Transfusion of red cells has saved countless lives owing to (a) the ability to perform far more extensive surgery; (b) treatment of acute hemorrhagic anemia, both civilian and military; and (c) treatment of illnesses of red cell destruction or impaired (including, but not limited to chemotherapy‐induced suppression of hemopoiesis) production. There are extensive, largely efficient, blood collection and banking systems in developed countries that ordinarily provide stored red cells (at 2‐6°C) for these functions, despite relatively brief periods of local or national shortages.
Blood banking systems require operational collection and processing facilities, sufficient number of healthy donors with access to a blood collection center, together with functional blood processing and transportation systems to provide the needed products in appropriate quantities. Depending on the circumstances, any or all of these are likely to be severely degraded or completely non‐operational in some civilian mass casualty events or on a military battlefield. These systems are also stressed at times of pathogen dissemination, owing to a decreased availability of healthy, acceptable donors, and healthy, non‐infected health care personnel. For example, considering only the donation issue, with the onset of the current coronavirus pandemic the American Red Cross “faced a severe blood shortage” 1 with the cancelation of thousands of blood drives, and a “precipitous decrease” in blood donations in Seattle was reported. 2 Earlier viral epidemics noted a 21% donation decrease in a prefecture in Japan, 3 and a greater than 90% decrease in Beijing 4 ; furthermore, COVID‐19 has been detected in asymptomatic donors whose donations had entered the blood supply in China. 5 Nevertheless, the U.S. blood banking community points to their past capability in domestic cases of mass casualty, such as the Twin Towers destruction on 9/11, focusing on blood supply and donations 6 , 7 , 8 and that the above functions remained preserved throughout the 9/11 crisis, but has paid far less attention to the isolated nature of this disaster. 9 Only 224 units of packed red blood cells (PRBCs) were required to satisfy the needs 6 , 7 (less than 300 units were needed for other U.S. disasters 10 , 11 , a ). In addition, the conditions are expected to be very different for other disasters (such as detonation of a thermonuclear device 12 , 13 , 14 ), when the number of injuries will be far greater and capabilities for donation, testing, and transportation will be degraded. While there have not been reports of an overwhelming strain on the blood supply following a nuclear event such as that at Chernobyl, b a nuclear detonation would be expected to result in a far larger mass casualty (including combined injuries) surge situation. 14 It has been estimated that a 10 kt nuclear detonation in a major city could require hundreds of thousands of units of plasma, and presumably a commensurate need for red cells. 19 , 20 , 21
The financial 22 and logistical 4 stresses on civilian blood collection and processing facilities add to the problem. c Early on in the COVID‐19 pandemic, volunteer blood donations at fixed sites and reduced demand with the cancellation of elective procedures enabled the blood supply to be successfully maintained in its early phases, but the effect on the blood supply if the pandemic intensifies or become more chronic could be problematic. Paradoxically, at least in the early phases of COVID‐19, societal lockdown may have unexpected results. In South Africa there was a marked initial decrease in trauma admissions and a reduced demand for blood. However, this does not rule out a later upsurge in blood demand coupled with a delayed lack of availability through inadequate donation and reduced transfusion service staffing. When elective surgery was restored at some institutions in the U.S., shortages did occur as some blood banks/centers were not prepared for the sudden demand increase and collection difficulties caused by new rules. Due to their unique operational challenges, military organizations appear to be far more cognizant of these challenges than are their civilian counterparts.
Nevertheless, these systems are unprepared to fulfill the needs should the systems become severely degraded, as has been recognized in coordinated US Government multi‐agency (NIH, FDA, BARDA, DoD) efforts to develop alternative products as countermeasures in these scenarios. 14 Limited shelf‐life and cold‐chain constraints make the systems dependent on continuous donor availability and uninterrupted laboratory operations. Blood collection, processing, and distribution is highly regulated. There are no products approved in the U.S. and most other countries that provide the primary physiologic functions of plasma or red cells that do not require continuous cold storage and a multitude of regulatory constraints and checks. However, there are products approved in some countries that fulfill these functions, have a prolonged shelf‐life at 22C, and do not require recipient blood typing and cross‐matching. Dried plasma is produced in Germany, France, and South Africa, with limited availability of these products outside these countries; a hemoglobin‐based oxygen carrier (HBOC) is approved for use in South Africa and Russia. d The need and rationale for a dried plasma has been published recently. 24 Here, we address the need and rationale for a non‐red cell oxygen carrier.
The quest for an efficacious non‐red cell oxygen carrier is many decades old. 25 , 26 , 27 Research and development intensified when it became apparent that hepatitis C 28 , 29 , 30 and HIV 31 could be transmitted by transfusion, with the intent that an artificial oxygen carrier could replace RBC transfusion. However, donor screening and testing has reduced greatly the risk of transmission of these pathogens. 32 That coupled with the perception of a slightly increased risk of infusion of an HBOC in comparison to red cell transfusion, 33 , 34 resulted in a substantial decrease of research and development and in the number of companies in this field despite the latter meta‐analysis having been widely criticized. 35 , 36 , 37 , 38 , 39 , 40 Subsequently, attention has turned to an alternative potential purpose for the use of such biologics, such as when RBC transfusion is not an option. For this indication, the appropriate comparator for the evaluation of product safety is untransfused severe anemia, rather than RBC transfusion. 43 , 44 e Recent data and analyses provide new perspectives on this potential for HBOC use.
Healthy volunteers incur neurocognitive deficits at a hemoglobin concentration (Hb) of 6 g/dL, 45 , 46 , 47 but do not demonstrate systemic metabolic consequences during a relatively brief reduction of Hb to 5 g/dL. 48 However, the clinical risk of untransfused severe anemia for more than a short period cannot be determined in a prospective randomized clinical trial, as it would not be ethical to allow this condition with its attendant irreversible consequences to remain untreated when RBC transfusion is available. The best information available for this risk are data from patients for whom RBC transfusion is not an option: those who refuse transfusion owing to religious beliefs, and cases where compatible RBCs cannot be made available in a clinically timely manner. It would not be feasible to conduct an adequately powered trial given the small number of patients and the limited number at any institution, raising yet another ethical issue as well: initiating a trial that is not properly powered, for which there is little or no hope of reaching a statistically valid conclusion. Additionally, many believe that equipoise does not exist for a randomized clinical trial involving these patients, based on their clinical experience and the published data. 44
Increasing mortality with decreasing nadir Hb has been well established in untransfused anemia. Analysis of two databases of 300 49 and 293 50 patients for whom RBC transfusion was not an option found nearly identical relationships (P = .39, HR 0.86 [95% CI: 0.44‐1.39]) 44 of a statistically significant association of increasing morbidity and mortality with decreasing post‐operative nadir Hb concentration. Two other databases have been examined to determine the association of mortality with anemia in mixed populations of both surgical and non‐surgical hospitalized patients for whom RBC transfusion was not an option (“all‐comers”). Underlying data from a New Zealand database of 103 patients 51 and a U.S. database (Duke University) of 29 patients also demonstrated increased mortality with decreasing nadir Hb concentration. 44 These two latter relationships (New Zealand and U.S.) were similar to each other when analyzed in the same manner (P = .34, HR 0.68 [0.26‐1.60]). However, the mortality in the “all‐comers” groups was significantly greater than in the surgical patients (P < .0001, HR 0.26 [0.040‐0.17]), 44 possibly owing to the former groups including patients with non‐reversible pathology, such as malignancy, contributing to the lower rate of survival.
There are three hemoglobin‐based oxygen carriers (HBOCs) that are in clinical development; however, only one (Hemopure, HbO2 Therapeutics, Souderton, MA, USA) for which there are sufficient published data for analyses. The best data for that product emanates from a randomized, clinical orthopedic trial, in which 350 patients received the HBOC and 338 received pRBCs. 52 Survival as a function of nadir native Hb was markedly improved in the HBOC group compared with that of untransfused surgical patients (P = .014, HR 0.42 [0.25‐0.85]). 44 The data for the HBOC administration for “all‐comer” hospitalized patients are less robust and come from “expanded access” use. 53 , 54 Many of these patients were given the HBOC (in the U.S., The Netherlands, Germany, Belgium, Canada, Lebanon, India, Guatemala, Chile) as a last resort, when their clinical condition had deteriorated severely and was already extremely grave. Nevertheless, a previous comparison of the survival of these patients with the combined above untransfused “all‐comer” anemic patients, found substantially improved survival with HBOC administration (P < .0001, HR 0.44 [0.15‐0.53]). 44
Recently, Guinn et al. have expanded their database examination to include 271 patients with untransfused substantial anemia, also finding a relationship of mortality to nadir Hb similar to previous findings. 55 We have performed analyses similar to those above, using that database. The relationship between mortality and nadir Hb in the expanded Duke database demonstrates a similar relationship as did the smaller database, and does not differ from the relationship in the New Zealand database (P = .93, HR 0.98 [0.58‐1.64]; Figure 1). These combined data also confirm the greater mortality in a mixed population than in post‐surgical patients (P < .0001, HR 0.34 [0.16‐0.36]; Figure 2) and that patients treated with the HBOC (Hemopure) in the expanded access program had improved survival compared to these untransfused patients; (P = .0002, HR 0.56 [95% CI: 0.32‐0.71] Figure 3).
FIGURE 1.
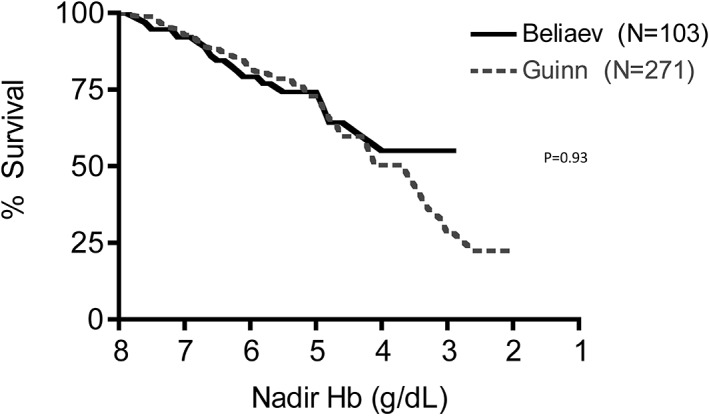
Mortality as a function of nadir native hemoglobin concentration in anemic patients for whom blood transfusion was not an option. This relationship from the New Zealand database 44 , 51 (——) and from Duke University 55 (‐‐‐‐) do not differ from each other (P = .93, HR 0.98 [95% CI: 0.58‐1.64]) [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 2.
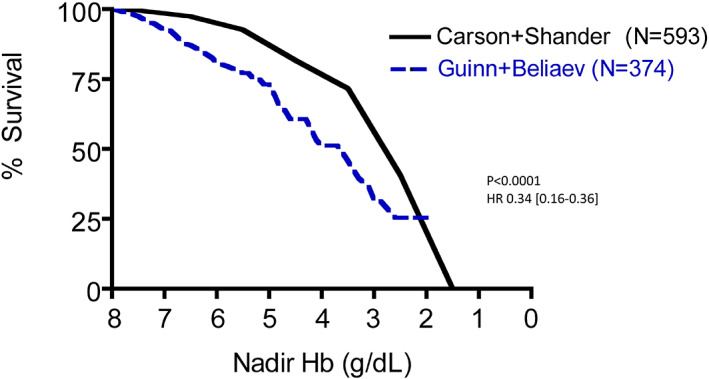
Mortality as a function of nadir native hemoglobin concentration in anemic patients for whom blood transfusion was not an option, comparing all hospitalized patients in the databases of Beliaev et al. 51 plus Guinn et al. 55 (——) with surgical patients in the databases of Carson et al. 49 plus Shander et al. 50 (‐‐‐‐) All hospitalized patients had decreased survival compared with surgical patients (P < .0001, HR 0.34 [95% CI: 0.16‐0.36]) [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 3.
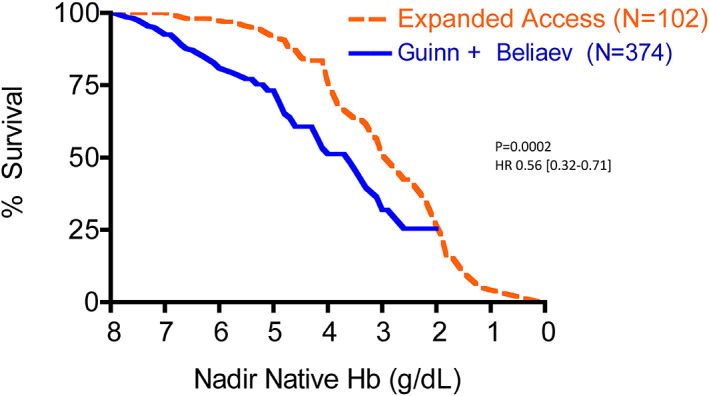
Mortality as a function of nadir native hemoglobin concentration in anemic patients for whom blood transfusion was not an option, comparing all hospitalized patients in the databases of Beliaev et al. 51 plus Guinn et al. 55 (——) with patients treated with an HBOC, Hemopure, in an expanded access program (‐‐‐‐). 44 Patients treated with the HBOC had improved survival (P = .0002, HR 0.56 [95% CI: 0.32‐0.71]) [Color figure can be viewed at wileyonlinelibrary.com]
The data for important morbidity for both untransfused anemia and HBOC use when RBC transfusion is not an option, are far less robust. In the surgical populations of Carson 49 and of Shander 50 morbidity increased with Hb <6 g/dL. Beliaev et al. noted increased cardiovascular complications in their untransfused anemic patients compared with matched transfused patients. 51 , 56 Guinn et al. found in untransfused anemic patients that myocardial infarction, as defined by troponin elevation, increased with decreasing nadir Hb below 8 g/dL, with an overall rate of 10.5%. 57 The HBOC orthopedic trial 52 did not analyze the adverse event data according to nadir native Hb, f and the data provided by clinicians who treated patients with HBOC in the expanded access program is inadequate for analysis. 58
The recent report of Guinn et al. has added an additional important novel piece of information to our knowledge of the mortality associated with severe anemia: the time to death as a function of Hb. 55 The analyses above have documented well that for Hb concentrations less than 8 g/dL, decreasing Hb is associated with increased mortality. However, those analyses do not provide information regarding the window for therapy, should any be available. Guinn et al. found that decreasing Hb was not only associated with increased mortality, but also with decreased time to death. With nadir Hb ≤5 g/dL median time to death from that Hb value was 2 days; at nadir Hb 5‐8 g/dL, median time to death was 4‐6 days. 55 These times are likely maximal for the period available for therapy, should any be available, as it is probable that the anemia‐induced damage became irreversible with death inevitable some time prior to death.
The above analyses and considerations regarding efficacy and safety of use of the HBOC for severe anemia when RBC transfusion is not an option (mortality is the ultimate expression of a severe adverse event) is supported by real‐world experience emanating from South Africa, where Hemopure is approved by the national regulatory authority for use in severe anemia when blood transfusion is not an option. Levien reviewed the hemovigilance program of 336 patients who received Hemopure for acute surgical anemia owing to when blood was not an option, when blood avoidance was medically desirable, or physician or patient preference, finding no pattern of significant adverse events attributable to the HBOC. 59 No deaths were attributable to the HBOC. 59 That review and a subsequent publication from South Africa 60 included patients for whom RBCs could not be provided in a timely manner, and for whom Hemopure provided a bridge to transfusion. The South African experience led their clinical experts to provide usage guidelines. 61
Zumberg et al. have recently reviewed the medical records of all patients who received at least 10 units (250 mL/unit) of Hemopure in the U.S. expanded access program. 62 There were 10 patients, given a mean of 16 units per patient, with a mean Hb before HBOC administration of 3.3 g/dL, during a mean administration duration of 8.2 days and an average administration of 2.0 units/d. All patients survived, with the expected increase in methemoglobin concentrations and blood pressure, but without HBOC‐attributed serious cardiac, CNS, respiratory, or renal adverse events. Mackenzie et al. recently reviewed the clinical experience of 1701 patients treated with Hemopure including an analysis of observed side effects, 40 that were clinically manageable and may have been attributable to vasoconstriction mediated by free Hb combining with nitric oxide, 63 , 64 or by heme oxidation. 65 When infused into patients with proven clinically significant myocardial vascular disease, Hemopure appears not to be harmful, but rather protective. 66 , 67 A small incomplete safety trial in hemorrhagic shock in trauma, in South Africa, found no mortality difference between those randomized to receive Hemopure (4/10) and those treated with standard of care (5/9; P > .99), but a 40% lesser need for crystalloid (11.5 L/patient v 19.3 L/patient, respectively; P < .0001). g The large crystalloid volume difference is of substantial importance, as increased crystalloid administration in trauma is associated with increased mortality. 69
The data presented and discussed suggest that HBOCs could fill the critical and unmet role of an emergency supply of oxygen carrying support during a catastrophe such as a nuclear event, military or environmental disaster, or pandemic. Other potential solutions have significant shortcomings. Depots of RBC stored at 4C would be useful for only 42 days based on current shelf life limitations, requiring substantial inventory management manipulations and inherent outdating. Freezing RBC would also not be a satisfactory solution owing to the need to remove glycerol with advanced technical equipment and support.
On the other hand, HBOCs can be stored for years at room temperature and could potentially be stored in a lyophilized state for longer periods, but immediately available for reconstitution with water for infusion. Although HBOCs could be available for rapid use in emergencies, their in vivo life span of only 12‐24 hours is a problematic shortcoming. Consequently, the HBOC discussed is suitable as a short‐term solution for acute anemia, but for chronic anemia only with repeated dosing to serve as a bridge until an alternate therapy is available. It is also possible that continued development efforts could be undertaken to develop prolonged in vivo survival of HBOCs and even more protracted storage life. Other side‐effects include nitric oxide‐induced vasoconstriction (as discussed above), underdosing, and volume overloading as may occur with the administration of any intravenous fluid, including rbcs or plasma. Nevertheless, our assessment is that for the described potential use, when red cell transfusion is not an option, the benefit:risk profile for the treatment of severe anemia by administration of the HBOC discussed is favorable, in comparison to that of untreated severe anemia.
In conclusion, there are international needs for ensuring the large‐scale availability of an artificial oxygen carrier for use when the civilian or military blood collection, processing, or delivery systems are degraded. The COVID‐19 pandemic has shown how vulnerable health care systems are to major disasters in the absence of proper preparation. Freeze‐dried plasma is approved in some countries, but HBOCs are approved only in two: South Africa and Russia.d There are products (FDP and HBOC) not approved in most countries that can be so positioned now, for such emergency purposes. It is our opinion that the evidence supports that the relatively small risk of doing so with these products even where unapproved, for emergency use, greatly outweighs the risk of untreated severe anemia or decrease in coagulation factors.
Key Message
|
STATEMENTS
All authors participated in the writing of this manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. No others contributed to the writing in any manner. No medical writers or editors have been involved in any way. RBW produced the figures. Nobody other than the authors have influenced the writing of this manuscript.
No funding or compensation of any kind (including from NIH, Wellcome Trust, or HHMI) was received by any of the authors for this manuscript.
The corresponding author confirms that he has had full access to all data and has had final responsibility for the decision to submit this manuscript for publication.
This manuscript has not been published elsewhere.
CONFLICTS OF INTEREST
R.B. Weiskopf has consulted for sponsors of hemoglobin‐based oxygen carriers in the past, but has not received any compensation from these entities in the past 3 years.
M.F.M. James has consulted with sponsors of hemoglobin‐based oxygen carriers in the past but has received no financial compensation for these activities at any time.
P. Ness has consulted for sponsors of hemoglobin‐based oxygen carriers in the past, but has not received any compensation from these entities in the past 3 years.
E. Glassberg, N. Guinn, and A.E. Pusateri declare no conflicts of interest.
Footnotes
The Las Vegas shooting injuries, perpetrated by only a single person, required 278 RBC units transfused, 67% in the first 24 hours, met by fully stocked in‐hospital and local blood centers’ supplies. 11
The nuclear disaster at Chernobyl 15 , 16 , 17 , 18 was an operator‐ and design‐caused steam explosion followed by a fire and a nine‐to‐ten day release of a substantial quantity of radioactive material with resulting long‐term medical issues including bone marrow suppression, but with relatively lesser immediate transfusion need, with two deaths in the first 5 hours, and apparently 31 in the first three days, predominantly from burns.
An HHS‐commissioned RAND study 23 concluded that disruption of the U.S. blood supply was unlikely, but, as pointed out by Klein et al. 22 that was shown to be off the mark when the system could not function properly during the Zika virus outbreak without a major financial infusion by the U.S. government.
Registration in Russia is currently inactive pending rebuilding of the manufacturing plant.
It is of interest to note that whole blood or packed red blood cells have never undergone the standard FDA process of approval based on data from randomized clinical trials to demonstrate efficacy and safety. Rather, it was recommended to be accepted in 1985 based on published and submitted information and “common experience.” 41 For further elucidation of this issue, see Weiskopf. 42
There was a greater SAE rate in the HBOC group compared with PRBCs, with most attributed to fluid overload or undertreatment.
This trial was apparently designed with a sample size of 50, but stopped because of slow recruitment and resultant financial issues. Limited data are available. 68 The P values shown here are those we calculated from the data presented; they differ from those in this cited reference.
REFERENCES
- 1. American Red Cross . American Red Cross faces a severe blood shortage as coronavirus outbreak threatens availability of nationʼs supply. 2020.
- 2. Pagano MB, Hess JR, Tsang HC, et al. Prepare to adapt: Blood supply and transfusion support during the first 2 weeks of the 2019 Novel Coronavirus (COVID‐19) pandemic affecting Washington State. Transfusion. 2020;60:908–911. [DOI] [PubMed] [Google Scholar]
- 3. Tsubokura M, Nakada H, Matsumura T, et al. The impact of H1N1 influenza A virus pandemic on the blood donations in Hyogo Prefecture, Japan. Transfusion. 2010;50:1803–1805. [DOI] [PubMed] [Google Scholar]
- 4. Shan H, Zhang P. Viral attacks on the blood supply: the impact of severe acute respiratory syndrome in Beijing. Transfusion. 2004;44:467–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chang L, Zhao L, Gong H, Wang L, Wang L. Severe acute respiratory syndrome coronavirus 2 RNA detected in blood donations. Emerg Infect Dis. 2020;26:1631–1633. 10.3201/eid2607.200839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Linden JV, Davey RJ, Burch JW. The September 11, 2001 disaster and the New York blood supply. Transfusion. 2002;42:1385–1387. [DOI] [PubMed] [Google Scholar]
- 7. Jones RL. September eleventh. Vox Sang. 2002;83(suppl 1):363–365. [DOI] [PubMed] [Google Scholar]
- 8. Glynn SA, Busch MP, Schreiber GB, et al. Effect of a national disaster on blood supply and safety: the September 11 experience. JAMA. 2003;289:2246–2253. [DOI] [PubMed] [Google Scholar]
- 9. Simonetti A, Ezzeldin H, Walderhaug M, Anderson SA, Forshee RA. An inter‐regional US blood supply simulation model to evaluate blood availability to support planning for emergency preparedness and medical countermeasures. Disaster Med Public Health Prep. 2018;12:201–210. [DOI] [PubMed] [Google Scholar]
- 10. Schmidt PJ. Blood and disaster‐‐supply and demand. N Engl J Med. 2002;346:617–620. [DOI] [PubMed] [Google Scholar]
- 11. Lozada MJ, Cai S, Li M, Davidson SL, Nix J, Ramsey G. The Las Vegas mass shooting: An analysis of blood component administration and blood bank donations. J Trauma Acute Care Surg. 2019;86:128–133. [DOI] [PubMed] [Google Scholar]
- 12. Gevirtz C. Blood and disaster. N Engl J Med. 2002;347:68–69; author reply −9. [PubMed] [Google Scholar]
- 13. Pusateri AE, Given MB, Macdonald VW, Homer MJ. Comprehensive US government program for dried plasma development. Transfusion. 2016;56(suppl 1):S16–S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pusateri AE, Homer MJ, Rasmussen TE, Kupferer KR, Hoots WK. The interagency strategic plan for research and development of blood products and related technologies for trauma care and emergency preparedness 2015‐2020. Am J Disaster Med. 2018;13:181–194. [DOI] [PubMed] [Google Scholar]
- 15. Gale RP. Immediate medical consequences of nuclear accidents. Lessons from Chernobyl. JAMA. 1987;258:625–628. [PubMed] [Google Scholar]
- 16. Anspaugh LR, Catlin RJ, Goldman M. The global impact of the Chernobyl reactor accident. Science. 1988;242:1513–1519. [DOI] [PubMed] [Google Scholar]
- 17. Young RW. Chernobyl in retrospect. Pharmacol Ther. 1988;39:27–32. [DOI] [PubMed] [Google Scholar]
- 18. United Nations Scientific Committee on the Effects of Atomic Radiation . The Chernobyl accident. [cited 2020 Aug 26]. Available from: https://wwwunscearorg/unscear/en/chernobylhtml.
- 19. Committee on Medical Preparedness for a Terrorist Nuclear Event of the Institute of Medicine of the National Academies . Assessing medical preparedness to respond to a terrorist nuclear event. workshop report. Washington, DC: The National Academies Press, 2009. [PubMed] [Google Scholar]
- 20. National Security Staff Interagency Policy Coordination Subcommittee for Preparedness & Response to Radiological and Nuclear Threats . Planning Guidance for Response to a Nuclear Detonation, 2nd ed. 2010.
- 21. U.S. Department of Health and Human Services . Medical planning and response manual for a nuclear detonation incident: A practical guide. 2011.
- 22. Klein HG, Hrouda JC, Epstein JS. Crisis in the sustainability of the U.S. blood system. N Engl J Med. 2017;377:1485–1488. [DOI] [PubMed] [Google Scholar]
- 23. Mulcahy AW, Kapinos KA, Briscombe B, et al. Toward a sustainable blood supply in the United States. An analysis of the current system and alternatives for the future. Santa Monica, CA: RAND Corporation, 2016. [Google Scholar]
- 24. Pusateri AE, Butler F, Shackelford SA, et al. The need for dried plasma — a national issue. Transfusion. 2019;59:1587–1592. [DOI] [PubMed] [Google Scholar]
- 25. Cannan R, Redish J. The large‐scale production of crystalline human hemoglobin: with preliminary observations on the effect of its injection in man. In: Mudd S, Thalhimer W, editors. Blood substitutes and blood transfusion. Springfield: Thomas, 1942. [Google Scholar]
- 26. Amberson W, Jennings J, Rhode C. Clinical experience with hemoglobin‐saline solutions. J Appl Physiol. 1949;1:469–489. [DOI] [PubMed] [Google Scholar]
- 27. Clark L, Gollan R. Survival of mammals breathing organic liquids equilibrated with oxygen a atmospheric pressure. Science. 1966;152:1755–1756. [DOI] [PubMed] [Google Scholar]
- 28. Aach RD, Szmuness W, Mosley JW, et al. Serum alanine aminotransferase of donors in relation to the risk of non‐A, non‐B hepatitis in recipients: the transfusion‐transmitted viruses study. N Engl J Med. 1981;304:989–994. [DOI] [PubMed] [Google Scholar]
- 29. Hollinger FB, Alter HJ, Holland PV, Aach RD. Non‐A, non‐B posttransfusion hepatitis in the United States. In: Gerety RJ, editor. Non‐A, non‐B hepatitis. New York, NY: Academic Press, 1981; p. 49–70. [Google Scholar]
- 30. Hollinger F, Mosley J, Szmuness W, et al. Non‐A, Non‐B hepatitis following blood transfusion: risk factors associated with donor characteristics. In: Szmuness W, Alter H, Maynard J, editors. Viral Hepatitis 1981 International Symposium. Philadelphia: The Franklin Institute Press, 1982; p. 361–376.
- 31. Curran JW, Lawrence DN, Jaffe H, et al. Acquired immunodeficiency syndrome (AIDS) associated with transfusions. N Engl J Med. 1984;310:69–75. [DOI] [PubMed] [Google Scholar]
- 32. Busch M, Glynn S, Stramer S, et al. A new strategy for estimating risks of transfusion‐transmittted viral infections based on rates of detection of recently infected donors. Transfusion. 2005;45:254–264. [DOI] [PubMed] [Google Scholar]
- 33. Silverman TA, Weiskopf RB, for the Planning Committee and Speakers . Hemoglobin based oxygen carriers: Current status and future directions. Anesthesiology. 2009;111:946–963. [DOI] [PubMed] [Google Scholar]
- 34. Natanson C, Kern SJ, Lurie P, Banks SM, Wolfe SM. Cell‐free hemoglobin‐based blood substitutes and risk of myocardial infarction and death: A meta‐analysis. JAMA. 2008;299:2304–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Levien LJ, Hodgson RE, James MFM. Hemoglobin‐based blood substitutes and risk of myocardial infarction and death. JAMA. 2008;300:1295. [DOI] [PubMed] [Google Scholar]
- 36. Keipert PE. Hemoglobin‐based blood substitutes and risk of myocardial infarction and death. JAMA. 2008;300:1295–1296. [DOI] [PubMed] [Google Scholar]
- 37. Lewis RJ, Fost N. Hemoglobin‐based blood substitutes and risk of myocardial infarction and death. JAMA. 2008;300:1296. [DOI] [PubMed] [Google Scholar]
- 38. Shander A, Thompson G. Hemoglobin‐based blood substitutes and risk of myocardial infarction and death. JAMA. 2008;300:1296–1297. [DOI] [PubMed] [Google Scholar]
- 39. Sauaia A, Moore EE, Banerjee A. Hemoglobin‐based blood substitutes and risk of myocardial infarction and death. JAMA. 2008;300:1297. [DOI] [PubMed] [Google Scholar]
- 40. Mackenzie C, Dube GP, Pitman A, Zafirelis M. Users guide to pitfalls and lessons learned about HBOC‐201 during clinical trials, expanded access, and clinical use in 1,701 patients. Shock. 2019;52(1S suppl 1):92–99. [DOI] [PubMed] [Google Scholar]
- 41. Food and Drug Administration . 21 CFR Parts 606, 610, and 640. Biological products; blood and blood derivatives; implementation of efficacy review. 1985:50FR52602‐723.
- 42. Weiskopf RB. The efficacy and safety of liquid stored blood and storage duration: a confused subject; are patients confused? Anesth Analg. 2014;119:224–229. [DOI] [PubMed] [Google Scholar]
- 43. Hill‐Pryor C, Pusateri AE, Weiskopf RB. Hemoglobin‐based oxygen carriers (HBOC)‐what the next generation holds: When red blood cells are not an option. Shock. 2019;52:4–6. [DOI] [PubMed] [Google Scholar]
- 44. Weiskopf RB, Beliaev AM, Shander A, et al. Addressing the unmet need of life‐threatening anemia with hemoglobin‐based oxygen carriers. Transfusion. 2017;57:207–214. [DOI] [PubMed] [Google Scholar]
- 45. Weiskopf RB, Kramer JH, Viele M, et al. Acute severe isovolemic anemia impairs cognitive function and memory in humans. Anesthesiology. 2000;92:1646–1652. [DOI] [PubMed] [Google Scholar]
- 46. Weiskopf RB, Feiner J, Hopf HW, et al. Oxygen reverses deficits of cognitive function and memory and increased heart rate induced by acute severe isovolemic anemia. Anesthesiology. 2002;96:871–877. [DOI] [PubMed] [Google Scholar]
- 47. Weiskopf RB, Feiner J, Hopf H, et al. Fresh blood and aged stored blood are equally efficacious in immediately reversing anemia‐induced brain oxygenation deficits in humans. Anesthesiology. 2006;104:911–920. [DOI] [PubMed] [Google Scholar]
- 48. Weiskopf RB, Viele MK, Feiner J, et al. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA. 1998;279:217–221. [DOI] [PubMed] [Google Scholar]
- 49. Carson JL, Noveck H, Berlin JA, Gould SA. Mortality and morbidity in patients with very low postoperative Hb levels who decline blood transfusion. Transfusion. 2002;42:812–818. [DOI] [PubMed] [Google Scholar]
- 50. Shander A, Javidroozi M, Naqvi S, et al. An update on mortality and morbidity in patients with very low postoperative hemoglobin levels who decline blood transfusion (CME). Transfusion. 2014;54:2688–2695. [DOI] [PubMed] [Google Scholar]
- 51. Beliaev AM, Marshall RJ, Gordon M, Smith W, Windsor JA. Clinical benefits and cost‐effectiveness of allogeneic red‐blood‐cell transfusion in severe symptomatic anaemia. Vox Sang. 2012;103:18–24. [DOI] [PubMed] [Google Scholar]
- 52. Jahr JS, Mackenzie C, Pearce LB, Pitman A, Greenburg AG. HBOC‐201 as an alternative to blood transfusion: efficacy and safety evaluation in a multicenter phase III trial in elective orthopedic surgery. J Trauma. 2008;64:1484–1497. [DOI] [PubMed] [Google Scholar]
- 53. Food and Drug Administration . 21 CFR Parts 312 and 316: Expanded access to investigational drugs for treatment use. 2009:74FR40900.
- 54. DHHS US, FDA, CDER, CBER . Guidance for Industry: Individual Patient Expanded Access Applications: Form FDA 392. 2017.
- 55. Guinn NR, Cooter ML, Weiskopf R. Lower hemoglobin concentration decreases time to death in severely anemic patients for whom blood transfusion is not an option. J Trauma Acute Care Surg. 2020;88:803–808. [DOI] [PubMed] [Google Scholar]
- 56. Beliaev AM, Marshall RJ, Smith W, Windsor JA. Mortality risk stratification in severely anaemic Jehovahʼs Witness patients. Intern Med J. 2012;42:e1–e3. [DOI] [PubMed] [Google Scholar]
- 57. Guinn NR, Cooter ML, Villalpando C, Weiskopf RB. Severe anemia associated with increased risk of death and myocardial ischemia in patients declining blood transfusion. Transfusion. 2018;58:2290–2296. [DOI] [PubMed] [Google Scholar]
- 58. Zafirelis Z. Personal communication 2020.
- 59. Levien LJ. South Africa: Clinical experience with Hemopure. ISBT Sci Ser. 2006;1:167–173. [Google Scholar]
- 60. Potgieter H, James M. The use of Hemopure(R) at Groote Schuur Hospital, Cape Town: 4 cases studies. South Afr J Anaesth Analg. 2009;15:13–15. [Google Scholar]
- 61. Mer M, Hodgson E, Wallis L, et al. Hemoglobin glutamer‐250 (bovine) in South Africa: Consensus usage guidelines from clinician experts who have treated patients. Transfusion. 2016;56:2631–2636. [DOI] [PubMed] [Google Scholar]
- 62. Zumberg M, Gorlin J, Griffiths EA, et al. A case study of 10 patients administered HBOC‐201 in high doses over a prolonged period: Outcomes during severe anemia when transfusion is not an option. Transfusion. 2020;60:932–939. [DOI] [PubMed] [Google Scholar]
- 63. Carlsen E, Comroe JH Jr. The rate of uptake of carbon monoxide and of nitric oxide by normal human erythrocytes and experimentally produced spherocytes. J Gen Physiol. 1958;42:83–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Viele MK, Weiskopf RB, Fisher D. Recombinant human hemoglobin does not affect renal function in humans: analysis of safety and pharmacokinetics. Anesthesiology. 1997;86:848–858. [DOI] [PubMed] [Google Scholar]
- 65. Mollan TL, Alayash AI. Redox reactions of hemoglobin: mechanisms of toxicity and control. Antioxid Redox Signal. 2013;18:2251–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Meliga E, Vranckx P, Regar E, Kint PP, Duncker DJ, Serruys PW. Proof‐of‐concept trial to evaluate haemoglobin based oxygen therapeutics in elective percutaneous coronary revascularisation. Rationale, protocol design and haemodynamic results. EuroIntervention. 2008;4:99–107. [DOI] [PubMed] [Google Scholar]
- 67. Serruys PW, Vranckx P, Slagboom T, et al. Haemodynamic effects, safety, and tolerability of haemoglobin‐based oxygen carrier‐201 in patients undergoing PCI for CAD. EuroIntervention. 2008;3:600–609. [DOI] [PubMed] [Google Scholar]
- 68. Briefing book for the 14 December 2006 Blood Products Advisory Committee (BPAC) meeting . 2006. Available from: https://wayback.archive‐it.org/7993/20170405060614/https://www.fda.gov/ohrms/dockets/ac/06/briefing/2006‐4270B1‐11.pdf. Accessed on 16 March 2020.
- 69. Jones DG, Nantais J, Rezende‐Neto JB, Yazdani S, Vegas P, Rizoli S. Crystalloid resuscitation in trauma patients: deleterious effect of 5L or more in the first 24h. BMC Surg. 2018;18:93. [DOI] [PMC free article] [PubMed] [Google Scholar]


