Abstract
Background and Purpose
The coronavirus disease 2019 (COVID‐19) pandemic challenges neurologists in counseling multiple sclerosis (MS) patients with respect to their risk for and by severe acute respiratory syndrome coronavirus 2 and in guiding disease‐modifying treatment (DMT). The objective was to determine the frequency and distribution of currently known risk factors for COVID‐19 mortality in an MS population.
Methods
Multiple sclerosis patients with at least one complete case report between January 1, 2015 and December 31, 2019 from the Innsbruck MS database were cross‐sectionally included. Frequencies of currently estimated COVID‐19 mortality risk factors were analyzed, and the cumulative risk was calculated by a recently developed score. For every risk group, the proportions of patients under DMT and immunosuppressive treatment were determined.
Results
Of 1931 MS patients, 63.4% had low risk of COVID‐19 mortality, 26% had mild risk, 8.8% had a moderate risk, whereas a combined 0.9% had high or very high risk of COVID‐19 mortality. Of the patients at high or very high risk, only one patient received DMT and none had an immunosuppressive therapy.
Conclusions
In a population‐based MS cohort, the proportion of patients at high risk of COVID‐19 mortality is below 1%. Importantly, the vast majority of these MS patients did not receive any DMT.
Keywords: comorbidities, COVID‐19, multiple sclerosis, risk, SARS‐CoV‐2
The coronavirus disease 2019 (COVID‐19) pandemic challenges neurologists in counseling multiple sclerosis (MS) patients with respect to their risk by severe acute respiratory syndrome coronavirus 2 and in guiding disease‐modifying treatment (DMT). Risk of COVID‐19 mortality and the respective proportions of patients under DMT and immunosuppressive treatment according to mortality risk were analyzed in a population‐based cohort of 1931 MS patients. Only 0.8% of MS patients displayed a high risk of COVID‐19 mortality, with less than 1% of these patients receiving DMT or immunosuppressive therapy.
![]()
INTRODUCTION
The pandemic spread of the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), with more than 25 million confirmed infections and more than 840,000 deaths as of August 31, 2020 from the consequences of the virus‐associated respiratory disease (coronavirus disease 2019 [COVID‐19]), has raised health concerns for patients with multiple sclerosis (MS). In Austria, with about 8.5 million inhabitants, there are more than 27,000 confirmed SARS‐COV‐2 infections, with over 700 deaths from COVID‐19 and about 14,500 MS patients [1].
Severity and mortality of COVID‐19 have varied greatly but appear to be strongly influenced by age, preexisting comorbidities, and thereby associated with certain therapies, in particular by ongoing immunosuppressive treatments [2, 3]. Although MS is typically diagnosed in young adults, a substantial number of individuals with MS are older than 60 years, a population with a demonstrated increased risk of severe morbidity and mortality from COVID‐19 [3, 4, 5, 6]. Immunomodulatory therapies and particularly, immunosuppressive therapies used in the treatment of MS, qualitatively and quantitatively alter the components of the immune system and are to some extent associated with a greater infectious risk, especially concerning viral pathogens [7].
Managing MS during the COVID‐19 pandemic poses an exceptional challenge with little published experience, no evidence‐based guidelines, and mounting pressure from patients and caregivers longing for specific counseling. The objective of this study was to determine the frequency and distribution of currently known risk factors as well as the cumulative risk for COVID‐19 mortality in a MS population in relation to the use of disease‐modifying treatment (DMT).
METHODS
As previously described in detail, the Innsbruck MS database (IMSD) was established at the MS Clinic of the Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria, which serves as both the primary and secondary reference center mainly for Western Austria and its catchment area [8]. The study was approved by the ethics committee of the Medical University Innsbruck (ethical approval number: AM3743‐281/4.3), and written informed consent was obtained from every patient. IMSD comprises a cohort of 2468 patients diagnosed with MS according to McDonald diagnostic criteria [9, 10, 11]. Given the prevalence of MS (148 per 100,000 people) and a population of about 1.6 million people in Western Austria, this database is likely to include most of the MS patients from this catchment area [1, 12]. IMSD case reports include demographic data, details of MS course, DMT history, and a detailed documentation of prior and current comorbidities. Comorbidities were confirmed by the respective medical reports stating the diagnosis or corroborated by appropriate comedication.
For this cross‐sectional study, we included all MS patients with at least one complete case report between January 1, 2015 and December 31, 2019. We extracted reported demographic and clinical factors, which are currently estimated as potential risk factors for COVID‐19 mortality: age, physical disability (Expanded Disability Status Scale [EDSS] score), smoking status, obesity (body mass index ≥30), DMT status, and presence of cardiovascular disease (coronary heart disease and/or ischemic heart failure and/or cardiac valve disease), chronic pulmonary disease (asthma or obstructive pulmonary disease [COPD]), diabetes, chronic kidney disease, and current malignancy.
Patients were classified regarding their risk of COVID‐19 mortality according to a modified version of a recently developed risk score categorizing patients as having either low (<1%), mild (<5%), moderate (~15%), high (~30%), or very high risk (~50%) of COVID‐19 mortality (Table 1) [13]. We analyzed the frequency of risk factors in the whole cohort and in three age subgroups (<40 years, 40–65 years, >65 years), and we calculated the proportion of the cohort in each COVID‐19 mortality risk group and contrasted that to the proportion of patients with DMT and, specifically, immunosuppressive treatment (alemtuzumab, cladribine, mitoxantrone, ocrelizumab, or rituximab).
TABLE 1.
Modified COVID‐19 mortality risk score
| Factor | Score |
| Diabetes and age <40 years | 5 |
| Age ≥65 years | 3 |
| Chronic kidney disease | 3 |
| Severe physical disability (EDSS >6) | 2 |
| Chronic obstructive pulmonary disease | 1 |
| Cardiovascular disease | 1 |
| Current malignancy | 1 |
| Obesity (BMI ≥30) | 1 |
| Diabetes | 1 |
| Smoking | 1 |
| Age <40 years | −6 |
| Risk category | Score interval |
| Low risk | ≤0 |
| Mild risk | 1–3 |
| Moderate risk | 4–7 |
| High risk | 8–11 |
| Very high risk | ≥12 |
Modified according to Bello‐Chavolla et al [13].
Abbreviations: BMI, body mass index; EDSS, Expanded Disability Status Scale.
Color shades correspond to the level of risk.
Statistical analysis
Statistical analysis was performed using SPSS 25.0 (IBM, Armonk, NY). Categorical variables were expressed in frequencies and percentages, and continuous variables as mean and standard deviation or median and range as appropriate. Frequencies of risk factors were compared across age groups by χ 2 test.
RESULTS
The inclusion process is depicted in detail in Figure 1. Characteristics of the final study cohort consisting of 1,931 MS patients are given in Table 2.
FIGURE 1.
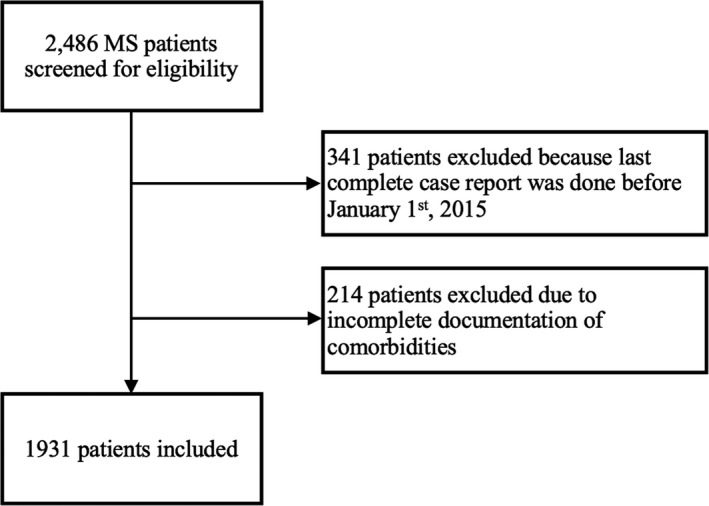
Study inclusion flowchart. MS, multiple sclerosis
TABLE 2.
Demographics and clinical characteristics of the cohort
| Whole cohort, n = 1931 | |
|---|---|
| Females a | 1394 (72.2) |
| Age, years b | 45 (15–88) |
| Disease duration, years b | 15 (1–54) |
| RRMS a | 1214 (62.9) |
| SPMS a | 578 (29.9) |
| PPMS a | 139 (7.2) |
| EDSS | 3.0 (0–9.5) |
| Current DMT | 1198 (62.0) |
| Immunosuppressive treatment | 91 (4.7) |
Abbreviations: DMT, disease‐modifying therapy; EDSS, Expanded Disability Status Scale; MS, multiple sclerosis; PPMS, primary progressive MS; RRMS, relapsing‐remitting MS; SPMS, secondary progressive MS.
Number (percentage).
Median and range.
The frequency of risk factors for COVID‐19 mortality in the whole MS cohort and their distribution among the different age categories are shown in Table 3. Prevalence of cardiovascular disease, chronic obstructive pulmonary disease, chronic kidney disease, diabetes, and concurrent malignancy as well as the proportion of patients with severe physical disability due to MS (i.e. EDSS > 6) significantly increased with age. The frequency of obesity and smoking did not significantly differ across age groups, whereas the rate of patients receiving DMT significantly decreased with increasing age.
TABLE 3.
Risk factors for COVID‐19 mortality in the multiple sclerosis population and age subgroups
| Whole cohort, n = 1,931 | Age group <40 years, n = 967 | Age group 40–65 years, n = 611 | Age group >65 years, n = 353 | p value b | |
|---|---|---|---|---|---|
| Cardiovascular disease a | 48 (2.5) | 3 (0.3) | 10 (1.6) | 35 (9.9) | <0.001 |
| Chronic obstructive pulmonary disease a | 116 (6.0) | 21 (2.2) | 37 (6.1) | 58 (16.5) | <0.001 |
| Chronic kidney disease a | 21 (1.1) | 1 (0.1) | 4 (0.6) | 16 (4.5) | <0.001 |
| Diabetes a | 119 (6.2) | 22 (2.3) | 45 (7.4) | 52 (14.7) | <0.001 |
| Obesity, BMI >30 a | 235 (12.2) | 111 (11.5) | 70 (11.5) | 54 (15.3) | 0.139 |
| Current malignancy a | 24 (1.2) | 2 (0.2) | 9 (1.5) | 13 (3.7) | <0.001 |
| Smoking a | 502 (26.0) | 234 (24.2) | 171 (28.0) | 97 (27.5) | 0.193 |
| EDSS >6 a | 335 (17.3) | 94 (9.7) | 128 (21.0) | 113 (32.0) | <0.001 |
| Receiving DMT a | 1198 (62.0) | 791 (81.2) | 353 (57.8) | 54 (15.3) | <0.001 |
Abbreviations: BMI, body mass index; DMT, disease‐modifying therapy; EDSS, Expanded Disability Status Scale.
Number (percentage).
Calculated by χ 2 test across age groups.
Calculating the COVID‐19 mortality risk according to the score, 63.4% had low risk and another 26% mild risk, whereas a combined 0.9% had high or very high risk of COVID‐19 mortality. Of the patients at high or very high risk, only one patient was under DMT, and none received immunosuppressive treatment (Table 4).
TABLE 4.
Distribution of patients at risk for COVID‐19 mortality with proportions under DMT
| Risk category | Patients at risk | Proportion under DMT | Proportion under immunosuppressive treatment |
|---|---|---|---|
| Low risk (score ≤0) | 1224 (63.4) | 863 (70.5) | 69 (5.6) |
| Mild risk (score 1–3) | 501 (26.0) | 291 (58.1) | 15 (2.9) |
| Moderate risk (score 4–7) | 170 (8.8) | 30 (17.7) | 7 (4.1) |
| High risk (score 8–11) | 13 (0.7) | 1 (8.3) | 0 (0) |
| Very high risk (score ≥12) | 3 (0.2) | 0 (0) | 0 (0) |
Immunosuppressive treatment, alemtuzumab, cladribine, mitoxantrone, ocrelizumab or rituximab.
Abbreviation: DMT, disease‐modifying therapy.
Color shades correspond to the level of risk.
DISCUSSION
Evidence regarding the specific morbidity and mortality from COVID‐19 in patients with MS with or without DMT is scarce. There are some anecdotal reports on the course of COVID‐19 in MS patients including some on commonly used DMTs, suggesting that MS patients do not have an increased risk of contracting symptomatic COVID‐19 or fatality from COVID‐19 compared with the population at large [14]. Recently, neurological disability, age, and obesity were described as risk factors for severe forms of COVID‐19 in a cohort study of 347 MS patients, but no association was found between DMT exposure and COVID‐19 severity [15]. Also, MS patients generally do not display a propensity for increased morbidity or mortality from other viral pathogens [14]. Thus, one can assume that the currently known risk factors in the general population can be extrapolated to the MS population.
In general, age ≥65 years seems to be the most important demographic risk factor for COVID‐19 severity and mortality [2, 3, 16]. Estimates indicate that up to 10% to 20% of MS patients are aged ≥65 years, with 18% in our cohort [6]. Whether increased COVID‐19 mortality in older patients reflects impaired regulation of immune responses and whether this may be accentuated or mitigated in patients with autoimmune diseases pertaining to some immunoregulatory deficits remains to be elucidated [14]. Besides age, presence of comorbidities (i.e. cardiovascular disease, COPD, chronic kidney disease, malignancy, obesity, and smoking) is associated with increased risk for COVID‐19 severity and mortality [2, 3, 16, 17]. The prevalence of these risk factors found in our cohort matches well the reported prevalence in MS populations [18, 19, 20, 21]. With respect to this prevalence and considering the population‐based nature of the IMSD cohort capturing more than 80% of the theoretical MS prevalence in Western Austria, our cohort is likely representative of a European, primarily Caucasian, MS population [1, 8, 12, 22].
In recent months, there have been various attempts to quantify the cumulative risk for COVID‐19 severity/mortality. Most of the studies combined demographic data and comorbidities with clinical, laboratory‐chemical, or radiographic findings at admission, which is not suitable for quantifying the risk in the general population. Hence, we used a recently developed risk score categorizing patients' risk of COVID‐19 mortality based on age and comorbidities [13]. The score was modified by adding severe physical disability (EDSS ≥6) as an additional risk factor relatively weighted according to a recent publication indicating that an EDSS ≥6 was associated with a twofold increased risk of severe COVID‐19 [15].
Encouragingly, the proportion of patients theoretically at high risk of COVID‐19 mortality if they contract the virus is below 1%, which is within the range of global mortality reports averaging about 3% in the general population [23]. Although there is no evidence‐based guidance, various expert committees have published recommendations on how to manage MS patients and DMT during the COVID‐19 pandemic. The initial approaches offered very conservative advice, with some even suggesting discontinuation of DMT, whereas published guidelines have now become less cautious as assuring experiences are growing [24]. Our data add to that, as they show that the overwhelming proportion of MS patients are not to be considered at high risk of COVID‐19 mortality. Also, most of the high‐risk group is not even receiving DMT, thus rendering the question of stopping DMT in patients with high risk of COVID‐19 mortality somewhat marginal. Theoretical considerations suggest that DMTs might even mitigate the overshooting immune response, which is likely pathophysiologically underlying the development of acute respiratory distress syndrome (ARDS) in COVID‐19 [14]. However, there are no conclusive data regarding the effect of DMTs on the frequency of serious COVID‐19 complications, including ARDS, to date. Still, in patients with low to moderate risk of COVID‐19 mortality, the benefit–risk ratio seems to be in favor of at least continuing and likely also initiating DMT when indicated by the course of MS in the respective individual patient. Importantly, the standard of care for MS patients including continuation of safety monitoring procedures needs to be upheld as far as possible depending on local circumstances [14, 24]. As always, treatment decisions should be extensively discussed with patients and individually tailored, taking into account all MS‐specific parameters as well as, for example, comorbidities, social circumstances, and personal risk perception [25].
As a limitation of this study, our data provide risk estimates based on theoretical weighting as opposed to actually observing the morbidity and mortality of COVID‐19 in MS patients. Also, the risk score applied was modified to include all the relevant reported demographic and comorbidity‐derived risk factors without formally validating the modifications. Furthermore, the original score was developed in a Mexican population, which might limit applicability to other ethnicities, especially if the distribution of risk factors varies between populations. Thus, our results naturally have to be interpreted cautiously. However, the goal of this study was to provide a general impression of the proportion of MS patients at risk rather than exact risk estimates.
In conclusion, we showed in a population‐based MS cohort that the proportion of patients at high risk of COVID‐19 mortality is below 1%, with the vast majority of the high‐risk group not receiving DMT, let alone immunosuppressive treatment.
CONFLICT OF INTEREST
G.B. has participated in meetings sponsored by and received speaker honoraria or travel funding from Biogen, Celgene, Merck, Novartis, Roche, Sanofi‐Genzyme, and Teva, and received honoraria for consulting from Biogen, Roche, and Teva. C.B. has nothing to disclose. H.H. has participated in meetings sponsored by and received speaker honoraria or travel funding from Bayer, Biogen, Merck, Novartis, Roche, Sanofi‐Genzyme, Siemens, and Teva, and received honoraria for consulting from Biogen and Teva. M.A. received speaker honoraria and/or travel grants from Biogen, Merck, Novartis, and Sanofi Genzyme. F.D.P. has participated in meetings sponsored by and received honoraria (lectures, advisory boards, consultations) or travel funding from Bayer, Biogen, Celgene, Merck, Novartis, Sanofi‐Genzyme, Roche, and Teva. P.R. has received honoraria for consultancy/speaking from AbbVie, Allmiral, Alexion, Biogen, Merck, Novartis, Roche, Sandoz, and Sanofi Genzyme, and has received research grants from Amicus, Biogen, Merck, Roche. F.D. has participated in meetings sponsored by or received honoraria for acting as an advisor/speaker for Almirall, Alexion, Biogen, Celgene, Genzyme‐Sanofi, Merck, Novartis, Roche, and Teva. His institution has received research grants from Biogen and Genzyme Sanofi. He is section editor of the MSARD journal (Multiple Sclerosis and Related Disorders). T.B. has participated in meetings sponsored by and received honoraria (lectures, advisory boards, consultations) from pharmaceutical companies marketing treatments for MS: Allergan, Bayer, Biogen, Bionorica, Celgene, MedDay, Merck, Novartis, Octapharma, Roche, Sanofi‐Genzyme, and Teva. His institution has received financial support in the past 12 months by unrestricted research grants from Bayer, Biogen, Merck, Novartis, Sanofi Aventis, and Teva, and for participation in clinical trials in multiple sclerosis sponsored by Alexion, Bayer, Biogen, Merck, Novartis, Octapharma, Roche, Sanofi‐Genzyme, and Teva.
AUTHOR CONTRIBUTIONS
Gabriel Bsteh: Conceptualization (lead); Data curation (lead); Formal analysis (lead); Methodology (lead); Writing‐original draft (lead). Christina Bitschnau: Data curation (equal); Writing‐review & editing (equal).
Bsteh G, Bitschnau C, Hegen H, et al. Multiple sclerosis and COVID‐19: How many are at risk? Eur J Neurol. 2021;28:3369–3374. 10.1111/ene.14555
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request from a qualified researcher.
REFERENCES
- 1. Salhofer‐Polanyi S, Cetin H, Leutmezer F, et al. Epidemiology of multiple sclerosis in Austria. Neuroepidemiology. 2017;49:40‐44. [DOI] [PubMed] [Google Scholar]
- 2. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. New Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical features of 69 cases with Coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71:769‐777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Compston A, Coles A. Multiple sclerosis. Lancet. 2002;359:1221‐1231. [DOI] [PubMed] [Google Scholar]
- 6. Minden SL, Frankel D, Hadden LS, Srinath KP, Perloff JN. Disability in elderly people with multiple sclerosis: an analysis of baseline data from the Sonya Slifka Longitudinal Multiple Sclerosis Study. NeuroRehabilitation. 2004;19:55‐67. [PubMed] [Google Scholar]
- 7. Grebenciucova E, Pruitt A. Infections in patients receiving multiple sclerosis disease‐modifying therapies. Curr Neurol Neurosci. 2017;17:88. [DOI] [PubMed] [Google Scholar]
- 8. Bsteh G, Ehling R, Lutterotti A, et al. Long term clinical prognostic factors in relapsing‐remitting multiple sclerosis: insights from a 10‐year observational study. PLoS ONE. 2016;11:e0158978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58:840‐846. [DOI] [PubMed] [Google Scholar]
- 10. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17:162‐173. [DOI] [PubMed] [Google Scholar]
- 12. Asamer E‐M, Astleithner F, Cetkovic P, et al. Quality assessment for register‐based statistics ‐ Results for the Austrian census 2011. Austrian J Statistics. 2016;45:3‐14. [Google Scholar]
- 13. Bello‐Chavolla OY, Bahena‐López JP, Antonio‐Villa NE, et al. Predicting mortality due to SARS‐CoV‐2: a mechanistic score relating obesity and diabetes to COVID‐19 outcomes in Mexico. J Clin Endocrinol Metab. 2020;105:2752‐2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Berger JR, Brandstadter R, Bar‐Or A. COVID‐19 and MS disease‐modifying therapies. Neurol Neuroimmunol Neuroinflammation. 2020;7:e761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Louapre C, Collongues N, Stankoff B, et al. Characteristics and outcomes in patients with Coronavirus disease 2019 and multiple sclerosis. JAMA Neurol. 2020;77:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tian J, Yuan X, Xiao J, et al. Clinical characteristics and risk factors associated with COVID‐19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21:893‐903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hamer M, Kivimäki M, Gale CR, Batty GD. Lifestyle risk factors, inflammatory mechanisms, and COVID‐19 hospitalization: a community‐based cohort study of 387,109 adults in UK. Brain Behav Immun. 2020;87:184‐187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marrie RA, Reider N, Cohen J, et al. A systematic review of the incidence and prevalence of cancer in multiple sclerosis. Multiple Scler. 2015;21:294‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marrie RA, Reider N, Cohen J, et al. A systematic review of the incidence and prevalence of cardiac, cerebrovascular, and peripheral vascular disease in multiple sclerosis. Multiple Scler. 2015;21:318‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marrie RA, Cohen J, Stüve O, et al. A systematic review of the incidence and prevalence of comorbidity in multiple sclerosis: overview. Multiple Scler. 2015;21:263‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Khurana SR, Bamer AM, Turner AP, et al. The prevalence of overweight and obesity in veterans with multiple sclerosis. Am J Phys Med Rehab. 2009;88:83‐91. [DOI] [PubMed] [Google Scholar]
- 22. Bsteh G, Algrang L, Hegen H, et al. Pregnancy and multiple sclerosis in the DMT era: a cohort study in Western Austria. Multiple Scler. 2018;127:135245851881661‐135245851881710. [DOI] [PubMed] [Google Scholar]
- 23. R. de B . COVID‐19 death rates worldwide as of June 10th, 2020, by country (online); 2010.
- 24. Amor S, Baker D, Khoury SJ, Schmierer K, Giovanonni G. SARS‐CoV‐2 and multiple sclerosis: not all immune depleting DMTs are equal or bad. Ann Neurol. 2020;87:794‐797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bsteh G, Monz E, Zamarian L, et al. Combined evaluation of personality, risk and coping in MS patients: a step towards individualized treatment choice – The PeRiCoMS‐Study I. J Neurol Sci. 2017;376:71‐75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request from a qualified researcher.


