Abstract
Aims
In patients with coronavirus disease 2019 (COVID‐19), the involvement of the cardiovascular system significantly relates to poor prognosis. However, the risk factors for acute myocardial injury have not been sufficiently studied. Thus, we aimed to determine the characteristics of myocardial injury and define the association between routine blood markers and cardiac troponin I, in order to perform a predictive model.
Methods and results
This retrospective cohort study included patients with confirmed COVID‐19 from Wuhan Tongji Hospital (Wuhan, China). Data were compared between those with and without myocardial injury. Kaplan–Meier analysis and Cox regression models were used to describe the association between myocardial injury and poor prognosis. Simple correlation analyses were used to find factors associated with high‐sensitivity cardiac troponin I levels. Univariate and multivariate logistic regression methods were used to explore the risk factors associated with myocardial injury. The area under the receiver operating characteristic curve was used to determine the predictive value of the model. Of 353 patients included in the study, 79 presented myocardial injury. Patients with myocardial injury had higher levels of inflammation markers, poorer liver and kidney function, and more complications compared with patients without myocardial injury. High‐sensitivity cardiac troponin I levels were significantly associated with neutrophil/lymphocyte ratio, creatinine, d‐dimer, lactate dehydrogenase, and inflammatory cytokines and negatively associated with oxygen saturation. It was significantly associated with poor prognosis after adjusting for age, sex, and complications. Multivariate regression showed that myocardial injury was associated with a high neutrophil/lymphocyte ratio (odds ratio 2.30, 95% CI 1.11–4.75, per standard deviation increase, P = 0.02), creatinine (3.58, 1.35–8.06, P = 0.01), and lactate dehydrogenase (3.39, 1.42–8.06, P = 0.01) levels. Using a predictive model, the area under the receiver operating characteristic curve was 0.92 (0.88–0.96).
Conclusions
In patients with COVID‐19, neutrophil/lymphocyte ratio, creatinine, and lactate dehydrogenase are blood markers that could help identify patients with a high risk of myocardial injury at an early stage.
Keywords: Myocardial injury, COVID‐19, Risk factors, Inflammation
Introduction
The outbreak of coronavirus disease 2019 (COVID‐19) has spread globally since December 2019. 1 The clinical spectrum of COVID‐19 appears to be wide, ranging from an asymptomatic infection, mild upper respiratory tract illness, or varying degrees of viral pneumonia to respiratory failure, multiple organ dysfunction, and death. 2 , 3 , 4 Identification and early management of risk factors for complications is key to improving survival in COVID‐19.
Like other respiratory tract infections, COVID‐19 can worsen underlying cardiovascular disease and even precipitate de novo cardiac complications. 5 , 6 Recent studies demonstrate that patients with COVID‐19 who suffer acute myocardial injury have increased death rates compared with those without myocardial injury. 7 , 8 Acute myocardial injury has been shown to be a strong negative prognostic marker in patients with COVID‐19. 9
Taking this into consideration, it would be imperative to risk stratify patients in order to identify those at highest risk who may require further tests as well as more intensive surveillance and support. Early recognition of high‐risk patients would aid in the clinical decision‐making process allowing prompt specific treatment. However, risk factors for myocardial injury in COVID‐19 remain unclear, as limited studies have been conducted to this purpose. Here, we aimed to determine the prevalence and characteristics of myocardial injury in patients with COVID‐19 in Wuhan, China, and investigated the association between routine blood markers and cardiac troponin I (cTNI) in order to perform a predictive model.
Methods
Study design and participants
This retrospective cohort study included patients diagnosed with COVID‐19 from 10 February to 5 March 2020, at Tongji Hospital (Wuhan, Hubei Province, China). Clinical diagnosis criteria were as follows: (i) fever or respiratory symptoms; (ii) leukopenia or lymphopenia; and (iii) a computerized tomography scan showing imaging features of viral pneumonia with characteristic changes. 10 , 11 Those with ≥2 clinical criteria and a positive result of either next‐generation sequencing or real‐time polymerase chain reaction assays were diagnosed with COVID‐19. 12
This study complied with the edicts of the 1975 Declaration of Helsinki 13 and was approved by the research ethics commission of Ruijin Hospital (Shanghai, China). Written informed consent was obtained from patients or patients' next of kin.
Data collection
Epidemiological, demographic, clinical, laboratory, treatment, complication, and outcome data were extracted from electronic medical records. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) RNA was detected in Wuhan Tongji Hospital laboratory using next‐generation sequencing or real‐time PCR methods, according to World Health Organization interim guidance. 14 Throat‐swab specimens were obtained after clinical remission of symptoms such as fever, cough, and dyspnoea.
Routine laboratory examinations consisted of complete blood count (Sysmex XN‐2000 and its original reagent, Kobe, Japan), renal and liver function tests (creatinine, alanine transaminase, and albumin) (Roche cobas 8000 and its original reagent, Basel, Switzerland), myocardial enzymes, coagulation profile (Stago STA‐R and its original reagent, Paris, France), lactate dehydrogenase (LDH) (Roche cobas 8000 and its original reagent, Basel, Switzerland), and inflammatory cytokines including high‐sensitivity C‐reactive protein (hsCRP) and several interleukins (Roche cobas e602 and its original reagent, Basel, Switzerland). hsCRP was tested using CRP‐LATEX X2 kit (612755, Denka Seiken Co., Tokyo, Japan), and high‐sensitivity cTNI was tested using STAT High Sensitive Troponin‐I Reagent Kit (3P25‐77, Abbott, Dublin, Ireland). The normal upper limits of cTNI in men and women were 34.2 and 15.6 pg/mL, respectively. Other reference intervals were specifically shown in Supporting Information, Table S1 . Chest computed tomography scans were performed in all patients. A trained team of physicians reviewed all the data.
Definitions
Fever was defined as axillary temperature ≥37.3°C. Myocardial injury was diagnosed if serum levels of cTNI were above the 99th percentile upper reference limit. 3 The severity of COVID‐19 was classified according to the Chinese guidelines for management of COVID‐19 (Version 7.0). 15 , 16 Severe cases were defined when at least one of the following was present: (i) respiratory rate >30 breaths per minute; (ii) oxygen saturation (SpO2) ≤93%; and (iii) PaO2/FiO2 ratio ≤300 mmHg. Critical cases were defined as those including at least one of the following: (i) respiratory failure requiring mechanical ventilation; (ii) shock; (iii) presence of other organ damage apart from respiratory; and (iv) admission to intensive care unit. Kidney injury was diagnosed according to the KDIGO clinical practice guidelines. 17 Acute respiratory distress syndrome was diagnosed according to the Berlin Definition. 18 Coagulopathy was defined as a 3 s extension of prothrombin time or a 5 s extension of activated partial thromboplastin time.
Statistical analysis
Continuous variables were log transformed to obtain a normal distribution and were presented as mean values ± standard deviation. Categorical variables were presented as number of individuals, with percentages within parentheses. Student's t‐test, χ 2 test, and Fisher's exact test were used to compare differences between patients with and without myocardial injury when appropriate. Kaplan–Meier analysis was used to compare the mortality rate in patients with and without myocardial injury. Univariate and multivariate Cox regression models were analysed to describe the association between cTNI and poor prognosis. Simple correlation analyses were used to explore the relationship between cTNI and other variables. Univariate and multivariate logistic regression models were used to explore risk factors associated with myocardial injury, and an area under the receiver operating characteristic curve was applied to determine the predictive value of the model. A two‐sided α of less than 0.05 was considered statistically significant. Statistical analyses were performed using the SPSS software (Version 20.0).
Results
Baseline characteristics
A total of 475 cases of suspected COVID‐19 were identified, of which 122 were excluded either because they lacked SARS‐CoV‐2 confirmation by RNA detection or because they were still hospitalized by 5 March. Therefore, 353 patients were included in the final analysis. Table 1 shows the clinical features of patients with confirmed COVID‐19. The mean age of these patients was 60.47 ± 10.05, ranging from 18 to 89 years. Co‐morbidities were present in nearly half of patients, with hypertension being the most prevalent condition, followed by diabetes and coronary heart disease. On admission, the most common symptoms were cough and fever; sputum, chest distress, fatigue, and dyspnoea also occurred in over 25% of the patients. Regarding laboratory findings, mean levels of alanine transaminase, LDH, myocardial enzymes, d‐dimer, and inflammatory cytokines were all significantly increased compared with normal levels. Lymphocytopenia (<0.8 × 109/L) occurred in 98 (27.8%) patients.
Table 1.
Demographic, clinical, and laboratory findings of patients with and without myocardial injury on admission
| Total (n = 353) | Without myocardial injury (n = 274) | With myocardial injury (n = 79) | P value | |
|---|---|---|---|---|
| Demographic and clinical characteristics | ||||
| Age (years) | 60.47 ± 10.05 | 57.65 ± 14.68 | 70.25 ± 11.96 | <0.01 |
| Sex, male (%) | 186 (52.7) | 136 (49.6) | 50 (63.3) | 0.03 |
| Exposure history (%) | 218 (61.8) | 165 (60.2) | 53 (67.1) | 0.27 |
| Co‐morbidity | ||||
| Hypertension (%) | 129 (36.5) | 87 (31.8) | 42 (53.2) | <0.01 |
| Diabetes (%) | 67 (19.0) | 49 (17.9) | 18 (22.8) | 0.33 |
| Coronary heart disease (%) | 40 (11.3) | 24 (8.8) | 16 (20.3) | 0.01 |
| Chronic obstructive lung disease (%) | 26 (7.4) | 18 (6.6) | 8 (10.1) | 0.29 |
| Chronic kidney disease (%) | 13 (3.7) | 3 (1.1) | 10 (12.7) | <0.01 |
| Carcinoma (%) | 11 (3.1) | 9 (3.3) | 2 (2.5) | 0.73 |
| Stroke (%) | 13 (3.7) | 10 (3.6) | 3 (3.8) | 0.95 |
| Symptom | ||||
| Fever (temperature ≥37.3°C) (%) | 271 (76.8) | 211 (77.0) | 60 (75.9) | 0.84 |
| Cough (%) | 288 (81.6) | 219 (79.9) | 69 (87.3) | 0.13 |
| Sputum (%) | 185 (52.4) | 141 (51.5) | 44 (55.7) | 0.51 |
| Dyspnoea (%) | 129 (36.5) | 90 (32.8) | 39 (49.4) | 0.01 |
| Pharyngalgia (%) | 45 (12.7) | 32 (11.7) | 13 (16.5) | 0.26 |
| Headache (%) | 51 (14.4) | 40 (14.6) | 11 (13.9) | 0.88 |
| Myalgia (%) | 71 (20.1) | 53 (19.3) | 18 (22.8) | 0.5 |
| Fatigue (%) | 135 (38.2) | 104 (38.0) | 31 (39.2) | 0.84 |
| Chest pain (%) | 63 (17.8) | 44 (16.1) | 19 (24.1) | 0.1 |
| Chest distress (%) | 144 (40.8) | 102 (37.2) | 42 (53.2) | 0.01 |
| Diarrhoea (%) | 81 (22.9) | 58 (21.2) | 23 (29.1) | 0.14 |
| Nausea or vomiting (%) | 49 (13.9) | 37 (13.5) | 12 (15.2) | 0.7 |
| CURB‐65 score | <0.01 | |||
| 0–1 (%) | 220 (62.3) | 205 (74.8) | 15 (19.0) | |
| 2 (%) | 77 (21.8) | 58 (21.2) | 19 (24.1) | |
| 3–5 (%) | 56 (15.9) | 11 (4.0) | 45 (56.9) | |
| Disease severity status | <0.01 | |||
| General (%) | 247 (70.0) | 229 (83.6) | 18 (22.8) | |
| Severe (%) | 56 (15.9) | 40 (14.6) | 16 (20.3) | |
| Critical (%) | 50 (14.1) | 5 (1.8) | 45 (57.0) | |
| Physical examination | ||||
| Systolic blood pressure (mmHg) | 130.86 ± 18.55 | 129.86 ± 17.97 | 134.32 ± 20.17 | 0.06 |
| Diastolic blood pressure (mmHg) | 79.11 ± 12.24 | 78.85 ± 11.77 | 79.99 ± 13.77 | 0.47 |
| Heart rate (b.p.m.) | 88.19 ± 14.29 | 86.76 ± 14.19 | 93.16 ± 13.61 | <0.01 |
| Respiratory rate (breaths per minute) | 20.96 ± 4.62 | 20.60 ± 4.31 | 22.20 ± 5.40 | 0.02 |
| Temperature (°C) | 36.80 ± 0.74 | 36.75 ± 0.71 | 36.96 ± 0.81 | 0.04 |
| Minimum SpO2 (%) | 90.39 ± 12.09 | 93.99 ± 5.86 | 77.78 ± 18.34 | <0.01 |
| Laboratory findings | ||||
| White blood cell count (× 109/L) | 6.40 ± 3.04 | 5.79 ± 1.99 | 8.56 ± 4.69 | <0.01 |
| Lymphocyte count (× 109/L) | 1.30 ± 0.57 | 1.42 ± 0.52 | 0.85 ± 0.49 | <0.01 |
| Neutrophil count (× 109/L) | 4.50 ± 3.08 | 3.75 ± 1.85 | 7.12 ± 4.71 | <0.01 |
| Minimum lymphocyte count (× 109/L) | 1.18 ± 0.55 | 1.33 ± 0.48 | 0.66 ± 0.44 | <0.01 |
| Maximum neutrophil/lymphocyte | 8.48 ± 15.38 | 3.75 ± 3.58 | 25.12 ± 25.96 | <0.01 |
| Monocyte count (× 109/L) | 0.62 ± 1.11 | 0.62 ± 1.15 | 0.62 ± 1.00 | 0.99 |
| Haemoglobin (g/L) | 126.59 ± 17.22 | 126.96 ± 15.31 | 125.29 ± 22.78 | 0.54 |
| Platelet count (× 109/L) | 250.89 ± 100.25 | 265.54 ± 91.74 | 199.32 ± 111.96 | <0.01 |
| Albumin (g/L) | 36.33 ± 5.22 | 37.56 ± 4.53 | 32.08 ± 5.22 | <0.01 |
| Alanine transaminase (U/L) | 55.73 ± 239.14 | 34.89 ± 34.44 | 127.87 ± 496.77 | <0.01 |
| Creatinine (μmol/L) | 90.07 ± 78.18 | 71.16 ± 18.79 | 155.42 ± 143.86 | <0.01 |
| Lactate dehydrogenase (U/L) | 338.54 ± 296.87 | 243.72 ± 84.14 | 655.05 ± 480.10 | <0.01 |
| High‐sensitivity cardiac troponin I (pg/mL) | 329.33 ± 3041.59 | 4.91 ± 4.12 | 1428.02 ± 6278.61 | <0.01 |
| Myoglobin (ng/mL) | 123.40 ± 246.35 | 43.30 ± 41.79 | 396.18 ± 408.17 | <0.01 |
| Creatine phosphokinase‐MB (ng/mL) | 1.87 ± 4.74 | 0.76 ± 0.78 | 5.62 ± 8.91 | <0.01 |
| N‐terminal pro‐brain natriuretic peptide (pg/mL) | 1727.72 ± 6878.03 | 125.24 ± 136.62 | 6913.51 ± 12914.95 | <0.01 |
| Prothrombin time (s) | 14.50 ± 5.26 | 13.59 ± 0.80 | 17.51 ± 10.35 | <0.01 |
| d‐dimer (μg/mL) | 3.80 ± 7.38 | 1.73 ± 5.07 | 10.80 ± 9.43 | <0.01 |
| High‐sensitivity C‐reactive protein (mg/L) | 43.62 ± 68.14 | 21.50 ± 37.54 | 120.22 ± 91.32 | <0.01 |
| IL‐1β (pg/mL) | 13.74 ± 69.43 | 8.31 ± 19.07 | 29.83 ± 133.89 | 0.01 |
| IL‐2R (U/mL) | 767.02 ± 788.73 | 490.81 ± 260.34 | 1585.79 ± 1176.23 | <0.01 |
| IL‐6 (pg/mL) | 189.38 ± 781.30 | 8.69 ± 20.74 | 728.22 ± 1438.00 | <0.01 |
| IL‐8 (pg/mL) | 102.87 ± 501.07 | 14.59 ± 28.17 | 364.57 ± 955.62 | <0.01 |
| TNF‐α (pg/mL) | 14.54 ± 36.95 | 7.76 ± 5.00 | 34.63 ± 69.73 | <0.01 |
IL, interleukin; SpO2, oxygen saturation; TNF, tumour necrosis factor.
Clinical features and laboratory characteristics in patients with and without myocardial injury
Cardiac injury occurred in 79 (22.4%) patents with COVID‐19. Compared with those without myocardial injury, these patients were predominantly male, had an older age, and presented a higher prevalence of hypertension and coronary artery disease. Although symptoms of COVID‐19 did not differ between the two groups, patients with myocardial injury exhibited more severe forms of the disease. Moreover, patients with myocardial injury demonstrated higher leucocyte and neutrophil counts and lower lymphocyte and platelet counts. Elevations in alanine transaminase, creatinine, and LDH were more frequent in patients with myocardial injury, as well as coagulation pathway abnormalities, including high d‐dimer and prolonged prothrombin time. Inflammatory cytokines including hsCRP, interleukin (IL)‐1β, IL‐2R, IL‐6, IL‐8, and tumour necrosis factor‐α were also significantly higher in this group of patients (Table 1 ).
Focusing on blood results at Day 2 of hospitalization, patients who developed myocardial injury had higher levels of hsCRP, higher leucocyte and neutrophil counts, and lower lymphocyte and platelet counts than patients who did not develop this complication. The monocyte count and haemoglobin level were similar between the two groups (Figure 1 ). In patients without myocardial injury, leucocyte count and neutrophil/lymphocyte ratio were highest on Day 7 and later improved, whereas these remained unchanged in those with myocardial injury. Moreover, the haemoglobin level gradually decreased in patients with myocardial injury.
Figure 1.
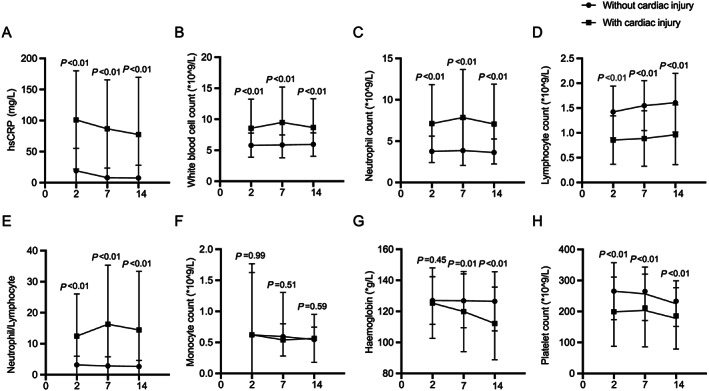
Temporal changes in laboratory markers from illness onset in patients with coronavirus disease 2019, with and without myocardial injury. Temporal changes in high‐sensitivity C‐reactive protein (A), white blood cell count (B), neutrophil count (C), lymphocyte count (D), neutrophil/lymphocyte ratio (E), monocyte count (F), haemoglobin levels (G), and platelet count (H). Differences between patients with and without myocardial injury were significant for all time points shown, except for monocyte count at all time points and haemoglobin at Day 2.
Treatment and outcome
Following current guidelines, 325 (92.1%) patients received antivirals including umifenovir, oseltamivir, ribavirin, and lopinavir/ritonavir; 271 (76.8%) received antibiotics; and 256 (72.5%) were treated with Chinese medicine. Corticosteroids, intravenous immunoglobulin, oxygen therapy, and mechanical ventilation were also used as appropriate (Table 2 ).
Table 2.
Treatments, complications, and outcomes of patients with and without myocardial injury
| Total (n = 353) | Without myocardial injury (n = 274) | With myocardial injury (n = 79) | P value | |
|---|---|---|---|---|
| Treatments | ||||
| Antiviral treatment | 325 (92.1) | 260 (94.9) | 65 (82.3) | <0.01 |
| Antibiotics | 271 (76.8) | 198 (72.3) | 73 (92.4) | <0.01 |
| Corticosteroids | 62 (17.6) | 24 (8.8) | 38 (48.1) | <0.01 |
| Intravenous immunoglobulin | 44 (12.5) | 18 (6.6) | 26 (32.9) | <0.01 |
| Chinese medicine | 256 (72.5) | 218 (79.6) | 38 (48.1) | <0.01 |
| Nasal cannula oxygen therapy | 240 (68.0) | 188 (68.6) | 52 (65.8) | 0.64 |
| Mask oxygen inhalation | 36 (10.2) | 16 (5.8) | 20 (25.3) | <0.01 |
| High‐flow nasal cannula oxygen therapy | 14 (4.0) | 2 (0.7) | 12 (15.2) | <0.01 |
| Non‐invasive mechanical ventilation | 29 (8.2) | 3 (1.1) | 26 (32.9) | <0.01 |
| Invasive mechanical ventilation | 26 (7.4) | 2 (0.7) | 24 (30.4) | <0.01 |
| Circulatory support | 11 (3.1) | 0 (0.0) | 11 (13.9) | <0.01 |
| Renal replacement therapy | 11 (3.1) | 0 (0.0) | 11 (13.9) | <0.01 |
| Complications | ||||
| Respiratory failure | 57 (16.1) | 8 (2.9) | 49 (62.0) | <0.01 |
| Acute liver damage | 92 (26.1) | 51 (18.6) | 41 (51.9) | <0.01 |
| Acute kidney injury | 53 (15.0) | 14 (5.1) | 39 (49.4) | <0.01 |
| Septic shock | 20 (5.7) | 2 (0.7) | 18 (22.8) | <0.01 |
| MODS | 46 (13.0) | 0 (0.0) | 46 (58.2) | <0.01 |
| Coagulopathy | 18 (5.1) | 5 (1.8) | 13 (16.5) | <0.01 |
| Outcomes | <0.01 | |||
| Recovery | 267 (75.6) | 239 (87.2) | 28 (35.4) | |
| Improvement | 35 (9.9) | 31 (11.3) | 4 (5.1) | |
| Stabilization | 1 (0.3) | 0 (0.0) | 1 (1.3) | |
| Aggravation | 1 (0.3) | 0 (0.0) | 1 (1.3) | |
| Death | 49 (13.9) | 4 (1.5) | 45 (57.0) | |
ARDS, acute respiratory distress syndrome; MODS, multiple organ dysfunction syndrome.
Liver damage was the most frequent complication, followed by myocardial injury, respiratory failure, and kidney injury. The frequency of complications was significantly higher in patients with myocardial injury than in those without it (Table 2 ). Overall, 49 patients (13.9%) died during hospitalization and 267 (75.6%) recovered. Mortality rates were markedly higher in the group with myocardial injury (Figure 2 ). Myocardial injury, as determined by cTNI levels, was significantly associated with mortality after adjusting for age, sex, and complications including respiratory failure, liver damage, kidney injury, coagulopathy, and septic shock, as well as the specific values of multiorgan function (Table 3 ).
Figure 2.
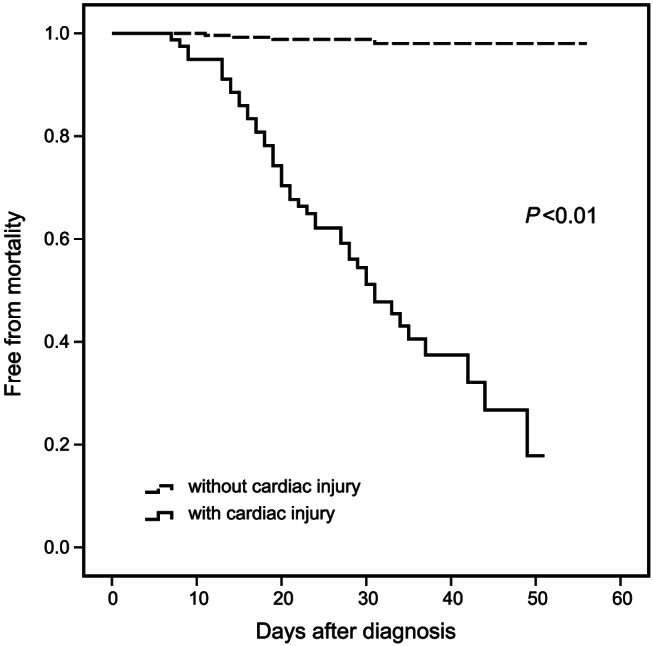
Prognosis of patients with and without myocardial injury. The mortality rates after diagnosis in groups with and without myocardial injury shown in Kaplan–Meier analysis.
Table 3.
Univariate and multivariate Cox proportional hazard models for cTNI as a predictor of mortality
| Log cTNI per SD | HR (95% CI) | P value |
|---|---|---|
| Univariable | 2.75 (2.30–3.29) | <0.01 |
| Adjusted for Model 1 | 2.57 (2.10–3.13) | <0.01 |
| Adjusted for Model 2 | 2.29 (1.81–2.89) | <0.01 |
| Adjusted for Model 3 | 1.65 (1.17–2.34) | <0.01 |
CI, confidence interval; cTNI, cardiac troponin I; HR, hazard ratio; SD, standard deviation; SpO2, oxygen saturation.
Model 1: adjusted for age and sex. Model 2: adjusted for age, sex, respiratory failure, liver damage, kidney injury, coagulopathy, and septic shock. Model 3: adjusted for age, sex, log alanine transaminase, log creatinine, log prothrombin time, and SpO2.
The predictive model of myocardial injury
In all patients with COVID‐19, cTNI levels were strictly correlated with age; minimum SpO2; CURB‐65 score; liver, kidney, and coagulation functions; lymphocytopenia; neutrophil/lymphocyte ratio; LDH; N‐terminal pro‐brain natriuretic peptide; and inflammatory markers such as hsCRP, IL‐6, and several cytokines (Supporting Information, Table S2 ).
In univariate analysis, myocardial injury was significantly more frequent in older patients with hypertension or coronary heart disease. In addition, myocardial injury was associated with low albumin levels and SpO2; an increased respiratory rate; a high neutrophil/lymphocyte ratio; and elevated levels of ALT, creatinine, LDH, d‐dimer, prothrombin time, and inflammatory markers (Table 4 ).
Table 4.
Risk factors associated with cardiac injury
| Univariable OR (95% CI) | P value | Adjusted for Model 1 OR (95% CI) | P value | Adjusted for Model 2 OR (95% CI) | P value | Adjusted for Model 3 OR (95% CI) | P value | |
|---|---|---|---|---|---|---|---|---|
| Demographic and clinical characteristics | ||||||||
| Log age per SD | 3.84 (2.34–6.29) | <0.01 | / | / | / | / | / | / |
| Male sex (vs. female) | 1.75 (1.05–2.93) | 0.03 | / | / | / | / | / | / |
| Co‐morbidity present (vs. not present) | ||||||||
| Hypertension | 2.44 (1.47–4.06) | <0.01 | — | — | / | / | / | / |
| Diabetes | 1.36 (0.74–2.49) | 0.33 | — | — | / | / | / | / |
| Coronary heart disease | 2.65 (1.33–5.28) | 0.01 | — | — | / | / | / | / |
| Chronic obstructive lung disease | 1.60 (0.67–3.84) | 0.29 | — | — | / | / | / | / |
| Respiratory rate (breaths per minute) | ||||||||
| ≤24 | 1 (ref) | 1 (ref) | ||||||
| >24 | 2.41 (1.24–4.68) | 0.01 | 1.99 (0.98–4.05) | 0.06 | — | — | — | — |
| Min SpO2 (%) | ||||||||
| >93 | 1 (ref) | 1 (ref) | 1 (ref) | |||||
| ≤93 | 8.15 (4.62–14.35) | <0.01 | 5.93 (3.28–10.73) | <0.01 | 5.77 (3.15–10.55) | <0.01 | — | — |
| Laboratory findings | ||||||||
| Log maximum neutrophil/lymphocyte per SD | 6.84 (4.47–10.46) | <0.01 | 6.32 (4.02–9.93) | <0.01 | 6.09 (3.92–9.45) | <0.01 | 2.30 (1.11–4.75) | 0.02 |
| Log alanine transaminase per SD | 1.80 (1.38–2.33) | <0.01 | 1.90 (1.41–2.56) | <0.01 | 1.92 (1.42–2.60) | <0.01 | — | — |
| Log creatinine per SD | 5.25 (3.30–8.35) | <0.01 | 6.79 (3.77–12.23) | <0.01 | 6.66 (3.71–11.97) | <0.01 | 3.58 (1.35–9.54) | 0.01 |
| Log lactate dehydrogenase per SD | 8.32 (4.87–14.21) | <0.01 | 8.27 (4.62–14.82) | <0.01 | 8.22 (4.52–14.91) | <0.01 | 3.39 (1.42–8.06) | 0.01 |
| Albumin per SD | 0.30 (0.22–0.41) | <0.01 | 0.37 (0.26–0.53) | <0.01 | 0.34 (0.24–0.49) | <0.01 | — | — |
| Log d‐dimer per SD | 4.76 (3.37–6.74) | <0.01 | 4.55 (3.10–6.69) | <0.01 | 4.41 (3.05–6.37) | <0.01 | — | — |
| Log prothrombin time per SD | 6.79 (3.66–12.60) | <0.01 | 4.52 (2.45–8.36) | <0.01 | 4.51 (2.36–8.60) | <0.01 | — | — |
| Log hsCRP per SD | 6.82 (4.22–11.01) | <0.01 | 5.82 (3.57–9.48) | <0.01 | 5.50 (3.42–8.84) | <0.01 | — | — |
| Log IL‐1β per SD | 1.66 (1.19–2.31) | <0.01 | 1.97 (1.34–2.88) | <0.01 | 1.93 (1.33–2.80) | <0.01 | — | — |
| Log IL‐2R per SD | 6.81 (3.90–11.88) | <0.01 | 5.28 (2.97–9.40) | <0.01 | 5.28 (2.97–9.40) | <0.01 | — | — |
| Log IL‐6 per SD | 6.68 (4.00–11.15) | <0.01 | 5.45 (3.17–9.36) | <0.01 | 5.34 (3.13–9.11) | <0.01 | — | — |
| Log IL‐8 per SD | 5.75 (3.36–9.84) | <0.01 | 4.35 (2.52–7.51) | <0.01 | 4.17 (2.39–7.27) | <0.01 | — | — |
| Log TNF‐α per SD | 7.23 (4.00–13.06) | <0.01 | 5.69 (3.09–10.45) | <0.01 | 5.59 (3.03–10.32) | <0.01 | — | — |
| Antiviral treatment | 0.25 (0.11–0.55) | <0.01 | 0.30 (0.13–0.73) | 0.01 | 0.30 (0.13–0.73) | 0.01 | — | — |
CI, confidence interval; hsCRP, high‐sensitivity C‐reactive protein; IL, interleukin; OR, odds ratio; SD, standard deviation; SpO2, oxygen saturation; TNF, tumour necrosis factor; /, divide; neutrophil/lymphocyte, neutrophil/lymphocyte ratio.
Model 1: adjusted for age and sex. Model 2: adjusted for age, sex, and conventional risk factors for cardiac injury including hypertension, diabetes, and coronary heart disease. Model 3: adjusted for all factors included in the univariable analysis.
In multivariate logistic regression models, variables were first adjusted for age and sex, then adjusted for age, sex, and co‐morbidities (including hypertension, diabetes, coronary heart disease, and chronic obstructive lung disease), and further analysed in a model that included all variables. Notably, we found that creatinine, LDH, and neutrophil/lymphocyte ratio were independently associated with increased odds of myocardial injury (Table 4 ).
We further analysed the predictive value of this model using an area under the receiver operating characteristic curve derived from multivariate analysis. The area under the receiver operating characteristic curve was 0.92 (0.88–0.96), and the sensitivity and specificity of the cut‐off predictive value were 0.75 and 0.96, respectively (Figure 3 ). The recommended reference cut‐off value of each parameter was 7.32 for maximum neutrophil/lymphocyte, 87.5 μmol/L for serum creatinine, and 278.5 U/L for serum LDH. Moreover, this model could predict the presence of myocardial injury in both patients with and without other organ dysfunction (Supporting Information, Table S3 ).
Figure 3.
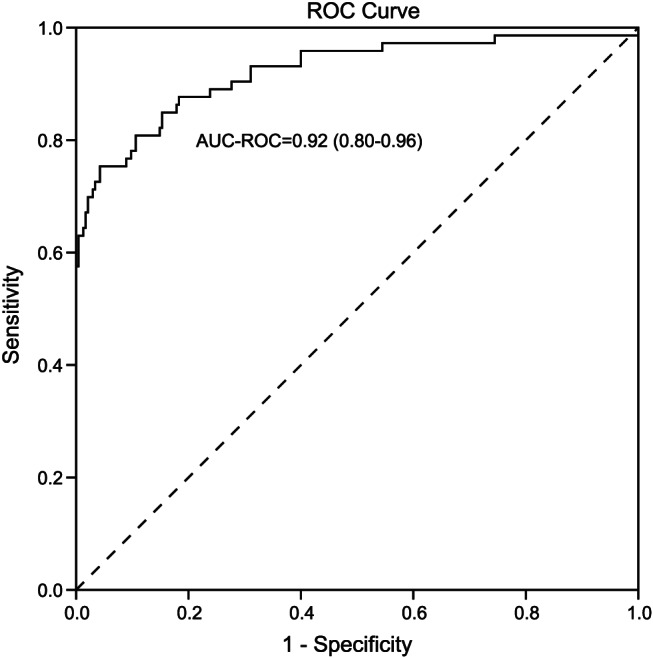
Predictive value of the multivariable regression model. Receiver operating characteristic (ROC) curves for multivariable analyses, including age, sex, co‐morbidities, neutrophil/lymphocyte ratio, creatinine, and lactate dehydrogenase as predictors of myocardial injury in all patients with coronavirus disease 2019. AUC‐ROC, area under the receiver operating characteristic curve.
Discussion
This retrospective cohort study identified several risk factors and performed a predictive model for myocardial injury in hospitalized patients with COVID‐19 in Wuhan, China. Notably, a high neutrophil/lymphocyte ratio and elevated levels of creatinine and LDH were associated with higher odds of myocardial injury. In addition, older age, lower SpO2, and elevated levels of ALT, d‐dimer, and inflammatory cytokines were significantly correlated with increased levels of cTNI. As expected, patients with myocardial injury suffered from more complications during hospitalization and had a poorer prognosis than those without myocardial injury.
Risk factors of cardiac events after pneumonia include older age, pre‐existing cardiovascular diseases, and greater severity of disease. 19 , 20 To date, the aetiology of cardiac disease in patients with COVID‐19 is likely to be multifactorial, and mechanisms underlying myocardial injury during the course of COVID‐19 infection remain unknown. 21
Severe acute respiratory syndrome coronavirus 2 may exert direct cytopathic effects on cardiac tissue, leading to the increase of cTNI levels. It has been shown that both SARS‐CoV and SARS‐CoV‐2 use angiotensin‐converting enzyme 2 (ACE2), which is highly expressed in the heart and vasculature, as a cell entry receptor. 22 , 23 Previous studies indicated that SARS‐CoV is detectable and ACE2 expression is down‐regulated in the heart of infected patients, leading to the activation of renin–angiotensin–aldosterone system and further exacerbating myocardial injury. 24 SARS‐CoV‐2 may as well bind to ACE2 expressed in the heart and induces ACE2 down‐regulation as well as renin–angiotensin–aldosterone system dysfunction, leading to cardiac dysfunction and pneumonia progression. Interstitial inflammatory cell infiltrates in heart tissue have also been documented in fatal cases of COVID‐19, supporting the possibility of direct myocardial infection. 25
The elevation of cTNI levels and the presence of myocardial injury may also be due to the indirect, infection‐related pathogenesis on myocardium. Associations between cTNI elevation and other features of the disease need to be systematically examined, with further analyses aiming to stratify patients with high risk of cardiovascular complications in COVID‐19. 26 The present study indicated the significant predictive value of neutrophil/lymphocyte ratio, creatinine, and LDH.
Many conclusions can be drawn from these results. First, this study confirmed that high levels of inflammation are associated with myocardial injury in patients with COVID‐19. cTNI levels were strictly correlated to levels of hsCRP and several cytokines, suggesting that the heart is affected by the systemic immune activation and cytokine storm. After the respiratory tract, immune organs are the second most affected system in COVID‐19. Research has revealed that, in severe cases with multiorgan involvement (including myocardial injury), peripheral blood lymphopenia is related to a significant reduction in the number of spleen and lymph node lymphocytes. 3 , 27 Additionally, in our study, the neutrophil count was positively associated with cTNI levels and occurrence of myocardial injury. Considering that neutrophil counts may increase in secondary infections, the neutrophil/lymphocyte ratio may not only be indicative of the degree of immune damage in COVID‐19 but may as well point to sepsis, which exerts its own inflammatory and immune negative effects. 28 Development of the uncontrolled overactivation of the immune system may partly account for the severe cardiovascular injury. Autopsy analysis of hearts from COVID‐19 patients also demonstrated the potential involvement of systematic inflammation in heart injury. 25
Second, increased creatinine levels predicted the occurrence of myocardial injury. Recent studies have observed a high prevalence of renal dysfunction in hospitalized patients with COVID‐19, and the presence of kidney disease was associated with a higher mortality and poorer outcome. 4 , 29 Renal injury may be indicative of overall disease severity: patients with higher creatinine level are likely to present multiple organ impairment, including cardiovascular damage. However, renal dysfunction is an independent risk factor for myocardial injury; it has been reported that a loss of glomerular filtration rate, either in acute or chronic kidney disease, independently accelerates the progression of cardiovascular disease. In patients with COVID‐19 and kidney injury, the accumulation of toxic metabolites, activation of inflammatory response, metabolic and nutritional changes, and altered haemodynamic, acid–base, or fluid status may altogether take a toll on the heart, leading to the occurrence and/or aggravation of myocardial injury. 30
Third, our study found that increased LDH levels were an important independent predictor of myocardial injury. To date, many studies have revealed that almost all hospitalized COVID‐19 patients show increased serum levels of LDH, a feature significantly associated with disease severity. 3 , 26 This may be due to the severity of systemic inflammation and tissue necrosis, thus leading to multiorgan dysfunction including the heart. 31 Moreover, LDH level was associated with hypoxia, which may be one of the causes of cardiac damage. We also found that minimum SpO2 was a risk factor for elevated cTNI and myocardial injury after adjusting for age, sex, and co‐morbidities. Both acute respiratory compromise and increased cardiometabolic oxygen demand may impair myocardial oxygen supply, leading to injury.
Fourth, in our study, elevated d‐dimer levels were positively correlated to cTNI. As a feature of disseminated intravascular coagulation, elevated d‐dimer levels are highly prevalent in COVID‐19, especially in severe/critical patients. 32 This is in accordance with the finding that coagulation abnormalities cause organ injury and are associated with a poor prognosis. 19
Fifth, although previous studies with unadjusted analyses suggested that hypertension was associated with severe forms of the disease, analyses adjusting for other risk factors revealed that hypertensive patients have no increased susceptibility to complications of COVID‐19. 26 , 33 In accordance with the latter statement, our study found that hypertension was not an independent risk factor of myocardial injury.
Finally, because COVID‐19 is a major global health and economic burden, strategies are urgently required to minimize unwarranted downstream diagnostic/therapeutic procedures and alleviate the strain on the healthcare system. Our present study found that the neutrophil/lymphocyte ratio and blood levels of creatinine and LDH could significantly predict the occurrence of myocardial injury, with good specificity and sensitivity. Because these are widely available tests, our model could serve as a quick risk stratification tool that helps reserve specific assays (such as cardiac biomarkers) for circumstances in which they would meaningfully contribute to management. Additionally, this model may help identify patients with high risk of myocardial injury who require early interventional therapies to prevent deterioration.
The present study has several limitations. Some patients were transferred to our hospital late in the course of their illness; in these cases, inadequate standard supportive therapy and late antiviral therapy might have contributed to myocardial injury and poor outcomes. Furthermore, although multivariate analysis was adjusted for many confounders, unknown or unmeasured variables might have contributed to myocardial injury. Further studies should be conducted to confirm these results and validate the prognostic utility of our model. Follow‐up data after hospital discharge were not available for analysis; therefore, the long‐term outcome of patients with myocardial injury was unknown. In addition, the precise impact of COVID‐19 on cardiac structure and function was not assessed in this study.
In conclusion, the prevalence of myocardial injury in hospitalized patients with COVID‐19 in Wuhan, China, was high. Blood cTNI levels were significantly associated with immune response, multiple organ dysfunction, and poor outcomes. After adjustment for confounders, the neutrophil/lymphocyte ratio, creatinine, and LDH emerged as independent predictors of myocardial injury. We consider that these variables may be useful for early detection and risk stratification of myocardial injury in hospitalized patients with COVID‐19.
Conflict of interest
None declared.
Funding
This study was supported by the National Natural Science Foundation of China (81570316 and 81770249 to R.Z.; 81400362 and 81670457 to X.Y.), a Shanghai Rising‐Star Program grant to X.Y. (17QA1402300), and a grant from the Municipal Human Resources Development Program for Outstanding Young Talents in Medical and Health Sciences in Shanghai (2017YQ017 to X.Y.). There are no relationships with industry.
Supporting information
Table S1. The specific kits and reference intervals of clinical measurements.
Table S2. The correlation between high‐sensitivity cardiac troponin I and clinical characteristics as well as laboratory findings.
Table S3. The area under the receiver operating characteristic (AUC‐ROC) derived from multivariate analysis in table 4 in subgroup patients.
Acknowledgements
We wish to acknowledge all the doctors and researchers of Ruijin Hospital, Shanghai Jiao Tong University, and Tongji Hospital who contributed to data collection.
Fan, Q. , Zhu, H. , Zhao, J. , Zhuang, L. , Zhang, H. , Xie, H. , Zhang, R. , Granada, J. F. , Xiang, X. , Hu, W. , and Yan, X. (2020) Risk factors for myocardial injury in patients with coronavirus disease 2019 in China. ESC Heart Failure, 7: 4108–4117. 10.1002/ehf2.13022.
Contributor Information
Xiaogang Xiang, Email: shine-xxg@163.com.
Weiguo Hu, Email: wghusurgeon@hotmail.com.
Xiaoxiang Yan, Email: cardexyanxx@hotmail.com.
References
- 1. Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA 2020; 323: 709–710. [DOI] [PubMed] [Google Scholar]
- 2. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bansal M. Cardiovascular disease and COVID‐19. Diabetes Metab Syndr 2020; 14: 247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. nciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, Cani DS, Cerini M, Farina D, Gavazzi E, Maroldi R, Adamo M, Ammirati E, Sinagra G, Lombardi CM, Metra M. Cardiac involvement in a patient with coronavirus disease 2019 (COVID‐19). JAMA Cardiol 2020; 5: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiol 2020; 5: 802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol 2020; 5: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Akhmerov A, Marban E. COVID‐19 and the heart. Circ Res 2020; 126: 1443–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lei J, Li J, Li X, Qi X. CT imaging of the 2019 novel coronavirus (2019‐nCoV) pneumonia. Radiology 2020; 295: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. IChung M, Bernheim A, Mei X, Zhang N, Huang M, Zeng X, Cui J, Xu W, Yang Y, Fayad ZA, Jacobi A, Li K, Li S, Shan H. CT imaging features of 2019 novel coronavirus (2019‐nCoV). Radiology 2020; 295: 202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. IPascarella G, Strumia A, Piliego C, Bruno F, Del Buono R, Costa F, Scarlata S, Agro FE. COVID‐19 diagnosis and management: a comprehensive review. J Intern Med 2020; 288: 192–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013; 310: 2191–2194. [DOI] [PubMed] [Google Scholar]
- 14. World Health Organization . Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. 2020https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected Accessed 28 January 2020.
- 15. Jin YH, Cai L, Cheng ZS, Cheng H, Deng T, Fan YP, Fang C, Huang D, Huang LQ, Huang Q, Han Y, Hu B, Hu F, Li BH, Li YR, Liang K, Lin LK, Luo LS, Ma J, Ma LL, Peng ZY, Pan YB, Pan ZY, Ren XQ, Sun HM, Wang Y, Wang YY, Weng H, Wei CJ, Wu DF, Xia J, Xiong Y, Xu HB, Yao XM, Yuan YF, Ye TS, Zhang XC, Zhang YW, Zhang YG, Zhang HM, Zhao Y, Zhao MJ, Zi H, Zeng XT, Wang YY, Wang XH. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019‐nCoV) infected pneumonia (standard version). Mil Med Res 2020; 7: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. National Health Commission of the People's Republic of China . Chinese management guideline for COVID‐19 (version 7.0) (accessed Feb 19, 2020; in Chinese). 2020. http://wwwnhcgovcn/yzygj/s7652m/202003/a31191442e29474b98bfed5579d5af95shtml (19 February 2020).
- 17. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012; 120: c179–c184. [DOI] [PubMed] [Google Scholar]
- 18. IRanieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012; 307: 2526–2533. [DOI] [PubMed] [Google Scholar]
- 19. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Corrales‐Medina VF, Musher DM, Shachkina S, Chirinos JA. Acute pneumonia and the cardiovascular system. Lancet 2013; 381: 496–505. [DOI] [PubMed] [Google Scholar]
- 21. Liu PP, Blet A, Smyth D, Li H. The science underlying COVID‐19: implications for the cardiovascular system. Circulation 2020; 142: 68–78. [DOI] [PubMed] [Google Scholar]
- 22. Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 579: 270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen L, Li X, Chen M, Feng Y, Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS‐CoV‐2. Cardiovasc Res 2020; 116: 1097–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus‐induced lung injury. Nat Med 2005; 11: 875–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020; 8: 420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guzik TJ, Mohiddin SA, Dimarco A, Patel V, Savvatis K, Marelli‐Berg FM, Madhur MS, Tomaszewski M, Maffia P, D'Acquisto F, Nicklin SA, Marian AJ, Nosalski R, Murray EC, Guzik B, Berry C, Touyz RM, Kreutz R, Wang DW, Bhella D, Sagliocco O, Crea F, Thomson EC, McInnes IB. COVID‐19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res 2020; 116: 1666–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W, Tian DS. Dysregulation of immune response in patients with COVID‐19 in Wuhan, China. Clin Infect Dis 2020; 71: 762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Terpos E, Ntanasis‐Stathopoulos I, Elalamy I, Kastritis E, Sergentanis TN, Politou M, Psaltopoulou T, Gerotziafas G, Dimopoulos MA. Hematological findings and complications of COVID‐19. Am J Hematol 2020; 95: 834–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, Li J, Yao Y, Ge S, Xu G. Kidney disease is associated with in‐hospital death of patients with COVID‐19. Kidney Int 2020; 97: 829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schefold JC, Filippatos G, Hasenfuss G, Anker SD, von Haehling S. Heart failure and kidney dysfunction: epidemiology, mechanisms and management. Nat Rev Nephrol 2016; 12: 610–623. [DOI] [PubMed] [Google Scholar]
- 31. Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J, Li Q, Jiang C, Zhou Y, Liu S, Ye C, Zhang P, Xing Y, Guo H, Tang W. Risk factors of critical & mortal COVID‐19 cases: a systematic literature review and meta‐analysis. J Infect 2020; 81: e16–e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020; 18: 844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. IKreutz R, Algharably EAE, Azizi M, Dobrowolski P, Guzik T, Januszewicz A, Persu A, Prejbisz A, Riemer TG, Wang JG, Burnier M. Hypertension, the renin–angiotensin system, and the risk of lower respiratory tract infections and lung injury: implications for COVID‐19. Cardiovasc Res 2020; 116: 1688–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The specific kits and reference intervals of clinical measurements.
Table S2. The correlation between high‐sensitivity cardiac troponin I and clinical characteristics as well as laboratory findings.
Table S3. The area under the receiver operating characteristic (AUC‐ROC) derived from multivariate analysis in table 4 in subgroup patients.


