoV‐2 in a pediatric healthcare network in the United States. [published online ahead of print June 19, 2020]. J Pediatric Infect Dis Soc. 10.1093/jpids/piaa074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Choi SH, Kim HW, Kang JM, Kim DH, Cho EY. Epidemiology and clinical features of coronavirus disease 2019 in children. Clin Exp Pediatr. 2020;63:125‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gujski M, Raciborski F, Jankowski M, Nowicka PM, Rakocy K, Pinkas J Epidemiological analysis of the first 1389 cases of COVID‐19 in Poland: a preliminary report. Med Sci Monitor. 2020;26:e924702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stokes EK, Zambrano LD, Anderson KN, et al. Coronavirus disease 2019 case surveillance—United States, January 22‐May 30, 2020. Morbidity Mortality Weekly Rep. 2020;69:759‐765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Docherty AB, Harrison EM, Green CA, et al. Features of 20133 UK patients in hospital with COVID‐19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239‐1242. [DOI] [PubMed] [Google Scholar]
- 24. Pan A, Liu L, Wang C, et al. Association of public health interventions with the epidemiology of the COVID‐19 Outbreak in Wuhan, China. JAMA. 2020;323:1915‐1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. COVID‐19 National Incident Room Surveillance Team . COVID‐19, Australia: epidemiology report 18 (fortnightly reporting period ending 7 June 2020). Commun Dis Intell. 2018;44:1‐27. [DOI] [PubMed] [Google Scholar]
- 26. Liu YJ, Chen P, Liu ZS, Li Y, Du H, Xu JL. Clinical features of asymptomatic or subclinical COVID‐19 in children. Chin J Contemp Pediatr. 2020;22:578‐582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mithal LB, Machut KZ, Muller WJ, Kociolek LK. SARS‐CoV‐2 infection in infants less than 90 days old. J Pediatr. 2020;224:150‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mizumoto K, Omori R, Nishiura H. Age specificity of cases and attack rate of novel coronavirus disease (COVID‐19). medRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang CJ, Ng CY, Brook RH. Response to COVID‐19 in Taiwan: big data analytics, new technology, and proactive testing. JAMA. 2020;323:1341‐1342. [DOI] [PubMed] [Google Scholar]
- 30. Zhang C, Gu J, Chen Q, et al. Clinical and epidemiological characteristics of pediatric SARS‐CoV‐2 infections in China: a multicenter case series. PLoS Med. 2020;17:e1003130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huff HV, Singh A. Asymptomatic transmission during the COVID‐19 pandemic and implications for public health strategies. [published online ahead of print May 28, 2020]. Clin Infect Dis. 10.1093/cid/ciaa654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kelvin AA, Halperin S. COVID‐19 in children: the link in the transmission chain. The Lancet Infect Dis. 2020;20:633‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhen‐Dong Y, Gao‐Jun Z, Run‐Ming J, et al. Clinical and transmission dynamics characteristics of 406 children with coronavirus disease 2019 in China: a review. J Infect. 2020;81:e11‐e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cai JH, Wang XS, Ge YL, et al. First case of 2019 novel coronavirus infection in children in Shanghai. Chine J Pediatr. 2020;58:E002. [DOI] [PubMed] [Google Scholar]
- 35. Mannheim J, Gretsch S, Layden JE, Fricchione MJ. Characteristics of hospitalized pediatric coronavirus disease 2019 cases in Chicago, Illinois, March–April 2020. [published online ahead of print June 1, 2020]. J Pediatric Infect Dis Soc. 10.1093/jpids/piaa070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lam HY, Lam TS, Wong CH, et al. The epidemiology of COVID‐19 cases and the Successful Containment Strategy in Hong Kong ‐ January to May 2020. Int J Infect Dis. 2020;98:51‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Supporting information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


