Abstract
COVID‐19 can occasionally be associated with cranial nerve involvement, but facial palsy, particularly if bilateral, is exceptional. We here report a patient who presented with severe bilateral facial palsy and evidence of SARS‐CoV‐2 infection preceded by upper respiratory symptoms. He also had serological evidence of coinfection with Epstein‐Barr virus, which could have also played a role in his neurological manifestations. PCR in the cerebrospinal fluid was negative for both EBV and SARS‐CoV‐2, which suggests an indirect, immune‐mediated mechanism rather than direct, viral‐induced damage. The patient was treated with prednisone 60 mg/24h with a tapering schedule and had a favorable outcome, with an almost complete recovery in 3 weeks. SARS‐CoV‐2 adds to the list of infectious agents causative of bilateral facial palsy. Coinfection with SARS‐CoV‐2 is not rare and should be considered in the differential diagnosis.
Keywords: bilateral facial palsy, COVID‐19, Epstein–Barr virus, peripheral facial palsy, SARS‐CoV‐2 virus
Bilateral facial palsy is a rare entity, accounting for 0.3 to 2% of all peripheral facial palsies. It can be caused by infections, inflammatory disorders (sarcoidosis), brainstem neoplasias, or can be idiopathic (bilateral Bell's palsy). SARS‐CoV‐2 adds to the list of infectious agents causative of bilateral facial palsy. Coinfection with SARS‐CoV‐2 is not rare (in this patient there was evidence of Epstein‐Barr virus coinfection) and should be considered in the differential diagnosis.
Case report
A 20‐year‐old male, with no relevant previous medical history, was admitted due to bilateral facial weakness. Two weeks before, he noticed odynophagia and fever of 39ºC without cough. He associated significant asthenia with headache, myalgia, nausea and vomiting and he was treated with levofloxacin 500 mg once a day for 7 days. One week after, during an initial improvement of the respiratory symptoms, he presented acute right facial weakness. He was diagnosed with right peripheral facial palsy and was treated with prednisone 60 mg/24 h with a tapering schedule. The following week he noted left facial weakness and was referred again to the emergency room. On neurological examination, there was a bilateral facial paresis (Figure 1). The rest of the examination was unremarkable, including other cranial nerves, strength, sensory examination and deep tendon reflexes. Based on the COVID‐19 pandemic at that time, a nasopharyngeal swab reverse transcriptase polymerase chain reaction (PCR) was performed for SARS‐CoV‐2 with positive results. Serum SARS‐CoV‐2 antibodies (qualitative immunoglobulin M and immunoglobulin G, IgM and IgG) were detectable at that time. He also had a positive heterophile test indicating a recent Epstein–Barr virus (EBV) infection.
Figure 1.
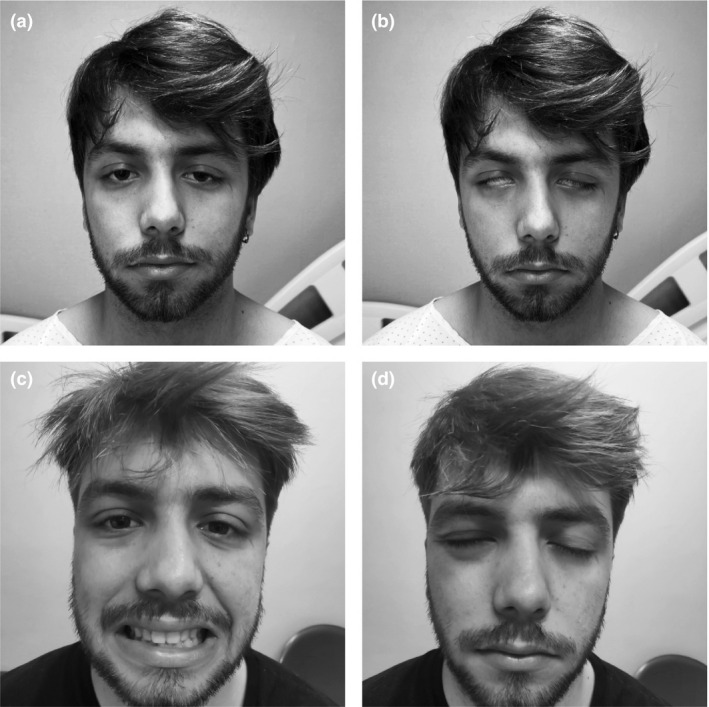
Patient with acute bilateral facial nerve paresis trying to smile (a) and closing eyes (b). (c), (d) The same gestures 3 weeks after discharge with a significant spontaneous improvement. Informed consent for publication was obtained from the patient.
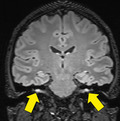
Brain magnetic resonance imaging raised the diagnosis of bilateral facial neuritis (Figure S1). The cerebrospinal fluid (CSF) showed protein levels of 80 mg/l and 9 cells/μl. CSF PCR was negative for SARS‐CoV‐2, EBV, enterovirus, herpes simplex and varicella zoster, cytomegalovirus and Parechovirus. Antiganglioside antibodies (IgM and IgG) were negative in serum and CSF. Neurophysiological studies revealed a severe neuropathy of the facial nerve bilaterally, with discrete signs of active denervation. The blink reflex showed delayed R1 and R2 responses and the absence of R2c response, with no peripheral nerve abnormalities.
A follow‐up examination 3 weeks later showed an almost complete facial palsy recovery (Figure 1). Neurophysiological studies showed facial nerve improvement with no peripheral nerve abnormalities.
Discussion
This patient presented with severe bilateral facial palsy, evidence of SARS‐CoV‐2 infection preceded by upper respiratory symptoms, and evidence of co‐infection with EBV. Interestingly, he had odynophagia but did not have the typical dry cough of COVID‐19.
Bilateral facial palsy is a rare entity, accounting for 0.3%–2% of all peripheral facial palsies [1]. There are several conditions identified as potential causes, including infections (Lyme disease, EBV, human immunodeficiency virus), inflammatory disorders (sarcoidosis), tumoural (brainstem tumours) and idiopathic (bilateral Bell's palsy) disorders. EBV infection is responsible for 0.5%–7.5% of peripheral facial palsies, and up to 35% are bilateral [2, 3]. This patient had serological evidence of recent EBV infection, although PCR for EBV in the CSF was negative.
Both viruses may have played a role in this patient. Co‐infection with SARS‐CoV‐2 is not rare. A recent study showed that 20.7% of a sample of 116 specimens positive for SARS‐CoV‐2 were also positive for one or more additional pathogens, amongst which EBV was not studied [4].
There are several reports of Guillain–Barré syndrome (GBS) associated with COVID‐19 infection [5], of which seven had facial diplegia (eight including ours), an incidence higher than the 3% reported in GBS [1]. One of the reported patients had isolated bilateral facial palsy and was interpreted as a variant of GBS known as bifacial weakness with paresthesias [6]. Our patient also meets the criteria (bifacial symmetrical weakness, absence of limb, neck or ocular weakness, evidence of infectious disease in the previous 3 days to 6 weeks, and albumino‐cytological dissociation in CSF), although he did not have paresthesias.
The fact that PCR in the CSF was negative for both EBV and SARS‐CoV‐2 may suggest an indirect, immune‐mediated mechanism rather than direct, viral‐induced damage.
SARS‐CoV‐2 infection should be suspected in patients with facial palsy or any suspicion of GBS in the times of the COVID‐19 pandemic since it may be the presenting feature in patients with mild respiratory symptoms. Co‐infections with other pathogens should be considered as they may require specific therapy.
Disclosure of conflicts of interest
The authors declare no conflict of interest regarding this paper.
Ethical approval
Informed consent for publication was obtained from the patient.
Author’s contributions
Cabrera Muras, Antonio; Carmona‐Abellán, María Mar; Collía Fernández, Alejandra; Uterga Valiente, Juan María: participated in data collection, drafting, and patient’s care. Antón Méndez, Lander: performed the MRI studies. García‐Moncó, Juan Carlos: participated in the study conception, drafting and writing.
Supporting information
Figure S1. Gadolinium‐enhanced T1 (a, coronal; c, axial) and FLAIR (b, coronal; d, axial) weighted sequences of a brain MRI. Bilateral and symmetric facial nerve enhancement can be noted along meatal and labyrinthine segments, as well as in both anterior genu. Findings are consistent with bilateral facial neuritis.
Data Availability Statement
Data available on request from the authors.
REFERENCES
- 1. Gaudin RA, Jowett N, Banks CA, Knox CJ, Hadlock TA. Bilateral facial paralysis: a 13‐year experience. Plast Reconstr Surg 2016; 138: 879–887. [DOI] [PubMed] [Google Scholar]
- 2. Coddington CT, Isaacs JD, Siddiqui AQ, Andrews TC. Neurological picture. Bilateral facial nerve palsy associated with Epstein–Barr virus infection. J Neurol Neurosurg Psychiatry 2010; 81: 1155–1156. [DOI] [PubMed] [Google Scholar]
- 3. Terada K, Niizuma T, Kosaka Y, Inoue M, Ogita S, Kataoka N. Bilateral facial nerve palsy associated with Epstein–Barr virus infection with a review of the literature. Scand J Infect Dis 2004; 36: 75–77. [DOI] [PubMed] [Google Scholar]
- 4. Kim D, Quinn J, Pinsky B, Shah NH, Brown I. Rates of co‐infection between SARS‐CoV‐2 and other respiratory pathogens. JAMA 2020; 323: 2085–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Toscano G, Palmerini F, Ravaglia S, et al. Guillain–Barré syndrome associated with SARS‐CoV‐2. N Engl J Med 2020; 382: 2574–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wakerley BR, Yuki N. Isolated facial diplegia in Guillain–Barré syndrome: bifacial weakness with paresthesias. Muscle Nerve 2015; 52: 927–932. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Gadolinium‐enhanced T1 (a, coronal; c, axial) and FLAIR (b, coronal; d, axial) weighted sequences of a brain MRI. Bilateral and symmetric facial nerve enhancement can be noted along meatal and labyrinthine segments, as well as in both anterior genu. Findings are consistent with bilateral facial neuritis.
Data Availability Statement
Data available on request from the authors.


