Abstract
Immunosuppressed patients such as solid organ transplant and hematologic malignancy patients appear to be at increased risk for morbidity and mortality due to coronavirus disease 2019 (COVID‐19) caused by SARS coronavirus 2 (SARS‐CoV‐2). Convalescent plasma, a method of passive immunization that has been applied to prior viral pandemics, holds promise as a potential treatment for COVID‐19. Immunocompromised patients may experience more benefit from convalescent plasma given underlying deficits in B and T cell immunity as well as contraindications to antiviral and immunomodulatory therapy. We describe our institutional experience with four immunosuppressed patients (two kidney transplant recipients, one lung transplant recipient, and one chronic myelogenous leukemia patient) treated with COVID‐19 convalescent plasma through the Expanded Access Program (NCT 04338360). All patients clinically improved after administration (two fully recovered and two discharged to skilled nursing facilities) and none experienced a transfusion reaction. We also report the characteristics of convalescent plasma product from a local blood center including positive SARS‐CoV‐2 IgG and negative SARS‐CoV‐2 PCR in all samples tested. This preliminary evidence suggest that convalescent plasma may be safe among immunosuppressed patients with COVID‐19 and emphasizes the need for further data on the efficacy of convalescent plasma as either primary or adjunctive therapy for COVID‐19.
Keywords: convalescent plasma, COVID‐19, immunocompromised, transplant
Abbreviations
- COVID‐19
Coronavirus disease 2019
- SARS‐CoV‐2
Severe acute respiratory syndrome coronavirus 2
- SOT
solid organ transplant
1. INTRODUCTION
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has led to a global pandemic with over 25 million reported cases and over 850 000 deaths. 1 , 2 Clinical coronavirus disease 2019 (COVID‐19) caused by SARS‐CoV‐2 varies from asymptomatic infection to critical illness. 3 , 4 Some groups of immunocompromised patients appear to be at increased risk for severe COVID‐19 disease. The mortality rate among solid organ transplant (SOT) recipients is reported to be as high as 60% 5 , 6 , 7 , 8 , 9 , 10 , 11 and mortality in hematologic malignancy patients is similarly high. 12
There are currently limited COVID‐19 therapies with proven efficacy. Early evidence suggests that the antiviral remdesivir can shorten the time to recovery 13 and dexamethasone can reduce 28‐day mortality. 14 Convalescent plasma (CP) is a passive immunization strategy used in the treatment of infectious diseases since the 1900s. CP is obtained via apheresis of infection survivors who have evidence of antibody production. The goal of therapy when applied early in infection is to neutralize virus and minimize the resulting inflammatory cascade. CP has been described to reduce mortality, decrease complications, and speed viral clearance of infections including the influenza pandemic of 1918, 15 SARS, 16 influenza H1N1, 17 and Ebola. 18
While there is increasing data on the safety and potential benefit of CP in patients with COVID‐19, 19 , 20 experience with this therapeutic among immunocompromised patients is limited. These populations may benefit more from CP compared to immunocompetent patients due to their inability to mount appropriate cellular and humoral immune responses to the virus. 21 Here, we report our institutional experience with four immunocompromised patients, three SOT recipients, and one hematologic malignancy patient, with COVID‐19 treated with CP.
2. MATERIALS AND METHODS
2.1. Study subjects and setting
2.1.1. Subjects
Hospitalized SOT and hematologic malignancy patients cared for at the University of California San Francisco (UCSF) were enrolled in the Expanded Access Program (EAP) for Convalescent Plasma (NCT 04338360, UCSF IRB #20‐30657). Included subjects were ≥18 years, with laboratory‐confirmed SARS‐CoV‐2 infection, and with or at risk of progression to severe or life‐threatening COVID‐19 disease. Severe disease was defined as the presence of dyspnea, respiratory rate ≥30/min, oxygen saturation ≤93%, PaO2/FiO2 < 300, or pulmonary infiltrates. Life‐threatening disease was defined by the presence of respiratory failure, septic shock, or multiple organ dysfunction/failure. Data on demographics, medical history, clinical results, treatment, and outcomes were extracted from the electronic medical record as approved under UCSF IRB #20‐30629.
2.1.2. COVID‐19 diagnostic testing
Patients were diagnosed with SARS‐CoV‐2 infection using RNA testing of nasopharyngeal (NP) or pooled nasopharyngeal/oropharyngeal (NP/OP) swab samples performed using a real‐time reverse transcriptase‐polymerase chain reaction (PCR) assay. 22 SARS‐CoV‐2 IgG was available but not conducted routinely.
2.1.3. Therapeutic approach
During the study period, our institutional treatment approach was to enroll patients with severe or life‐threatening COVID‐19 disease into clinical trials, if possible. The main trial has been the National Institute of Allergy and Infectious Diseases Adaptive COVID‐19 Treatment Trial (ACTT), a randomized controlled trial of remdesivir (NCT 04280705). Patients who did not qualify or consent for the study were considered for remdesivir through either the EUA or EAP program. They were also considered for EAP CP. All admitted COVID‐19 patients were provided with aggressive supportive care.
2.2. Convalescent plasma
2.2.1. Donor selection and qualification
All CP units transfused were collected per US Food & Drug Administration guidance from donors with confirmed COVID‐19 diagnosis based on positive PCR. If donating within 14‐28 days post‐resolution of symptoms, a negative PCR was required prior to donation. If >28 days post‐resolution of symptoms, repeat PCR was not required.
2.2.2. Collection and storage
CP was collected using standard apheresis methods and stored at −18°C within 8 hours of collection. Frozen units from Stanford Blood Center (SBC) and the American Red Cross (ARC) were shipped to UCSF using validated coolers. Units were stored in a freezer at UCSF and when ready to use, removed and placed in a plasma thawer.
Donor CP samples from SBC were tested for antibodies against the receptor‐binding domain of SARS‐CoV‐2 spike protein using a validated laboratory‐developed enzyme‐linked immunosorbent assay (cut‐off of 0.30 optical density). Binding titers were performed using the same assay at 1:100, 1:200, and 1:400 dilutions. A research PCR test targeting the SARS‐CoV‐2 envelope gene was also performed in EDTA plasma mini pools of six individuals (Stanford IRB#55550). Donor CP sample from ARC did not have PCR or antibody titers done.
2.2.3. Administration
In accordance with the EAP program, one unit (approximately 200 mL) of ABO‐compatible CP was administered at a rate of 100 mL/h using standard blood administration procedures.
3. CASE SERIES
3.1. Case 1
A 44‐year‐old African‐American man with deceased donor renal transplant for IgA nephropathy 19 years prior to presentation and hypertension presented to an outside institution 6 days after onset of fever, cough, dyspnea, and diarrhea and was diagnosed with COVID‐19 by PCR (Figure 1A). Maintenance immunosuppression consisted of mycophenolate, tacrolimus, and prednisone. Chest X‐ray demonstrated patchy left lower lobe infiltrate and he received hydroxychloroquine (days 6‐20), lopinavir/ritonavir (days 13‐18), tocilizumab (days 12‐13), methylprednisolone (days 17‐20), and broad‐spectrum antibiotics. On day 20 of illness, he was intubated and transferred to our center. Hydroxychloroquine and steroids were discontinued, mycophenolate was held, and patient received CP on day 27 for life‐threatening COVID‐19. The patient did not qualify for ACTT‐1 or EAP remdesivir due to acute kidney injury requiring hemodialysis. His course was complicated by right common femoral vein thrombosis (day 23) requiring anticoagulation then inferior vena cava filter placement, Klebsiella ventilator‐associated pneumonia (day 24), gastrointestinal bleed (day 25), and candidemia (day 41). He underwent tracheostomy on day 50. On day 73 of illness, the patient was on room air and discharged to long‐term acute care facility.
FIGURE 1.
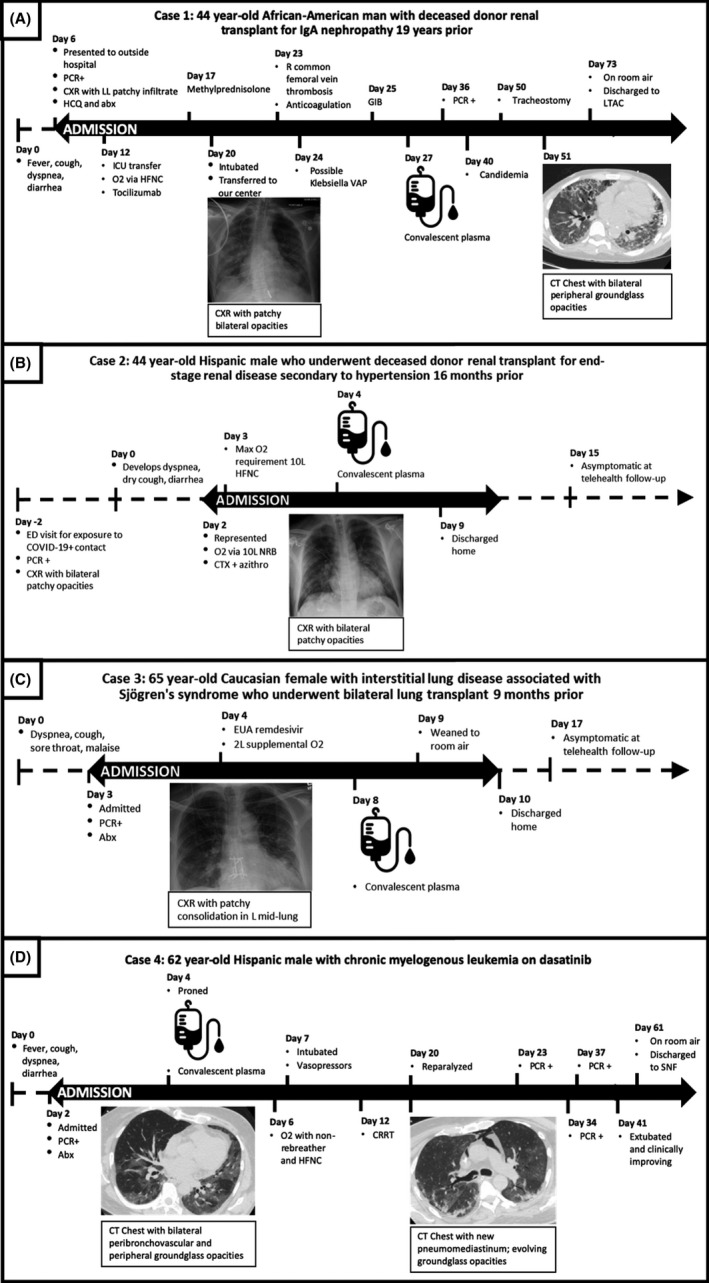
Clinical course of four immunosuppressed COVID‐19 patients treated with convalescent plasma. Abbreviations: CXR, chest X‐ray; HCQ, hydroxychloroquine; ICU, intensive care unit; HFNC, high‐flow nasal cannula; VAP, ventilator‐associated pneumonia; GIB, gastrointestinal bleed; LTAC, long‐term acute care facility; CT, computed tomography; ED, emergency department; CTX, ceftriaxone; azithro, azithromycin; Abx, antibiotics; EUA, emergency use authorization; 2L, 2 liters; CRRT, continuous renal replacement therapy; SNF, skilled nursing facility
3.2. Case 2
A 44‐year‐old Hispanic male who underwent a deceased donor renal transplant for end‐stage renal disease secondary to hypertension 16 months prior presented asymptomatically to the emergency department after exposure to a roommate with COVID‐19 (Figure 1B). Maintenance immunosuppression consisted of mycophenolate, tacrolimus, and prednisone. On initial asymptomatic presentation, PCR testing was positive and chest X‐ray demonstrated bilateral patchy opacities, and was discharged home. Two days later, he developed dyspnea, dry cough, and diarrhea and represented for care. On admission, chest X‐ray demonstrated bilateral patchy opacities and he required supplemental oxygen via non‐rebreather. In the ICU, oxygen requirement reached a maximum of 10 liters per minute via high‐flow nasal cannula. Tacrolimus and mycophenolate were held. ACTT was not enrolling during the patient's hospitalization and he did not qualify for EAP remdesivir (not intubated). CP was administered on day 4 for severe COVID‐19 disease. On day 9, the patient was oxygenating well on room air and discharged home. During telehealth visit on day 15, the patient was feeling well without symptoms.
3.3. Case 3
A 65‐year‐old Caucasian female with interstitial lung disease associated with Sjögren's syndrome who underwent bilateral lung transplant 9 months prior presented with 2 days of dyspnea on exertion, cough, sore throat, and malaise (Figure 1C). She received induction with basiliximab and was on maintenance immunosuppression with mycophenolate, tacrolimus, and prednisone. Chest X‐ray demonstrated increased patchy left mid lung and retrocardiac consolidation and she required supplemental oxygen via nasal cannula for comfort (maximum 2 L). Mycophenolate was held and the patient received EUA remdesivir on day 4 of illness without significant clinical change, followed by CP on day 8 for severe COVID‐19 disease. The patient was discharged home on day 10. During telehealth visit on day 17, the patient was feeling well without symptoms.
3.4. Case 4
A 62‐year‐old Hispanic male with CML on dasatinib, stage IV chronic kidney disease, hypertension, and poorly controlled diabetes presented with 2 days of fatigue, diarrhea, and anorexia (Figure 1D). On presentation, the patient was afebrile but developed rapidly progressive hypoxemia requiring oxygen administered via high‐flow nasal cannula. Computed tomography of the chest demonstrated bilateral peribronchovascular groundglass opacities and peripheral nodular consolidation. Patient did not qualify for EAP remdesivir due to renal failure, and CP was administered on day 3 of illness for life‐threatening COVID‐19 disease. The patient was subsequently intubated (day 7), required continuous renal replacement therapy (day 12), with course complicated by the shock of unclear etiology (day 15) and severe autonomic instability with systolic blood pressure ranges from 70 to 300 for which extensive work‐up (toxicology consultation, CT, MRI, and EEG) was negative. The patient was extubated on day 41 and discharged to a skilled nursing facility with no oxygen requirement on day 61.
4. CONVALESCENT PLASMA
CP was supplied by SBC for three patients (Cases 1, 2, and 4) and ARC for one patient (Case 3). CP from SBC was collected from donors who had confirmed COVID‐19 by PCR testing. All SBC CP units were positive for IgG against the SARS‐CoV‐2 receptor‐binding domain protein and negative for SARS‐CoV‐2 PCR. Table 1 provides information on binding titer and samples have been retained to perform neutralizing antibody titers as required by the EAP.
TABLE 1.
COVID‐19 convalescent plasma characteristics
| Case | Supplier | DONOR | PRODUCT | ADMINISTRATION | |||||
|---|---|---|---|---|---|---|---|---|---|
| Evidence of prior COVID‐19 infection | Days symptom free at donation | SARS‐CoV2 IgG |
SARS‐CoV2 RT‐PCR |
Infusion duration (minutes) | Volume (mL) | Reaction | |||
| Result (OD) | Titer | ||||||||
| 1 | Stanford | NP PCR | 14‐28 | Positive (1.19) | >1:400 | Negative | 146 | 214 | No |
| 2 | Stanford | NP PCR | >28 | Positive (1.63) | >1:400 | Negative | 124 | 207 | No |
| 3 | American Red Cross | Not documented | >28 | Not done | Not done | Not done | 101 | 208 | No |
| 4 | Stanford | NP PCR | >28 | Positive (0.74) | >1:400 | Negative | 120 a | 206 | No |
Abbreviations: NP PCR, nasopharyngeal PCR; OD, optical density.
Estimated infusion time.
5. DISCUSSION
We report our institutional experience with CP for the treatment of COVID‐19 in four immunocompromised patients, including two renal transplant recipients, one lung transplant recipient, and one hematologic malignancy patient.
Increasing data indicate that CP is safe among patients with COVID‐19, with <1% rate of transfusion reaction and thrombosis, and ~3% rate of cardiac events. 23 However, clear data on clinical benefit are lacking. A randomized controlled trial in China of 103 patients suggested a trend toward improvement (HR 1.4, P = .26), particularly among patients with severe disease (HR 2.15, P = .03). 19 However, results were limited by the lack of target enrollment and also confounded by variations in therapy. Another study from Texas reporting 25 patients with severe and/or life‐threatening COVID‐19 treated with CP found that 76% experienced at least a 1‐point improvement in the WHO ordinal scale in the 14 days post‐transfusion. 20 Other smaller case series predominantly from China 24 , 25 , 26 , 27 , 28 document improvement in clinical, radiologic, and virologic COVID‐19 parameters after CP administration including among elderly 29 and pregnant 24 , 30 patients.
Although immunocompromised SOT patients can develop IgG response to SARS‐CoV‐2, 31 their underlying functional deficits in B and T cell immunity 21 may make them a population that experiences particular benefit from passive immunization from CP. In a prior report of three kidney transplant recipients with COVID‐19 who received CP, all three recovered and exhibited positive SARS‐CoV‐2 IgG (no pre‐transfusion baseline), though notably, one patient experienced acute dyspnea after CP transfusion. 32 The patients we report received CP at a median of 5.5 days from COVID‐19 symptom onset and none experienced any reactions to CP transfusion. Two patients received other antiviral or immunomodulatory therapy in addition to CP (Cases 1 and 3). All patients were clinically improving at the time of this report, with two discharged home and fully recovered (Cases 2 and 3) and two discharged to skilled nursing facilities (Cases 1 and 4). Our results represent preliminary evidence that CP may be safe for immunosuppressed patients.
This study has several limitations. As a small case series, we are unable to draw clear conclusions about the therapeutic efficacy of CP. Patients varied significantly with regards to COVID‐19 disease severity and timecourse at the time of CP administration, and received potential confounding COVID‐19 therapies.
Despite recent EUA for CCP, 33 larger randomized studies remain crucial to understand the role of CP in the treatment of COVID‐19 and which, if any, subgroups (degree of immunosuppression, comorbidities, infection severity) of immunocompromised patients would benefit. There remains a significant gap in knowledge about the therapeutic mechanism of CP, characteristics predictive of efficacy such as titer of neutralizing antibodies, as well as potential downstream effects such as attenuated humoral immunity. Furthermore, CP donation and supply chains need to be further optimized at the local and national level. For the transplant community, it is worth examining whether the existing infrastructure for a living donation could be leveraged to serve as a source of CP during this critical period.
In summary, there is currently limited evidence on both safety and efficacy of CP among immunosuppressed COVID‐19 patients. We report our experience with three SOT and one hematologic malignancy patient with COVID‐19 treated with CP, all of whom are clinically improving after administration and did not experience any transfusion reactions. Additional research is urgently needed to better understand and optimize CP for the treatment of COVID‐19 among immunosuppressed patients.
DISCLOSURE
PCH is a site investigator for convalescent plasma EAP. The remaining authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
MF and PCH conceived and designed the analysis. MF, AN, SP, JMA, and JT collected the data. MF, AN, and PCH performed the analysis. MF wrote the paper. CF, JR, SC, SRH, EB, CD, ARG, DY, TJH, and JMB participated in the clinical care of the patient and provided significant edits to the manuscript.
Fung M, Nambiar A, Pandey S, et al. Treatment of immunocompromised COVID‐19 patients with convalescent plasma. Transpl Infect Dis.2021;23:e13477. 10.1111/tid.13477
Funding informationThis study was supported in part by a US Department of Health and Human Services (HHS), Biomedical Advanced Research and Development Authority (BARDA) contract 75A50120C00096 (MJ Joyner, PI).
REFERENCES
- 1. World Health Organization . WHO statement regarding cluster of pneumonia cases in Wuhan, China. https://www.who.int/china/news/detail/09‐01‐2020‐who‐statement‐regarding‐cluster‐of‐pneumonia‐cases‐in‐wuhan‐china. Published 2019. Accessed August 4, 2020
- 2. Dong E, Du H, Gardner L. An interactive web‐based dashboard to track COVID‐19 in real time. Lancet Infect Dis. 2020;20(5):533‐534. 10.1016/S1473-3099(20)30120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guan W‐J, Ni Z‐Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China. JAMA. 2020;323(13):1239. 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 5. Nair V, Jandovitz N, Hirsch JS, et al. COVID‐19 in kidney transplant recipients. Am J Transplant. 2020;20(7):1819‐1825. 10.1111/ajt.15967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Akalin E, Azzi Y, Bartash R, et al. Covid‐19 and kidney transplantation. N Engl J Med. 2020;382(25):2475‐2477. 10.1056/NEJMc2011117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pereira MR, Mohan S, Cohen DJ, et al. COVID‐19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020;20(7):1800‐1808. 10.1111/ajt.15941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Montagud‐Marrahi E, Cofan F, Torregrosa J, et al. Preliminary data on outcomes of SARS‐CoV‐2 infection in a Spanish single centre cohort of kidney recipients. Am J Transplant. 2020. 10.1111/ajt.15970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Webb GJ, Moon AM, Barnes E, Barritt AS, Marjot T. Determining risk factors for mortality in liver transplant patients with COVID‐19. Lancet Gastroenterol Hepatol. 2020;1253(20):30125. 10.1016/S2468-1253(20)30125-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alberici F, Delbarba E, Manenti C, et al. A single center observational study of the clinical characteristics and short‐term outcome of 20 kidney transplant patients admitted for SARS‐CoV2 pneumonia. Kidney Int. 2020;1–6. 10.1016/j.kint.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fernández‐Ruiz M, Andrés A, Loinaz C, et al. COVID‐19 in solid organ transplant recipients: a single‐center case series from Spain. Am J Transplant. 2020;20(7):1849‐1858. 10.1111/ajt.15929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. He W, Chen L, Chen L, et al. COVID‐19 in persons with haematological cancers. Leukemia. 2020;34(6):1637‐1645. 10.1038/s41375-020-0836-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beigel JH, Tomashek KM, Dodd LE, et al.Remdesivir for the treatment of Covid‐19 — preliminary report. N Engl J Med. 2020; 10.1056/NEJMoa2007764 [DOI] [PubMed] [Google Scholar]
- 14. RECOVERY Collaborative Group , Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid‐19 ‐ preliminary report. N Engl J Med. July 2020. 10.1056/NEJMoa2021436. [DOI] [Google Scholar]
- 15. Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta‐analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med. 2006;145(8):599‐609. 10.7326/0003-4819-145-8-200610170-00139 [DOI] [PubMed] [Google Scholar]
- 16. Soo YOY, Cheng Y, Wong R, et al. Retrospective comparison of convalescent plasma with continuing high‐dose methylprednisolone treatment in SARS patients. Clin Microbiol Infect. 2004;10(7):676‐678. 10.1111/j.1469-0691.2004.00956.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hung IF, To KK, Lee C‐K, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52(4):447‐456. 10.1093/cid/ciq106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Griensven J, Edwards T, de Lamballerie X, et al. Evaluation of convalescent plasma for Ebola virus disease in guinea. N Engl J Med. 2016;374(1):33‐42. 10.1056/NEJMoa1511812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li L, Zhang W, Hu Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life‐threatening COVID‐19. JAMA. 2020;324(5):460. 10.1001/jama.2020.10044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Salazar E, Perez KK, Ashraf M, et al. Treatment of COVID‐19 patients with convalescent plasma. Am J Pathol. May 2020:2020.05.08.20095471. 10.1016/j.ajpath.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fishman JA. Infection in organ transplantation. Am J Transplant. 2017;17(4):856‐879. 10.1111/ajt.14208 [DOI] [PubMed] [Google Scholar]
- 22. Centers for Disease Control and Prevention (CDC) . Real‐Time RT‐PCR Panel for Detection 2019‐Novel Coronavirus.
- 23. Joyner MJ, Bruno KA, Klassen SA, et al. Safety update. Mayo Clin Proc. 2020;95(9):1888‐1897. 10.1016/j.mayocp.2020.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang B, Liu S, Tan T, et al. Treatment With convalescent plasma for critically ill patients with severe acute respiratory syndrome coronavirus 2 infection. Chest. 2020;158(1):e9‐e13. 10.1016/j.chest.2020.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID‐19 with convalescent plasma. JAMA. 2020;323(16):1582. 10.1001/jama.2020.4783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID‐19 patients. Proc Natl Acad Sci USA. 2020;117(17):9490‐9496. 10.1073/pnas.2004168117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ye M, Fu D, Ren Y, et al. Treatment with convalescent plasma for COVID‐19 patients in Wuhan, China. J Med Virol. 2020;92(10):1890‐1901. 10.1002/jmv.25882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zeng Q‐L, Yu Z‐J, Gou J‐J, et al. Effect of convalescent plasma therapy on viral shedding and survival in patients with coronavirus disease 2019. J Infect Dis. 2020;222(1):38‐43. 10.1093/infdis/jiaa228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kong Y, Cai C, Ling L, et al. Successful treatment of a centenarian with coronavirus disease 2019 (COVID‐19) using convalescent plasma. Transfus Apher Sci. 2020;102820. 10.1016/j.transci.2020.102820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Anderson J, Schauer J, Bryant S, Graves CR. The use of convalescent plasma therapy and remdesivir in the successful management of a critically ill obstetric patient with novel coronavirus 2019 infection: a case report. Case Reports Women’s Heal. 2020;27:e00221. 10.1016/j.crwh.2020.e00221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fung M, Chiu CY, DeVoe C, et al. Clinical outcomes and serologic response in solid organ transplant recipients with COVID‐19: a case series from the United States. Am J Transplant. 2020; 10.1111/ajt.16079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Naeem S, Gohh R, Bayliss G, et al. Successful recovery from COVID‐19 in three kidney transplant recipients who received convalescent plasma therapy. Transpl Infect Dis. 2020; 10.1111/tid.13451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mahase E. Covid‐19: US approves emergency use of convalescent plasma despite warnings over lack of evidence. BMJ. 2020;370:m3327. 10.1136/bmj.m3327 [DOI] [PubMed] [Google Scholar]


