Abstract
Remdesivir is a direct‐acting nucleoside RNA polymerase inhibitor with activity against the novel severe acute respiratory syndrome‐coronavirus 2 (SARS‐CoV‐2) virus used in the treatment of coronavirus disease 2019 (COVID‐19) pneumonia. Here, we present two cases of suspected remdesivir‐associated acute liver failure (ALF) in which the liver failure improved after continuous infusion acetylcysteine and withdrawal of remdesivir. Both patients had significant increases in transaminases between day 3 and day 10 of remdesivir therapy accompanied by coagulopathy and encephalopathy. After initiation of continuous infusion acetylcysteine, the transaminases of both patients rapidly improved. Ultimately, one patient fully recovered while the other died of suspected septic shock. Due to its novel nature and only recent widespread use, there are very little data on the risk of ALF from remdesivir. Additionally, the data for the use of acetylcysteine to manage non‐acetaminophen‐induced ALF are limited. It is important to consider the risk of remdesivir‐associated ALF when weighing the risk versus benefits of use, and acetylcysteine may have a role in its management.
Keywords: adverse drug reactions, toxicology, COVID‐19, Remdesivir, acute liver failure, acetylcysteine, coronavirus, SARS‐CoV‐2
The novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) was initially identified in Wuhan, China, in December 2019 and has since led to the coronavirus disease 2019 (COVID‐19) pandemic experienced worldwide. 1 The first case of COVID‐19 pneumonia in the United States was documented in January of 2020, and cases have since exploded to over 5.8 million in the United States with over 180,000 deaths by August 28, 2020. 2 There are no currently approved treatments for SARS‐CoV‐2, with recent guidelines recommending supportive care for most patients and the use of remdesivir and dexamethasone for patients with severe COVID‐19 pneumonia. 3 , 4
As cases of COVID‐19 have increased exponentially, the health care infrastructure of the United States has been strained, leading to the desire by many clinicians to use experimental, unapproved therapies in addition to supportive care. One such experimental therapy is remdesivir (Gilead Sciences, Inc., Foster City, California, USA), which is an antiviral with potent activity against SARS‐CoV‐2. 5 Remdesivir has been shown to be superior to placebo in shortening time to recovery in adults hospitalized with COVID‐19, although there was no difference seen in mortality. 6 Remdesivir, although not currently approved by the US Food and Drug Administration (FDA), has been made available for clinical use through an Emergency Use Authorization (EUA) program through the FDA. In both preclinical and clinical studies, nonserious elevations of both aspartate transaminase (AST) and alanine transaminase (ALT) were noted, and the EUA fact sheet also reports that there have been several cases of severe liver‐related laboratory abnormalities. 7 The EUA program includes monitoring of both AST and ALT as a requirement for use in patients, with a focus on ALT. We report two cases of suspected remdesivir‐associated acute liver failure (ALF) in patients with COVID‐19 in which the liver failure improved with the addition of continuous infusion acetylcysteine and discontinuation of potentially hepatotoxic medications.
1. Case #1
A 68‐year‐old woman with a history of hypertension, diabetes mellitus, hyperlipidemia, and coronary artery disease (CAD) presented to an outside hospital with a chief complaint of increasing shortness of breath and tightness across her chest. On admission, she was found to have an oxygen saturation in the 70s and a systolic blood pressure > 200 mm Hg, both treated with bilevel positive airway pressure ventilation (BiPAP) and a nitroglycerin continuous infusion, respectively. The patient also reported chills, fatigue, and body aches for the past few days but with no sick contacts or known COVID‐19 exposure. A chest radiograph was performed that found bilateral, diffuse interstitial infiltrates consistent with COVID‐19 pneumonia, but the COVID‐19 reverse transcription polymerase chain reaction (RT‐PCR) performed on the day of admission was negative. An initial troponin was collected at a level of 0.2 ng/ml that increased to 1.35 ng/ml over the course of 24 hours, thought to be a type II non‐ST‐elevation myocardial infarction due to demand ischemia. On day 4 of admission, the patient was taken for cardiac catheterization and was found to have severe multivessel CAD, and the patient was transferred to our hospital for coronary artery bypass grafting (CABG). By day 4, the patient had been completely weaned from supplemental oxygen.
On hospital day 6, the patient was taken to the operating room for a planned CABG. Another COVID‐19 RT‐PCR was sent before the patient went to the operating room and was subsequently reported as positive after induction of anesthesia. It was determined that the patient’s CAD was critical enough to proceed with the revascularization, and the patient underwent a successful four‐vessel CABG. After the operation, the patient was admitted to the COVID‐19 intensive care unit (ICU) where she was extubated to 4 liters nasal cannula without incident on day 7 (postoperative day 1).
The patient’s oxygen requirements increased by day 8 to 8 liters nasal cannula and she developed a new cough and worsening infiltrates on chest radiograph. A loading dose of remdesivir 200 mg intravenous (i.v.) was administered the same day due to worsening COVID‐19 pneumonia, and a maintenance of remdesivir 100 mg i.v. every 24 hours was started on day 9 for a planned total of 5 days of therapy. Before remdesivir administration, the patient’s liver function enzymes were within normal limits. On day 10, the patient had increasing oxygen demands and an amiodarone infusion was initiated for new‐onset, postoperative atrial fibrillation. The amiodarone was initiated with a 150 mg i.v. loading dose over 10 minutes, and the continuous infusion (450 mg/250 ml) was started at 1 mg/min for 6 hours followed by 0.5 mg/min. By day 10, the patient was requiring humidified, high flow nasal cannula at 60 L/min at 100% fraction of inspired oxygen (FiO2). A complete metabolic panel (CMP) showed an AST greater than 5000 units/L and ALT of 2400 units/L (Figure 1). Over the course of the next 24 hours, both the AST and ALT were greater than 5000 units/L along with a total bilirubin of 3.1 mg/dL, and International Normalized Ratio (INR) of 2.3, and an ammonia of 161 μmol/L. Both remdesivir and amiodarone were discontinued and on the evening of day 10, a continuous infusion of acetylcysteine was initiated for the suspected remdesivir‐induced ALF using the 21 hours acetaminophen toxicity protocol of 150 mg/kg over 1 hour, 50 mg/kg over 4 hours, and 100 mg/kg over 16 hours.
Figure 1.
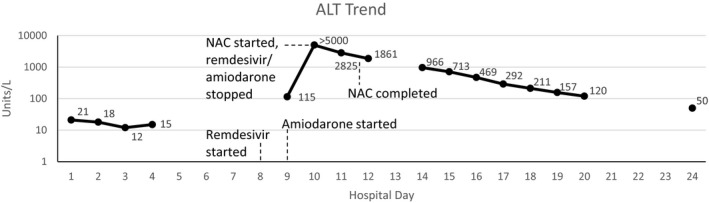
Alanine transaminase (ALT) trend by day in case #1 along with significant events (logarithmic trend). NAC = acetylcysteine.
By the end of the 21‐hour acetylcysteine protocol, the patient’s AST and ALT had decreased to 1348 and 1861 units/L, respectively. By day 14, the AST and ALT had decreased to 235 and 966 units/L, respectively, along with a total bilirubin of 2.1 mg/dL and an ammonia of 69 μmol/L. Over the course of the rest of her hospital stay, the patient’s oxygen requirements improved until she was weaned to room air on day 20 and her liver function tests, total bilirubin, and INR returned to within normal limits by day 24 of hospitalization. The patient was then discharged to home with home health care.
2. Case #2
An 80‐year‐old woman with a history of hypertension, diabetes mellitus, and hyperlipidemia presented to the emergency department with low back pain, fatigue, shortness of breath, and difficulty with urination. She endorsed fever and chills but no sick contacts or recent travel. Five days previously, the patient had been admitted to our institution for atypical chest pain and was found to have COVID‐19 pneumonia confirmed by RT‐PCR, and the patient was subsequently discharged the next day.
Upon this current admission, the patient’s chest radiograph showed persistent bibasilar and left perihilar infiltrates. She was found to have an oxygen saturation of 82% on room air, initiated on supplemental oxygen of 15 L/min, admitted to a step‐down COVID unit, and started on dexamethasone 6 mg orally every day. By day 3 of admission, she was requiring humidified, high flow nasal cannula at 55 L/min at 100% FiO2. Over the next few days, the patient’s oxygen requirements remained relatively unchanged. On day 5, she was initiated on remdesivir, with a 200 mg i.v. loading dose and 100 mg i.v. daily for the next 4 days (finishing on hospital day 9). She was also consented for convalescent plasma, which was administered on day 7. While receiving remdesivir, her transaminases remained stable and within normal limits, however, starting on day 5 of remdesivir therapy, her alkaline phosphatase began trending up (but remaining within normal limits during therapy) as well as her total bilirubin.
On day 11, the patient’s oxygen requirements continued to escalate, and she was admitted to the ICU where she was placed on BiPAP to maintain her oxygen saturation. She also required norepinephrine and vasopressin continuous infusions due to suspected distributive/septic shock (increasing to a maximum norepinephrine dose of 0.8 μg/kg/min for around 1 hour on day 13 and then steadily weaned to less than 0.2 μg/kg/hr within 24 hours). On day 12, the patient was given tocilizumab 640 mg (7.8 mg/kg) i.v. once for suspected cytokine storm due to COVID‐19. She continued to have worsening respiratory failure, ultimately requiring intubation and mechanical ventilation on day 13. On day 14, the patient had a CMP that showed an AST/ALT of 2178/1510 units/L (Figure 2), an alkaline phosphatase of 109 units/L, a total bilirubin of 2.1 mg/dL, an INR of 1.7, and an ammonia of 128 μmol/L. Due to suspected remdesivir‐induced ALF, she was initiated on a continuous infusion of acetylcysteine of 150 mg/kg over 1 hour, 50 mg/kg over 4 hours, and 100 mg/kg over 16 hours. Approximately 12 hours after finishing the 21‐hour acetylcysteine protocol, she had an AST/ALT of 546/871 units/L, alkaline phosphatase of 151 units/L, a total bilirubin of 2.3 mg/dL, and an ammonia of 127 μmol/L. Her ammonia further decreased to 85 μmol/L by 36 hours after finishing the acetylcysteine infusion (a follow up INR was not performed). From day 14 to day 17, the patient’s ventilation settings were able to be weaned down from an FiO2 of 100% to 60%, however, during this time she continue to require vasopressors for distributive shock and went into atrial fibrillation with rapid ventricular rate. On day 17, the patient became more hypotensive requiring reinitiation of a vasopressin infusion, and her arterial blood gas showed a severe metabolic acidosis. Her ventilatory settings escalated back up to requiring 100% FiO2, and she was found to be hypoglycemic. Despite our efforts, the patient went into cardiac arrest on the evening of day 17 and died.
Figure 2.
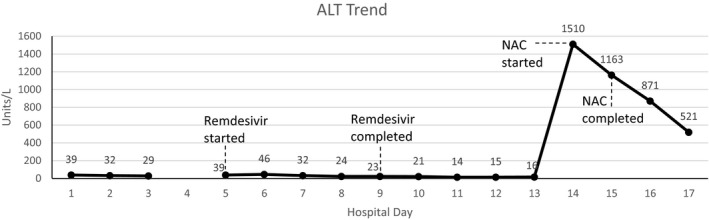
Alanine transaminase (ALT) trend by day in case #2 along with significant events (linear trend). NAC = acetylcysteine.
3. Discussion
It has been reported that infection with SARS‐CoV‐2 is associated with elevations in transaminases, similar to what has been seen in the original SARS‐CoV and Middle East respiratory syndrome coronavirus (MERS‐CoV) infections. 8 , 9 In most reports, however, transaminases have only been moderately elevated (5–10 times the upper limit of normal), suggesting liver injury instead of ALF. Although it appears that SARS‐CoV‐2 has some properties that can affect the liver and hepatic function, it is also important to examine if the therapies currently being used for the treatment of COVID‐19 may also cause liver dysfunction either independently or in conjunction with the natural course of the virus.
Remdesivir is a novel direct‐acting nucleoside RNA polymerase inhibitor with activity against the novel SARS‐CoV‐2 virus used in the treatment of COVID‐19 pneumonia. In phase I clinical trials in healthy volunteers, grade 1 and 2 transaminase elevations were observed, which resolved after discontinuation. 7 In a randomized, double‐blind, placebo‐controlled trial of 1063 hospitalized patients with COVID‐19, there was no difference in the incidence of more than or equal to grade 3 nonserious elevations of AST, ALT, or both between remdesivir and placebo (4.1% vs 5.9%). 6 Finally, in a randomized open‐label study of remdesivir 5 days versus 10 days for the treatment of hospitalized patients with COVID‐19, the incidence of grade 3 or 4 elevations of AST or ALT ranged between 2 and 6%. 10 The EUA fact sheet provided by the manufacturer states that in the original compassionate use program of remdesivir in patients with severe or critical illness with COVID‐19, liver function test abnormalities were reported in 12% of patients with a time to onset from first dose ranging from 1 to 16 days. This included cases of serious liver‐related laboratory abnormalities. 7
Outside of experiences discussed in the EUA fact sheet, very little has been published regarding the risk of ALF from remdesivir. One published case report by Leegwater discusses a case of acute liver failure 5 days after initiation of remdesivir in a patient with severe COVID‐19 pneumonia. 11 In that case, a dramatic increase in the AST and ALT to 1305 and 1461 units/L (respectively) were noted on day 5 of remdesivir therapy. The authors also noted that amiodarone was initiated on day 2 of remdesivir therapy and postulated that amiodarone‐induced inhibition of P‐glycoprotein efflux transporters (of which remdesivir is a known substrate in vitro) reduces efflux of remdesivir from hepatocytes, increasing remdesivir to toxic concentrations. This is only theoretical, as remdesivir is listed as only a minor substrate of P‐glycoprotein efflux transporters and has a relatively short half‐life before being converted to active metabolites. 12
In the two cases we present, the time course of COVID‐19 infection, remdesivir administration, and liver function abnormalities appears to point to remdesivir as a possible contributing cause of ALF. When utilizing the Naranjo algorithm to determine the possibility of a drug‐induced effect, both cases scored as a “probable” adverse drug reaction with a score of 6 each. 13 In the first case presented, ALF was noted on day 3 of remdesivir therapy. Although amiodarone can rarely cause ALF, it is unlikely to be the cause in this case as the ALT was already beginning to trend upward on the day amiodarone was initiated. In addition, the ALT began to resolve very rapidly with discontinuation of remdesivir and amiodarone and initiation of acetylcysteine; as amiodarone has a half‐life of 9 to 142 days (depending on the formulation and duration of therapy), if amiodarone was the cause of the ALF, it is not expected that discontinuation would lead to such rapid resolution. 14 In the second case, the sudden increase in transaminases was 5 days after completing remdesivir but still within the time range described in the EUA fact sheet. The patient did experience severe distributive shock the day before evidence of overt liver failure, requiring high dose vasopressors for a short time. Hepatic ischemia from distributive shock could have been a contributing factor in development of ALF, but increasing markers of liver injury (increasing alkaline phosphatase several days before vasopressor initiation) before the patient experienced shock along with the timing of the recent remdesivir exposure indicates that there is a possibility of remdesivir also contributing to ALF. The patient also received tocilizumab 2 days before experiencing ALF. Tocilizumab has been associated with mild transaminitis (AST/ALT 1–5 times the upper limit of normal) in acute use that tends to resolve quickly, and there have been cases of severe liver injury linked to tocilizumab use. 15 In these cases, however, severe liver injury was usually associated with several months of tocilizumab therapy, where our patient only received a single dose before ALF.
Drug‐induced liver injury (DILI) is a serious adverse effect of medications that can range in presentation from mild transaminitis to fulminant liver failure and represents one of the most common causes of ALF. 16 Acetaminophen is the leading cause of ALF in the United States and Europe, although there are many other medications associated with DILI and ALF. Acetylcysteine is the antidote for acetaminophen toxicity, and although acetylcysteine is only indicated for treatment of acetaminophen‐induced liver failure, some guidelines recommend the use of acetylcysteine in non‐acetaminophen‐induced ALF (NAI‐ALF) whereas others recommend its use only in the context of clinical trials. 16 , 17 , 18
Although there are different etiologies of ALF, the loss of hepatocellular function commonly leads to multisystem organ failure if not addressed. Whereas treatment of DILI revolves around stopping the offending agent, other supportive measures are often needed, including administration of an antidote if available, achieving hemodynamic stability, reversing coagulopathies, treating encephalopathies, and consideration of liver transplantation. 19 Because acetylcysteine acts as a glutathione precursor in acetaminophen toxicity, its mechanism of action in NAI‐ALF is not as clear, although it is hypothesized that acetylcysteine’s antioxidant and vasodilatory properties may provide some benefit. 19
Acetylcysteine use in NAI‐ALF is controversial due to the lack of data conclusively showing benefits. Initial trials assessing the use of acetylcysteine in NAI‐ALF in adults found conflicting results. A prospective, randomized, double‐blind trial by Lee et al found higher transplant‐free survival only in patients with coma grades I to II (representing earlier stage liver failure) treated with i.v. acetylcysteine compared to placebo. 20 This contrasts with a study by Mumtaz et al who conducted a prospective trial assessing an oral acetylcysteine protocol compared to a historical control group. Although the primary cause of ALF in this trial was acute hepatitis, the authors found that not using acetylcysteine was an independent predictor of mortality while also reporting no significant adverse effects from its use. 21 Subsequent trials in adults with NAI‐ALF have continued to support the hypothesis of benefit from acetylcysteine, although they have been retrospective or small. Darweesh et al conducted a prospective observational trial with historical control of adults admitted with NAI‐ALF. They found that patients given i.v. acetylcysteine on admission had a significant improvement in transplant‐free survival (96.4% vs 23.3% in the placebo group) along with reduction in hospital length of stay, bleeding, and encephalopathy in the acetylcysteine group. 22 These results show a much more significant benefit than previously seen, although this could be due to the small, observational nature of the trial. Finally, Nabi et al conducted a prospective, randomized trial comparing i.v. acetylcysteine to placebo for treatment of NAI‐ALF. The authors found that the acetylcysteine group had significantly lower mortality and hospital length of stay compared with the placebo group and that not using acetylcysteine was an independent risk factor for death. 23 It is important to note that suspected DILI only accounted for anywhere from 6 to 40% of NAI‐ALF cases in the different studies, indicating the difficulty in applying the limited literature to a case of suspected ALF from a novel, unapproved medication used in an emergency setting.
To date, it remains unclear what the true risk and prevalence of ALF from remdesivir use is in the setting of COVID‐19. Remdesivir has been studied in humans previously for the treatment of the Ebola virus, but it has only been widely available for clinical use during the SARS‐CoV‐2 pandemic. Although remdesivir has been shown to be relatively safe in the context of limited use, there is a possibility that widespread use will reveal new safety concerns that must be accounted for when weighing the risks versus benefits of use. The incidence of transaminitis seen in previous clinical trials of remdesivir suggests there is a possibility of unexpected ALF. Whereas there is very limited evidence supporting the use of acetylcysteine for NAI‐ALF (and there are no data on its use in remdesivir‐associated ALF), both of these cases of remdesivir‐associated acute, fulminant ALF showed improvement in ALF with its use in conjunction with discontinuation of potentially hepatotoxic medications without either patients experiencing any adverse effects.
4. Conclusion
The use of the novel antiviral remdesivir in the treatment of COVID‐19 pneumonia may put patients at risk of drug‐associated ALF. In the two cases presented, the liver failure experienced improved with the administration of acetylcysteine and discontinuation of potentially hepatotoxic medications. More data on the risk of remdesivir‐associated ALF and its management with acetylcysteine are needed.
Conflict of interest: The authors declare no conflicts of interest.
References
- 1. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China. JAMA 2020;323(13):1239. [DOI] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention . Cases in the U.S. Updated August 28th, 2020. Available from https://www.cdc.gov/coronavirus/2019‐ncov/cases‐updates/cases‐in‐us.html
- 3. Bhimraj A, Morgan RL, Shumaker AH, et al. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID‐19. Updated June 25 2020. Available from https://www.idsociety.org/COVID19guidelines [DOI] [PMC free article] [PubMed]
- 4. COVID‐19 Treatment Guidelines Panel . Coronavirus disease 2019 (COVID‐19) treatment guidelines. National Institutes of Health. Available from https://www.covid19treatmentguidelines.nih.gov/. Accessed September 11, 2020. [PubMed] [Google Scholar]
- 5. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCOV) in vitro. Cell Res 2020;30:269–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid‐19 — preliminary report. N Engl J Med 2020; Published online 5/22/2020. Available from https://www.nejm.org/doi/full/10.1056/NEJMoa2007764 [DOI] [PubMed] [Google Scholar]
- 7. Gilead Sciences, Inc . Fact sheet for health care providers Emergency Use Authorization (EUA) of Veklury® (remdesivir). Revised 07/2020. Available from https://www.gilead.com/‐/media/files/pdfs/remdesivir/eua‐fact‐sheet‐for‐hcps.pdf
- 8. Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int 2020;40:998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goldman JD, Lye DC, Hui DS, et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid‐19. N Engl J Med 2020. Published online 5/27/2020. Available from https://www.nejm.org/doi/full/10.1056/NEJMoa2015301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leegwater E, Strik A, Wilms E, et al. Drug‐induced liver injury in a COVID‐19 patient: potential interaction of remdesivir with P‐glycoprotein inhibitors. Clin Infect Dis 2020. Published online 6/28/2020. Available from https://academic.oup.com/cid/advance‐article/doi/10.1093/cid/ciaa883/5864408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Remdesivir . Lexi‐Drugs. Hudson, OH: Lexicomp. Wolters Kluwer Health, Inc.; 2020. [Google Scholar]
- 13. Naranjo CA, Busto U, Sellers EM, et al. A method for estimated the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30(2):239–45. [DOI] [PubMed] [Google Scholar]
- 14. Amiodarone . Lexi‐Drugs. Hudson, OH: Lexicomp. Wolters Kluwer Health, Inc.; 2020. [Google Scholar]
- 15. LiverTox: Clinical and Research Information on Drug‐Induced Liver Injury [Internet]. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2012. Tocilizumab. [Updated 2020 Apr 18]. Available from https://www.ncbi.nlm.nih.gov/books/NBK548243/. [PubMed] [Google Scholar]
- 16. Lee WM, Larson AM, Stravitz RT. AASLD Position Paper: the management of acute liver failure: update 2011. Hepatology 2011. [DOI] [PubMed] [Google Scholar]
- 17. European Association for the Study of the Liver . EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J Hepatol 2017;66:1047–81. [DOI] [PubMed] [Google Scholar]
- 18. Flamm SL, Yang Y, Singh S, Falck‐Ytter Y. American Gastroenterological Association Institute Guidelines for the Diagnosis and Management of Acute Liver Failure. Gastroenterology 2017;152:644–7. [DOI] [PubMed] [Google Scholar]
- 19. Sales I, Dzierba AL, Smithburger PL, Rowe D, Kane‐Gill S. Use of acetylcysteine for non‐acetaminophen‐induced acute liver failure. Ann Hepatol 2013;12(1):6–10. [PubMed] [Google Scholar]
- 20. Lee WM, Hynan LS, Rossaro L, et al. Intravenous N‐acetylcysteine improves transplant‐free survival in early stage non‐acetaminophen acute liver failure. Gastroenterology 2009;137:856–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mumtaz K, Azam Z, Hamid S, et al. Role of N‐acetylcysteine in adults with non‐acetaminophen‐induced acute liver failure in a center without the facility of liver transplantation. Hepatol Int 2009;3:563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Darweesh SK, Ibrahim MF, El‐Tahawy MA. Effect of N‐acetylcysteine on mortality and liver transplantation rate in non‐acetaminophen‐induced acute liver failure: a multicenter study. Clin Drug Investig 2017;37:473–82. [DOI] [PubMed] [Google Scholar]
- 23. Nabi T, Nabi S, Rafiq N, Shah A. Role of N‐acetylcysteine treatment in non‐acetaminophen‐induced acute liver failure: a prospective study. Saudi J Gastroenterol. 2017;23(3):169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]


