Abstract
A significant proportion of patients infected with SARS‐CoV‐2 develop severe respiratory symptoms due to an excessive immune response. Treatment of this condition may include immunosuppressive therapies, such as IL‐6 receptor antagonists and corticosteroids, which pose a risk for patients with active or past hepatitis B virus (HBV) infection. In this prospective cohort study, we analysed the risk of HBV reactivation in patients with severe COVID‐19 and resolved HBV infection undergoing immunosuppressive therapy. From 15th March to 30th April 2020, 600 patients with severe COVID‐19 were admitted to our hospital and treated with immune modulators. Data regarding HBV infection were available in 484, of whom 69 (14%) were HBsAg negative/anti‐HBc positive. For these patients, HBV reactivation prophylaxis with entecavir was strongly recommended. Complete follow‐up was available in 61 patients: 72% were male, median age was 67 years, and anti‐HBs was >10 IU/mL in 72%. The immunosuppressive drug most used was tocilizumab (72%). Despite HBV prophylaxis recommendation, 38 (62%) patients received entecavir and 23 (38%) did not. Baseline features of both groups were similar. At follow‐up, we found no cases of HBsAg seroreversion and only 2 (3%) patients (no prophylaxis group) had detectable serum HBV‐DNA (<15 IU/mL). Both were anti‐HBs negative and had normal aminotransferase levels. Our data show that the risk of HBV reactivation in patients with severe COVID‐19 and resolved HBV infection undergoing immunosuppressive treatment is low. However, if a systematic follow‐up after hospital discharge is unfeasible in patients without anti‐HBs, a short course of antiviral prophylaxis may be a safe option.
Keywords: COVID‐19, hepatitis B, immunotherapy, reactivation
Abbreviations
- HBV
hepatitis B virus
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has spread globally, causing a pandemic with more than 34 million infections worldwide and 1 millon fatalities so far. COVID‐19 (the disease caused by SARS‐CoV‐2) is usually mild, but around 20% of infected individuals develop severe respiratory symptoms that require hospitalization and, in some cases, intensive care support. 1 , 2 Although the pathogenesis of SARS‐CoV‐2 infection is not yet completely understood, in some patients there is an excessive immune response to the virus, known as a ‘cytokine storm’. The latter mediates severe lung inflammation, and for this reason, patients are treated with immunosuppressive therapies aimed at limiting immune‐mediated damage. 3 Corticosteroids, IL‐6 and IL‐1 receptor antagonists, and Janus kinase inhibitors are some of the drugs that have been used in patients with severe COVID‐19. The use of these drugs may pose a risk for patients with active or past hepatitis B virus (HBV) infection.
Hepatitis B reactivation associated with immune suppressive and biological therapies is an important cause of morbidity and mortality in patients with current or past exposure to HBV. 4 In HBsAg‐positive patients, HBV reactivation is defined as a sudden and rapid increase in HBV‐DNA levels in patients with previously detectable DNA or reappearance of HBV‐DNA in individuals who did not have viremia before the initiation of immune suppressive therapy. 5 In individuals who are initially negative for HBsAg and anti‐HBc positive, HBV reactivation is defined by appearance of HBsAg and/or HBV‐DNA. Following HBV reactivation, ALT elevation can occur and, in some cases, this can evolve into a fulminant hepatic failure.
The risk of HBV reactivation is highly dependent on the status of HBV infection, as well as on the type of immune suppressive therapy. The highest risk of reactivation occurs in patients with active or past HBV infection treated with B‐cell–depleting agents such as rituximab, and in HBsAg‐positive patients who receive high‐dose corticosteroids, anthracyclines or potent TNF‐alfa inhibitors. 5 , 6 , 7 Except for high‐dose corticosteroids, all the immune modulators used to treat cases of severe COVID‐19 are considered of low risk, particularly in HBsAg‐negative anti‐HBc‐positive patients.
Since past HBV infection is a relatively common condition (≈10%), 8 our aim was to assess the risk of HBV reactivation and the utility of nucleoside analog (NUC) prophylaxis in HBsAg‐negative/anti‐HBc‐positive patients who were admitted to the hospital for severe COVID‐19 and underwent immune suppressive therapy.
2. PATIENTS AND METHODS
This is a prospective cohort study conducted in a single centre (Hospital Clínic of Barcelona). All patients admitted to our centre from 15th March to 30th April 2020 with a diagnosis of COVID‐19 who had an indication for immune modulatory therapy were tested for markers of present (HBsAg) or past (anti‐HBc) hepatitis B virus infection. HBsAg‐ or anti‐HBc‐positive patients were included in the study.
The following drugs were considered as immunosuppressive therapy: (a) interleukin‐6 receptor antagonists (tocilizumab, siltuximab); (b) interleukin‐1 receptor antagonists (anakinra); (c) Janus kinase inhibitors (baricitinib); and (d) high‐dose corticosteroids. Tocilizumab was administered in a single dose of 400 mg (600 mg if weight >75 kg); some patients received a second dose within the next 24 hours if there was an unfavourable clinical course. Siltuximab was administered as a single dose of 800 mg. Anakinra was administered at a dose of 200 mg/12 h the first day and 200 mg/d the following 2 days. Baricitinib was administered at a dose of 4 mg/d for 3 days. Regarding corticosteroid therapy, methylprednisolone was dosed at 250 mg/d for 3 days, followed by 30 mg/d for a week. In patients with suspected organizing pneumonia, treatment with prednisone 0.5 mg/kg/d was initiated and extended for at least 1 month.
Due to the complex and rapidly evolving situation during the pandemic's peak, the immunosuppressive treatment regimen was decided according to the physicians' criteria, availability of drugs at the time of admission, or based on inclusion in ongoing clinical trials.
2.1. HBV reactivation prophylaxis
Hepatitis B virus reactivation prophylaxis was mandatory for HBsAg‐positive patients and strongly recommended for those who were HBsAg‐negative/anti‐HBc‐positive (independently of their anti‐HBs status). For HBsAg‐positive patients, we indicated entecavir 0.5 mg/d for at least 6 months. For patients with isolated positive anti‐HBc markers, entecavir 0.5 mg/d was administered for 1 month. Doses of entecavir were adjusted if renal failure was present according to label.
For HBsAg‐negative/anti‐HBc‐positive patients, HBV reactivation was defined as: (a) detectable HBV‐DNA or (b) reverse HBsAg seroconversion (reappearance of HBsAg). 9
2.2. Follow‐up
Between 1 and 2 months after the last dose of the immune modulator therapy, all HBsAg‐positive or HBsAg‐negative/anti‐HBc‐positive patients at hospital admission underwent a blood test including liver enzymes and HBV infection markers (HBsAg, HBeAg, anti‐HBe, anti‐HBc, anti‐HBs and HBV viral load). HBsAg, HBeAg, anti‐HBc, anti‐HBe and anti‐HBs were determined by Atellica (Siemens); HBV‐DNA was determined by real‐time PCR with a lower limit of quantification of 10 and a lower limit of detection of 3 IU/mL (Cobas‐HBV; Roche).
2.3. Ethics
The study was approved by the Hospital Ethics Committee. All patients gave oral consent to participate in the study. Once patients were discharged from the centre, a written informed consent form was provided to all patients in order to obtain their signature.
3. RESULTS
From 15th March to 30th April 2020, 600 patients with severe COVID‐19 were admitted into our Hospital and were treated with an immune modulator. Data of HBV infection status were available in 490 (81%) patients, 3 of whom (0.6%) were HBsAg positive and 69 (14%) were HBsAg negative/anti‐HBc positive (Figure 1). As expected in our geographical area, the prevalence of anti‐HBc increased with age and was higher than 15% in individuals over 60 years (Figure S1).
FIGURE 1.
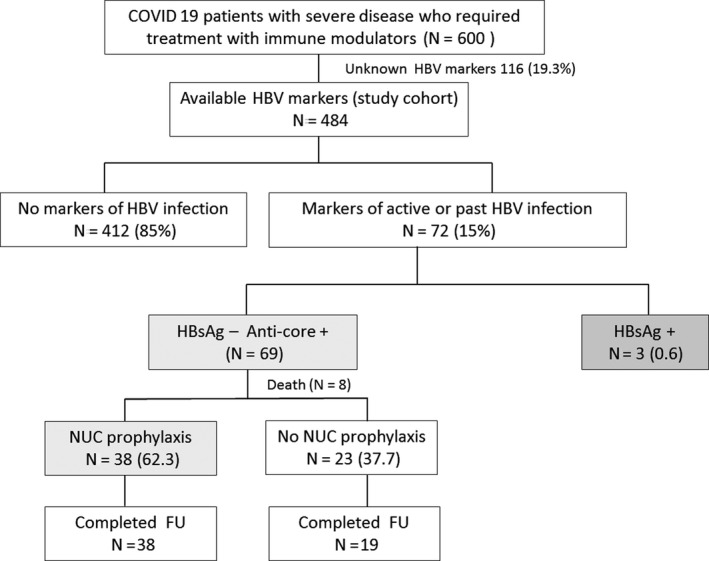
Flow chart of the study
Among the 69 patients with isolated anti‐HBc, eight died during the admission and follow‐up was not available. Thus, the final study cohort consisted in 61 HBsAg‐negative/anti‐HBc‐positive patients. Their baseline features are depicted in Table 1: 72% were men, median age 67 (59‐75) years, the most frequent comorbidities were arterial hypertension (62%), diabetes mellitus (36%) and dyslipidemia (33%). Fifty‐three (87%) patients had abnormal ALT levels, 35 (57%) with values three times above the ULN. Regarding HBV infection markers, 44 (72%) of the 61 patients were anti‐HBs positive. The most frequently used immunosuppressant drug was tocilizumab (72%); 7 (11%) patients had received a second drug (anakinra). Twenty‐five patients (41%) received high doses of steroids (methylprednisolone 250 mg/d for 3 days) alone (n = 5) or in combination with other immune modulators (n = 20). Twenty‐two patients (36%) received 0.5 mg/kg/d of prednisone for 1 month or longer due to suspected organizing pneumonia; 15 of them had undergone high‐dose steroid therapy (Table 1).
TABLE 1.
Baseline characteristics of patients
|
Anticore pos/HBsAg− N (%)/median (IQR) |
Total (61) | NUC prophylaxis (38) | No NUC prophylaxis (23) |
|---|---|---|---|
| Age | 67 (59‐75) | 69 (61‐75) | 62 (58‐74) |
| <40 | 1 (2) | 1 (3) | 0 (0) |
| 40‐49 | 7 (11) | 3 (8) | 4 (17) |
| 50‐59 | 8 (13) | 5 (13) | 3 (13) |
| 60‐69 | 19 (31) | 11 (29) | 8 (35) |
| 70‐79 | 18 (30) | 11 (29) | 7 (30) |
| >80 | 8 (13) | 7 (18) | 1 (4) |
| Sex (M) | 44 (72) | 28 (70) | 16 (74) |
| Comorbidities | |||
| Arterial hypertension | 38 (62) | 21 (55) | 17 (74) |
| Diabetes mellitus | 22 (36) | 15 (39) | 7 (30) |
| Hypercholesterolaemia | 20 (33) | 14 (37) | 6 (26) |
| Cardiovascular disease | 9 (15) | 8 (21) | 1 (4) |
| Chronic renal disease | 6 (10) | 3 (8) | 3 (13) |
| Days of hospitalization | 16 (10‐23) | 16 (10‐22) | 15 (10‐37) |
| ICU admission | 20 (33) | 12 (32) | 8 (35) |
| Mechanical ventilation | 14 (23) | 9 (24) | 5 (22) |
| Discharge to nursing home | 19 (31) | 11 (29) | 8 (35) |
| HBV baseline markers | |||
| HBV‐DNA a | 2# | 2# | 0 |
| Anti‐HBs >10 IU/mL | 44 (72) | 27 (71) | 17 (74) |
| Laboratory | |||
| Baseline ALT | 33 (21‐58) | 29 (19‐50) | 42(23‐69) |
| ALT peak | 144 (67‐194) | 142 (53‐189) | 146 (73‐224) |
| >ULN | 53 (87) | 31 (82) | 22 (96) |
| >3 ULN | 35 (57) | 20 (53) | 15 (65) |
| Lymphocytes | 600 (500‐900) | 600 (500‐900) | 800 (600‐1100) |
| <1000 | 46 (79) | 30 (83) | 16 (73) |
| <500 | 12 (22) | 9 (27) | 3 (14) |
| Immune modulator | |||
| Tocilizumab | 44 (72) | 27 (71) | 17 (74) |
| Siltuximab | 8 (13) | 6 (16) | 2 (9) |
| Baricitinib | 2 (3) | 2 (5) | 0 (0) |
| Anakinra | 1(2) | 1 (3) | 0 (0) |
| Second IS | |||
| Anakinra | 7 (11) | 5 (13) | 2 (9) |
| Steroids b | |||
| High dose | 25 (41) | 16 (42) | 9 (39) |
| Medium dose | 22 (36) | 13 (34) | 9 (39) |
| Low dose | 4 (7) | 0 (0) | 4 (17) |
| Organizing pneumonia | 16 (26) | 14 (37) | 2 (9) |
| Follow‐up HBV | 57 | 38 | 19 |
| HBsAg seroreversion | 0 (0) | 0 (0) | (0) |
| Detectable HBV‐DNA | 2 (3) | 0 (0) | 2 (10) |
| ALT | 28 (18‐48) | 24 (18‐47) | 35 (18‐49) |
| ALT >ULN | 20 (35) | 11 (29) | 9 (47) |
| ALT >3ULN | 2 (4) | 2 (5) | 0 (0) |
Data of 28 patients, # detectable, <10 IU/mLa
The doses of steroids were classified as high: 250 mg in bolus for 3 d followed by 30 mg/d for at least 1 wk; medium: 0.5 mg/kg/d more 1 mo or longer; low: less than 20 mg oral prednisone, less than 1 mo. High‐dose steroids were administered in combination with other immune modulators (20) or given as monotherapy (5).b
Thirty‐eight (62%) patients received entecavir prophylaxis, and 23 (38%) did not (Figure 1). Although the hospital protocol recommended NUC prophylaxis for anti‐HBc‐positive patients undergoing immune modulator treatments, the decision to indicate HBV prophylaxis depended on the treating physician. As shown in Table 1, relevant baseline features of both groups (prophylaxis/no prophylaxis) were similar regarding age, gender, presence of comorbidities, immune modulator regimen and laboratory tests. After being discharged, 19 (31%) patients were transferred to a nursing home or rehabilitation centre for continuing their recovery; the proportion was similar in patients who did or did not receive entecavir prophylaxis.
Follow‐up data after hospital discharge were available in 57 (93%) of the 61 patients (4 patients were lost after discharge). Among these 57 patients, we did not identify a single case of HBsAg seroreversion (positive HBsAg), and we only detected two cases of positive HBV‐DNA. In both, HBV viral load was below the quantification limit (<10 IU/mL). None of these two patients had undergone entecavir prophylaxis, and both were negative for anti‐HBs. One case (male, 77 years old) had received siltuximab and methylprednisolone 250 mg/d (3 days) followed by prednisone 0.5 mg/kg due to suspected organizing pneumonia, whereas the second case (male, 64 years old) was treated with a single dose of tocilizumab.
Concerning aminotransferase elevations, the proportion of patients with abnormal ALT levels (above the ULN) decreased from 87% (peak during hospitalization) to only 35% at follow‐up (4% with ALT values three times above ULN). ALT values were within the normal range in the 2 individuals with detectable HBV‐DNA.
Regarding the three HBsAg‐positive patients, two had positive HBV‐DNA (109 IU/mL and 15 IU/mL, respectively) whereas one patient (who was coinfected with HIV undergoing tenofovir therapy) had undetectable HBV‐DNA. Entecavir prophylaxis was initiated in the two patients with positive HBV‐DNA, and they are currently followed at our liver clinic.
4. DISCUSSION
The results of our study show that a short course of immune modulator therapy does not appear to increase the risk of HBV reactivation in patients with severe COVID‐19 with markers of past HBV infection. Drugs used to treat the disproportionate immune response after SARS‐CoV‐2 infection (mainly IL‐6 receptor antagonists or high‐dose corticosteroids) are considered of moderate risk for HBV reactivation in HBsAg‐negative/anti‐HBc‐positive individuals. 5 , 6 Nevertheless, we decided to recommend NUC prophylaxis for several reasons. First, a significant proportion of patients admitted to the hospital with severe COVID‐19 are old males with significant comorbidities, which are considered risk factors for HBV reactivation. Second, SARS‐CoV‐2 infection induces significant lymphopenia, which might increase the possibility of HBV reactivation. Finally, due to the extremely high burden of the pandemics on our healthcare system, we could not guarantee a proper follow‐up after patient discharge from the hospital, particularly older patients who returned to a nursing home. The latter is highly recommended for patients at risk of HBV reactivation if no prophylaxis is indicated.
Despite the strong recommendation to include HBV prophylaxis in patients undergoing immune suppressive treatment, not all treating physicians indicated entecavir. The latter allowed us to compare outcomes of individuals with and without HBV prophylaxis. The main baseline features of both groups were comparable, and we only detected positive HBV‐DNA in two patients who had not received entecavir prophylaxis. The two cases were anti‐HBs negative. In both cases, HBV‐DNA remained below the limit of quantification and was not accompanied by ALT elevations.
Our data regarding the low risk of HBV reactivation using IL‐6 receptor antagonists are supported by some reports in patients with rheumatoid arthritis. 10 , 11 In a recent study, which included 152 patients with resolved HBV infection treated with disease‐modifying anti‐rheumatic drugs (25 with tocilizumab), the risk of HBV reactivation was very low (<5%). 10 Nevertheless, patients who were anti‐HBs negative showed a significantly higher incidence of HBV reactivation (15%), as already reported in other studies. 7 Reactivation, defined as detectable HBV‐DNA at any point during therapy, tended to occur several months after immunosuppressive treatment initiation (in contrast with the short course therapy in our scenario). In cases where HBV‐DNA did not rise above the limit of quantification, antiviral treatment was not indicated and the final outcome was excellent. A completely different scenario applies to patients with active infection (HBsAg positive), in whom cases of reactivation and even fulminant hepatic failure have been reported during tocilizumab therapy. 12
Regarding the high prevalence of abnormal aminotransferases, our results are consistent with other articles were the liver impairment has been reported in up to 75% in patients with severe COVID‐19. 13 The mechanism of liver damage during COVID‐19 is not fully understood but it seems related, at least in part, with the highly activated inflammatory status 14 (though it can be influenced by multiple additional factors, as drug toxicity). The elevation of aminotransferases is usually mild and transient, 15 as seen in our patients.
Our study has several limitations. First, the small sample size, which does not allow to draw definitive conclusions. Second, the fact that this was not a randomized study: the decision to indicate NUC prophylaxis in HBsAg‐negative anti‐HBc‐positive patients depended on the treating physician, although we did not find significant differences between treated and untreated patients. Third, the duration of NUC prophylaxis is arguable. We based our decision on the fact that most patients received a single dose of immune modulator. Moreover, except for patients with suspected organizing pneumonia, duration of corticosteroid treatment did not overcome 1 month. In addition, due to characteristics of patients with severe COVID‐19 (old age, comorbidities, residency at nursing homes), we chose to minimize pill burden and medical interventions.
In summary, our data show that the risk of HBV reactivation in patients with severe COVID‐19 and resolved HBV infection undergoing immune modulator treatment is low. Follow‐up after patient discharge is recommended, but if this is not possible due to the burden of the pandemic, a short course of antiviral prophylaxis may be a safe alternative in patients without anti‐HBs.
CONFLICTS OF INTEREST
XF has acted as advisor for Gilead and Abbvie. SR‐T received speaker fees from Gilead and Abbvie. ZM received speaker fees and acted as advisor for Gilead and Abbvie. SL acted as advisor for Gilead and Abbvie. ML has received fees to give lectures from Gilead, MSD, ViiV, Abbvie and Janssen‐Cilag.
Supporting information
Fig S1
Rodríguez‐Tajes S, Miralpeix A, Costa J, et al. Low risk of hepatitis B reactivation in patients with severe COVID‐19 who receive immunosuppressive therapy. J Viral Hepat 2021;28:89–94. 10.1111/jvh.13410
Funding information
This study was sponsored by the Instituto de Salud Carlos III (ISCIII) through the Plan Estatal de Investigación Científica y Técnica y de Innovación grant PI18/00079 (FRF), co‐funded by the European Regional Development Fund (ERDF). XF also received support by Secretaria d'Universitats i Recerca del Departament d'Economia i Coneixement (grant 2017_SGR_1753) and CERCA Programme/Generalitat de Catalunya. SRT was funded by the Rio Hortega programme (fellowship CM17/00015) of the ISCIII.
REFERENCES
- 1. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA. 2020;10022(20):E1‐E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McGonagle D, Sharif K, O'Regan A, et al. The role of cytokines including interleukin‐6 in COVID‐19 induced pneumonia and macrophage activation syndrome‐like disease. Autoimmun Rev. 2020;19(6):102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560‐1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Loomba R, Liang TJ. Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: current concepts, management strategies, and future directions. Gastroenterology. 2017;152:1297‐1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Perrillo RP, Gish R, Falck‐Ytter YT. American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148(1):221‐244.e3. [DOI] [PubMed] [Google Scholar]
- 7. Wong GLH, Wong VWS, Yuen BWY, et al. Risk of hepatitis B surface antigen seroreversion after corticosteroid treatment in patients with previous hepatitis B virus exposure. J Hepatol. 2019;72(1):57‐66. [DOI] [PubMed] [Google Scholar]
- 8. Domínguez À, Bruguera M, Vidal J, et al. Community‐based seroepidemiological survey of HCV infection in Catalonia, Spain. J Med Virol. 2001;65(4):688‐693. [DOI] [PubMed] [Google Scholar]
- 9. Raimondo G, Locarnini S, Pollicino T, et al. Update of the statements on biology and clinical impact of occult hepatitis B virus infection. J Hepatol. 2019;71(2):397‐408. [DOI] [PubMed] [Google Scholar]
- 10. Watanabe T, Fukae J, Fukaya S, et al. Incidence and risk factors for reactivation from resolved hepatitis B virus in rheumatoid arthritis patients treated with biological disease‐modifying antirheumatic drugs. Int J Rheum Dis. 2019;22(4):574‐582. [DOI] [PubMed] [Google Scholar]
- 11. Ahn SS, Jung SM, Song JJ, et al. Safety of tocilizumab in rheumatoid arthritis patients with resolved hepatitis B virus infection: data from real‐world experience. Yonsei Med J. 2018;59(3):452‐456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sonneveld MJ, Murad SD, van der Eijk AA, et al. Fulminant liver failure due to hepatitis B reactivation during treatment with tocilizumab. ACG Case Rep J. 2019;6(12):e00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cai Q, Huang D, Yu H, et al. COVID‐19: abnormal liver function tests. J Hepatol. 2020;73:566‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang C, Shi L, Wang FS. Liver injury in COVID‐19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5(5):428‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lei F, Liu YM, Zhou F, et al. Longitudinal association between markers of liver injury and mortality in COVID‐19 in China. Hepatology. 2020;72:389‐398. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1


