Abstract
The coronavirus disease 2019 pandemic has had an impact on all facets of our health care system, including life‐saving procedures like organ transplantation. Concerns for potential exposure to the causative severe acute respiratory syndrome coronavirus type 2 have profoundly altered the process of organ donation and recovery that is vital to the execution of organ transplantation. Issues regarding adequate donor evaluation and consent, organ recovery, organ procurement organization, and donor hospital resources as well as the transplant center’s acceptance of organ offers for their candidates have all required new practice paradigms. Consequently, the ability to treat patients with organ failure, in particular patients with end‐stage liver disease in whom no temporizing treatments exist, and to obtain expected excellent outcomes for new liver transplant recipients has been challenged during this time. Conclusion: We summarize some of the negative effects of the current pandemic on organ recovery and liver transplantation as well as offer considerations and strategies for their mitigation that could have a lasting impact on the field even after the coronavirus disease 2019 has waned.
Abbreviations
- BAL
bronchoalveolar lavage
- COVID‐19
coronavirus disease 2019
- DCD
donation after circulatory death
- ICU
intensive care unit
- MELD
Model for End‐Stage Liver Disease
- OPO
organ procurement organization
- OPTN
Organ Procurement Transplant Network
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus type 2
- UNOS
United Network for Organ Sharing
The coronavirus disease 2019 (COVID‐19) in recent months has had a substantial impact on the transplant community. The potential for viral transmission from donor to recipients or to and from health care workers has imposed new and significant risks, altering the ability to safely recover organs and perform transplants. Solid organ transplantation is considered as highly necessary and essential, earning the designation by the Center for Medicare Services (CMS) as a tier 3b procedure that should continue even when other elective surgical procedures are curtailed.( 1 ) Although this was a necessary condition to be able to continue the process of organ recovery and transplantation, this designation was likely too broad and did not apply to all transplants. Patients with stable disease as well as for those awaiting scheduled elective living donor transplants have in many cases been able to safely defer surgery until after the incidence peak of the pandemic subsided.
The decision to proceed with transplantation was judiciously determined by each transplant center. In most cases, living donor transplantation, especially liver donation, was deferred, enabling healthy donors to follow recommended stay‐at‐home guidelines. In contrast, deceased donor liver transplantation has been continually pursued in those with the greatest disease severity (highest Model for End‐Stage Liver Disease [MELD] scores). The pandemic caused by severe acute respiratory syndrome coronavirus type 2 (SARS‐CoV‐2) has caused a major disruption of the process of organ donation and recovery, resulting in declining rates of overall organ recovery (Fig. 1).( 2 ) This disease process affected multiple regions in the United States to varying degrees and at different time points, depending on the incidence and prevalence of COVID‐19. As organ recoveries and living donation have markedly diminished, liver transplantation decreased nationally by more than 25% between February and April 2020 (Fig. 2). A similar interruption in delivery of transplantation may recur in the presence of another wave of COVID‐19.
FIG. 1.
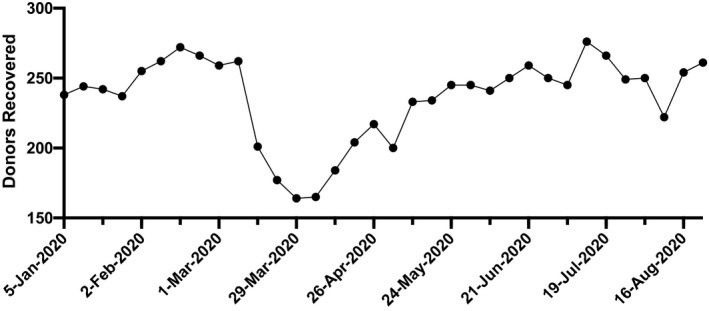
Weekly rates of deceased donor organ recovery, January to August 2020. Significant decreases in organ recovery rates were noted at the height of the COVID pandemic in March 2020. A 25% reduction in organ recovery was noted between February and April 2020. (Source: UNOS, https://unos.org/covid, Accessed September 4, 2020.).
FIG. 2.
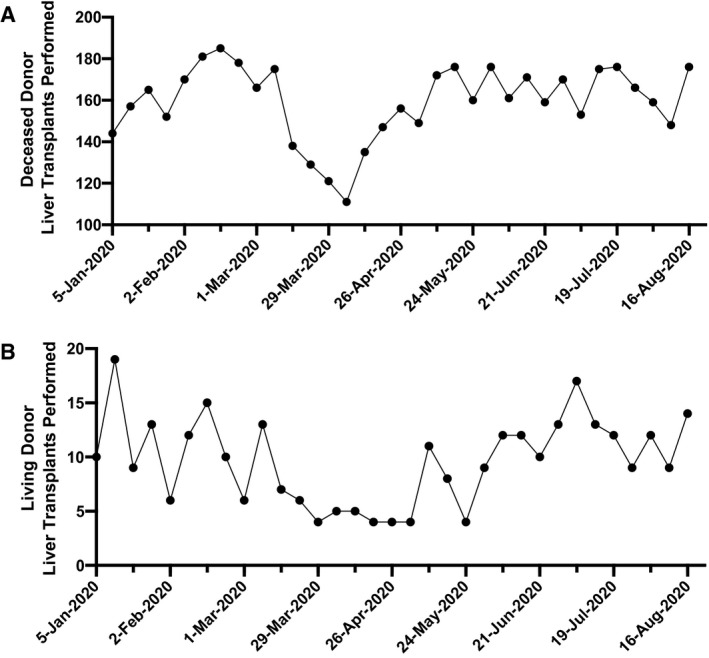
Weekly rates of US deceased donor (A) and living donor (B) liver transplants performed January to June 2020. Marked reductions in liver transplantation were observed in March and April 2020. Living donor liver transplantation has continued to be performed seldomly throughout the month of April 2020. (Source: UNOS, https://unos.org/covid, Accessed July 5, 2020.).
In recognition of these challenges, the United Network for Organ Sharing (UNOS)/Organ Procurement Transplant Network (OPTN) rapidly approached the COVID‐19 pandemic with a multifaceted strategy to support the patients on the transplant waiting lists, the transplant centers, and the organ procurement organizations (OPOs) with policy changes, guideline developments, and easily accessible resources. UNOS/OPTN created three new refusal codes for transplant centers to use to track COVID‐19‐related issues in candidates, donors, or OPO/transplant hospital operational issues.( 3 ) Use of these codes has been noted with increasing frequency and has documented the marked decrease in both living and deceased donation.
Donor history and COVID‐19 testing have become a routine part of the process of donor evaluation. Ideally, there should be no history of exposure to SARS‐CoV‐2 or symptoms suggestive of coronavirus disease, rapid access to reliable diagnostics, and necessary imaging studies. However, even with testing, some transmission of coronavirus has occurred from asymptomatic patients through direct interaction. In addition, testing protocols are not standardized nationally, and current tests have significant false‐negative results. Moreover, access to sensitive imaging such as computed tomography of the chest is not always readily available, although society guidelines do not recommend its use as a routine screening strategy.( 4 )
Due to the risk of transmission to staff during recovery when teams move between centers and regions, there is now a stronger emphasis on local organ recovery for organ procurements. Moreover, the perioperative care and staffing of recovery and intensive care units required for organ recipients has become more limited due to safety from exposure to COVID‐19 and staff reassignment to areas of need in hospitals. Collectively, these have forced the need for greater vigilance in organ acceptance for wait‐listed registrants and in some instances has deferred the execution of transplants. The issues that have directly or indirectly resulted from the COVID‐19 pandemic, which have altered practices surrounding organ donation and transplantation, are the subject of the following review.
Donor Evaluation
Guidelines from UNOS/OPTN, the American Association for the Study of Liver Disease, and the American Society of Transplant Surgeons (ASTS) strongly recommend that donor history and rapid COVID‐19 testing be a routine part of the donor and recipient evaluation.( 5 , 6 ) Assessment of exposure to SARS‐CoV‐2 has been challenging due to the potential for asymptomatic patients to spread disease, and initially there were great difficulties in obtaining timely testing for this virus. Many local medical centers and OPOs are only now beginning to obtain consistent access to rapid COVID‐19 testing kits and machines for running the assays. Prior methodology turnaround required up to 12 hours before a negative test result was confirmed. There is also significant variability in false negative rate ranging from 2% up to 30%‐40% in testing for SARS‐CoV‐2 by nasopharyngeal (NP) swabs.( 7 , 8 ) This limits reliance on these tests, particularly in the setting of high clinical suspicion. In settings with high suspicion for COVID‐19 exposure, bronchoalveolar lavage (BAL) is the most effective method for viral detection and has a very low false‐negative rate. However, performing BAL carries the risk of aerosolization of respiratory secretions and is often challenging to obtain in a short time window. Furthermore, BAL has the greatest sensitivity later in the course of the infection, whereas NP swab testing has greatest sensitivity during the early period. There is recent evidence that sputum testing for SARS‐CoV‐2 is even more reliable and sensitive during both the early and late periods of the disease.( 9 ) Development of newer, more accurate, and rapid testing will hopefully improve in the near future.
Organ Recovery
Procurement of deceased donor organs for transplantation has traditionally relied on surgical teams from the recipient centers traveling sometimes significant distances to execute recovery procedures at the donor medical centers, many of which do not have sufficient protective equipment, requiring the donor surgical teams to bring their own for the travel and the operations. The current epidemic has imposed greater risks to donor recovery teams. An incident of a team from a high‐prevalence region with an asymptomatic but COVID‐19‐positive health care worker exposing health care workers in the organ recovery operating room who later became infected with COVID‐19 has been reported. This led to strong recommendations by UNOS/OPTN, and later the ASTS, for greater collaboration and reliance on local donor recovery teams.( 5 ) This move to local donor recovery necessitates improved communications between teams and OPOs as well as the use of technology for digital imaging, digital microscopy, and even video sharing across secure platforms like DonorNet, which are actively being implemented.
Hospital and OPO Resources for Organ Recovery
Successful organ donation relies on donor hospitals for the timely relocation of potential donors to non‐COVID‐19 intensive care units (ICUs), and to obtain the necessary testing as screening measures to exclude SARS‐CoV‐2 and pneumonitis caused by the virus.
In the face of the COVID‐19 pandemic, it is not uncommon for patients meeting brain death criteria to take an additional 36‐48 hours of ICU bed and ventilator time, so that appropriate testing and history taking can be done. Moreover, family consent and organ‐placement processes are often initiated before recovery operating room times can be set. Under the constraints of COVID‐19, hospital bed and ventilator shortages, as well as staffing shortages, made demands for a potential organ donor evaluation that were often unable to be accommodated. Additionally, availability of blood products at times was limited due to decreased donation rates in areas affected by COVID‐19. Furthermore, hospital visitor restrictions of family members or next‐of‐kin posed a barrier to obtaining timely donation consent.
The performance of donation after circulatory death (DCD) donation was made even more difficult, as it required the ICU physician to be removed from the clinical unit so that the prospective donor could be removed from mechanical ventilatory support and allowed to die. This required time away from clinical care for the ICU physician who was needed for the care of other critical patients in the ICU. Due to staffing shortages, many hospitals declined opportunities for organ donation. Overall, UNOS data revealed a 25% decrease in the number of deceased donor livers recovered between February and April 2020.( 2 )
Moving forward, establishment of neutral recovery sites run by OPOs may minimize exposure to both donor and recipient teams and enable consistent recovery and testing practices within individual regions. One current impediment in moving this forward has been the lack of financial reimbursement for transplant centers sending deceased donor organs to centralized recovery facilities for procurement, which are not counted in the hospital’s usable organ count as “organs sent to OPOs.”( 10 ) Altering the language for the CMS protocol that would allow transplant centers to count donors as in‐house among those who were “consented,” rather than only if organs are “recovered,” would mitigate the financial burdens on already strained health care systems that opt to use centralized procurement facilities.
Organ recovery centers, whether off site or on site at the transplant centers, have the potential to allow more efficient, expeditious, and timely recoveries of solid organs that could also mitigate many of the issues created by COVID‐19 to protect OPO and hospital staff, allowing better communication with families, and liberating demands faced by transplant centers to focus on recipient patients.( 11 ) Doing so would limit transportation to package movements, rather than staffed teams, which would be much less costly and easier to obtain.
Organ Acceptance Practices
UNOS/OPTN organ refusal codes were implemented on March 25, 2020, in the setting of the COVID‐19 pandemic, in recognition of donor or recipient risk factors as well as medical centers’ ability to perform transplants safely.( 3 ) Rates of wait‐list inactivation were noted to rise before this in patients with positive SARS‐CoV‐2 testing, new onset clinical symptoms compatible with COVID‐19, recent exposure to affected individuals, and travel to high risk areas. Inactivated registrants due to COVID‐19 now make up approximately 5% of the US waiting list and 10% of all registrants in higher‐incidence regions such as the Northeast and Northern Midwest.( 2 )
Currently, most of the programs have deferred all living donor transplants, given the increased risks posed to healthy donors for contracting SARS‐CoV‐2, as well as those for the recipient receiving induction immunosuppression. For liver transplantation around the world, living donation postponement was been considered the ethically appropriate action during the pandemic. In many centers where community incidence of COVID‐19 is now lower, a COVID minimal‐exposure pathway was created for resumption of living donor transplantation. As this procedure resumes at more centers, we will need to be vigilance about keeping patient safety at the forefront, and parameters should be set to determine when program suspension might be necessary should a new wave of viral activity occur.
UNOS/OPTN created a few other emergency policy changes to protect patients and assist transplant centers by allowing lab collections for the purposes of upgrading MELD score calculations to be performed at the center’s discretion, so that patients would not be required to risk viral exposure by coming to centers or laboratories for blood testing. Centers could therefore use the prior labs to extend their MELD listing over longer periods of time. There was also reassurance by the OPTN membership and professional standard committee that transplant volume activities be considered in accordance with the timing of the COVID‐19 pandemic, such that centers should not fear being penalized for low volumes while practicing in a manner to protect their patients during the critical phases.( 2 )
Future Adaptation of Liver Transplant Practice
As our community has learned to co‐exist with COVID‐19 and the incidence has diminished, we have seen that liver transplant volumes have begun to increase, approaching the rates before the pandemic. Lessons learned from this COVID‐19 pandemic for liver transplantation as we evolve into a new normal are many. We have learned to rapidly improve communication with our teams and the hospital resource managers, between transplant centers and OPOs, across our oversight organizations and professional societies, and most importantly with our patients. We have navigated assimilation of telehealth into our practices and learned how often we actually need physical visits and labs compared with what we had become accustomed to using. Incorporation of telehealth has required weighing the advantages of mitigating exposure risks, increasing patient convenience, and decreasing care costs with the challenges of incorporating new technology platforms and reducing the ability to perform clinical physical exams. We learned how to connect with technology between donor teams and transplant centers and what other forms of transportation could work effectively to transport organs rather than entire organ recovery teams, and will realize the financial effects of that change in practice.
Use of DCD livers and living donors will continue to serve an unmet need for access to transplant but may be variable in the current environment, given uneven regional trends in viral mitigation and the potential resurgence of new infections. The postponement of DCD organ use for fear of organ quality compromise and poor transplant outcomes, not to mention the admissions and exposures in the hospitals of the candidates who are hoping the donor will progress in time with minimal ischemic injury, will certainly lead to a total decrease in deceased liver transplantation in the United States. Moreover, the delay in living‐donor liver transplantation, for caution against exposing a normal healthy donor to COVID‐19 as they recover from major surgery, will also contribute to a decline in total liver transplantation. If ever a donor becomes infected with significant sequelae of SARS‐CoV‐2, it will have serious ramifications and affect living donation all across the country. Therefore, we will need to develop and maintain confident measures to prevent infection in the hospital and clinics, and in the communities to which donors return to postoperatively, as we proceed with resumption of living donor liver transplantation. As for transplantation in general in the time of COVID, whether these metrics have been reached to continue with the resumption of living donor transplantation or suspension of activities should involve a careful review by individual transplant centers. In addition, there should be inclusion of the risk of COVID disease in discussions for consent of donors and recipients for living donor transplantation in general.
Use of COVID‐19 refusal codes will be helpful in identifying center activities affected by reductions in volumes, to ensure equitable treatment among payers and regulatory bodies and to promote thoughtful planning in delivering transplantation to this high‐risk patient population. In a recent report from Agopian et al., not all centers experienced a dramatic drop in liver transplantation due to the COVID‐19 pandemic. They noted that many showed increased activity in areas where COVID prevalence was low, and even a few where hospitals were able to preserve resources to continue liver transplant and shunt COVID‐19‐afflicted patients elsewhere.( 12 )
The need to better understand the risks posed by COVID‐19 to liver transplant recipients has spawned the establishment of several registries, including SECURE Cirrhosis and COVID‐Hep. Report of some of the COVID‐positive cases in liver‐transplant recipients from these registries have revealed a 23% mortality.( 13 ) Given the small numbers of reported cases thus far, guidelines relating to treatment of transplant patients who test positive and risks posed to health care providers and family contacts have not been established. Each center must adapt their own individual practice until more data become available.
Perhaps more widespread and reliable testing, vaccination, and more successful treatments will restore patient and health care personnel confidence in our medical systems. The future may bring a workforce of organ recovery surgeons managed by the OPOs with streamlined technologies to share images and micrographs, and better point‐to‐point transportation of organs that is less expensive due to less need to transport organ recovery teams. There will likely be regional centers for organ regeneration/resuscitation that will have multiple organ perfusion devices to assess and improve organ function before sending them to the recipient centers for transplantation. This would enhance the use of potentially marginal livers and livers obtained by deceased cardiac donation as well as improve timing of the transplant operation, to ensure that optimal resources and personnel are available at the transplanting center.
In summary, successful liver transplantation is a complex process that is reliant on many factors that have been challenged in the COVID‐19 pandemic. These factors include adequate donor evaluation, organ recovery, the availability OPO and donor hospital resources, as well as the transplant center’s acceptance of organ offers for their candidates and their own resources for performing transplants (e.g., ICU beds, ventilator availability, blood product availability, staff), all of which have been significantly altered by the COVID‐19 pandemic. To successfully improve organ donation in the era of COVID, we must facilitate reliable and timely testing that is widespread, limit travel for personnel from transplant centers, and promote local or centralized organ recoveries. To balance risks and benefits, we must also reprioritize wait‐listed registrants and determine the relative resources and risks of performing transplantation at a given transplant center, depending on the geographic constraints and local information regarding the incidence of COVID‐19 in the hospital and community at a given time. Navigating these hurdles can help the transplant community educate staff and the public about challenges facing organ donation and hopefully improve and maximize the availability of needed resources, to ensure that patients with end‐stage liver disease continue to receive timely and safe liver transplantation. There are many lessons that will be learned during this pandemic that will change transplantation as a whole for the better and allow more patients to receive life‐saving organs with excellent long‐term outcomes.
Potential conflict of interest: Dr. Schilsky received grants from Alexion and GMPO.
References
Author names in bold designate shared co‐first authorship.
- 1. Center for Medicare Services . https://www.cms.gov/files/document/covid‐elective‐surgery‐recommendations.pdf. Accessed September 13, 2020.
- 2. United Network for Organ Sharing . Current state of donation and transplantation; 2020. https://unos.org/covid/. Accessed September 13, 2020. [Google Scholar]
- 3. United Network for Organ Sharing . COVID‐19 refusal codes for transplant hospitals implemented March 25; 2020. https://unos.org/news/covid19/covid‐19‐refusal‐codes‐for‐txc/. Accessed September 13, 2020. [Google Scholar]
- 4. American College of Radiology . ACR recommendations for the use of chest radiography and computed tomography (CT) for suspected COVID‐19 infection 2020 [updated March 22, 2020]. https://www.acr.org/Advocacy‐and‐Economics/ACR‐Position‐Statements/Recommendations‐for‐Chest‐Radiography‐and‐CT‐for‐Suspected‐COVID19‐Infection. Accessed September 13, 2020. [Google Scholar]
- 5. American Association for the Study of Liver Disease . Clinical best practice advice for hepatology and liver transplant providers during the COVID‐19 pandemic: AASLD expert panel consensus statement 2020. https://www.aasld.org/sites/default/files/2020‐05/AASLD‐COVID19‐ClinicalInsights‐May42020‐FINAL.pdf. Accessed September 13, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. American Society of Transplant Surgeons . Organ retrieval for transplantation in the COVID‐19 era: 2020. https://asts.org/advocacy/covid‐19‐resources/asts‐covid‐19‐strike‐force/asts‐covid‐19‐strike‐force‐organ‐retrieval‐guidance#.XrG7wS2ZOt8. Accessed September 13, 2020. [Google Scholar]
- 7. Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, et al. Detection of SARS‐CoV‐2 in different types of clinical specimens. JAMA 2020;323:1843‐1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Iwasaki S, Fujisawa S, Nakakubo S, Kamada K, Yamashita Y, Fukumoto T, et al. Comparison of SARS‐CoV‐2 detection in nasopharyngeal swab and saliva. J Infect 2020;81:e145‐e147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wyllie AL, Fournier J, Casanovas‐Massana A, Campbell M, Tokuyama M, Vijayakumar P, et al. Saliva is more sensitive for SARS‐CoV‐2 detection in COVID‐19 patients than nasopharyngeal swabs 2020. https://www.medrxiv.org/content/10.1101/2020.04.16.20067835v1. Accessed September 13, 2020. [Google Scholar]
- 10. Center for Medicare Serices . Provider Reimbursement Manual 15‐2, §4028.3. Sect. Chapter 40. 2018. https://www.cms.gov/Regulations‐and‐Guidance/Guidance/Manuals/Paper‐Based‐Manuals‐Items/CMS021935. Accessed September 13, 2020.
- 11. Marsolais P, Durand P, Charbonney E, Serri K, Lagace AM, Bernard F, et al. The first 2 years of activity of a specialized organ procurement center: report of an innovative approach to improve organ donation. Am J Transplant 2017;17:1613‐1619. [DOI] [PubMed] [Google Scholar]
- 12. Agopian V, Verna E, Goldberg D. Changes in liver transplant center practice in response to COVID‐19: unmasking dramatic center‐level variability. Liver Transpl 2020;26:1052‐1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Webb GJ, Moon AM, Barnes E, Barritt SA, Marjot T. Determining risk factors for mortality in liver transplant patients with COVID‐19. Lancet Gastroenterol Hepatol 2020;5:643‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]


