Abstract
Background and Aims
Coronavirus disease 2019 (COVID‐19) is associated with liver injury, but the prevalence and patterns of liver injury in liver transplantation (LT) recipients with COVID‐19 are open for study.
Approach and Results
We conducted a multicenter study in the United States of 112 adult LT recipients with COVID‐19. Median age was 61 years (interquartile range, 20), 54.5% (n = 61) were male, and 39.3% (n = 44) Hispanic. Mortality rate was 22.3% (n = 25); 72.3% (n = 81) were hospitalized and 26.8% (n = 30) admitted to the intensive care unit (ICU). Analysis of peak values of alanine aminotransferase (ALT) during COVID‐19 showed moderate liver injury (ALT 2‐5× upper limit of normal [ULN]) in 22.2% (n = 18) and severe liver injury (ALT > 5× ULN) in 12.3% (n = 10). Compared to age‐ and sex‐matched nontransplant patients with chronic liver disease and COVID‐19 (n = 375), incidence of acute liver injury was lower in LT recipients (47.5% vs. 34.6%; P = 0.037). Variables associated with liver injury in LT recipients were younger age (P = 0.009; odds ratio [OR], 2.06; 95% confidence interval [CI], 1.20‐3.54), Hispanic ethnicity (P = 0.011; OR, 6.01; 95% CI, 1.51‐23.9), metabolic syndrome (P = 0.016; OR, 5.87; 95% CI, 1.38‐24.99), vasopressor use (P = 0.018; OR, 7.34; 95% CI, 1.39‐38.52), and antibiotic use (P = 0.046; OR, 6.93; 95% CI, 1.04‐46.26). Reduction in immunosuppression (49.4%) was not associated with liver injury (P = 0.156) or mortality (P = 0.084). Liver injury during COVID‐19 was significantly associated with mortality (P = 0.007; OR, 6.91; 95% CI, 1.68‐28.48) and ICU admission (P = 0.007; OR, 7.93; 95% CI, 1.75‐35.69) in LT recipients.
Conclusions
Liver injury is associated with higher mortality and ICU admission in LT recipients with COVID‐19. Hence, monitoring liver enzymes closely can help in early identification of patients at risk for adverse outcomes. Reduction of immunosuppression during COVID‐19 did not increase risk for mortality or graft failure.
Abbreviations
- ALI
acute liver injury
- ALT
alanine aminotransferase
- ALP
alkaline phosphatase
- AST
aspartate aminotransferase
- CI
confidence interval
- CLD
chronic liver disease
- COLD
consortium of investigators to study COVID‐19 in chronic liver disease
- COVID‐19
coronavirus disease 2019
- DM
diabetes mellitus
- HTN
hypertension
- ICU
intensive care unit
- IQR
interquartile range
- LT
liver transplantation
- OR
odds ratio
- PPIs
proton pump inhibitors
- ULN
upper limit of normal
Coronavirus disease 2019 (COVID‐19) has now claimed >1,250,000 lives around the world, with >230,000 deaths in the United States alone.( 1 ) Data on clinical outcomes and disease severity of COVID‐19 in liver transplantation (LT) recipients are limited, but initial reports raise concern for high rates of adverse outcomes.( 2 , 3 , 4 ) Bhoori et al. first reported the death of 3 LT recipients in the epicenter of COVID‐19 in Lombardy, Italy in March 2020 and postulated that posttransplant metabolic complications may drive adverse outcomes with COVID‐19 rather than immunosuppression.( 5 ) A subsequent study of 38 LT recipients from the international SECURE‐CIRRHOSIS registry showed a mortality rate of 24%.( 6 ) Other recent studies have reported mortality rates ranging from 12% to 20% and have identified factors like older age, comorbid active cancer, or renal injury to be predictive of adverse outcomes.( 2 , 3 , 4 ) Dose or type of immunosuppression do not appear to be associated with adverse outcomes, and most guidelines recommend continuing immunosuppression during COVID‐19.( 7 ) However, real‐world data on how immunosuppression was modified during COVID‐19 and the impact of these changes on graft function or outcomes are not yet clear.
Early studies have reported that COVID‐19 is associated with liver injury, which, in turn, is predictive of severe disease.( 8 , 9 , 10 ) However, it is not clear whether severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), the virus that causes COVID‐19, causes liver injury in a direct fashion or whether other factors like hepatotoxic medications and comorbid metabolic conditions play a major role. LT recipients present a unique challenge given that they are immunosuppressed and hence might not be able to mount an adequate immune response against the virus. Moreover, their immunosuppression is likely to be modified during COVID‐19, placing them at risk for acute rejection. In addition, the propensity for drug‐drug interactions is higher in these patients, raising concern for hepatotoxicity from medications used during the management of COVID‐19. More studies are needed to understand the risk for graft injury in LT recipients who acquire COVID‐19.
Here, we report data from a large U.S. multicenter study of LT recipients with COVID‐19 and define their patterns of liver enzyme abnormalities, determine the impact of changes in immunosuppression, and identify predictors of liver injury and mortality.
Materials and Methods
Study Design
This is a multicenter, observational cohort study on clinical outcomes of COVID‐19 in patients who have undergone LT. This study was carried out by the consortium of investigators to study COVID‐19 in chronic liver disease (COLD; registered Clinicaltrials.gov, NCT04439084). Inclusion criteria for this study constituted: age >18 years; laboratory‐confirmed diagnosis of COVID‐19, and history of LT (Supporting Fig. S1). The COLD registry collected deidentified data on patients within the inclusion criteria diagnosed with COVID‐19 before May 30, 2020. Only patients with COVID‐19 confirmed by PCR‐based laboratory diagnosis were included. All participating institutions independently identified patients meeting inclusion criteria and collected data. Death was attributed to COVID‐19 if it was clinically related to the COVID‐19 illness and there were no other unrelated causes of death.( 11 )
Data Collection
We collected deidentified data using 170 structured and text variables in 10 different categories: demographic data; clinical course of COVID‐19; comorbidities; laboratory tests within 6 months before the diagnosis of COVID‐19; at diagnosis of COVID‐19 and after COVID‐19; transplant status; immunosuppression; hepatotoxic medications; vasopressor use (if >12 hours); and treatment of COVID‐19. For the analysis on liver injury, only patients who had laboratory values for liver tests before and during COVID‐19 infection were included. A control group of nontransplant patients with chronic liver disease (CLD) who also had laboratory values for liver tests at the same points was used (Supporting Fig. S1).
Statistical Analysis
A predefined statistical data analysis plan was used. Continuous variables are expressed as medians and interquartile ranges (IQRs) or mean and SD, as appropriate. Categorical variables are summarized as counts and percentages. Statistical significance of differences between groups was evaluated using the independent t test or the Mann‐Whitney U test for continuous variables and the chi‐square test for categorical variables.
The primary outcome studied was the presence of acute liver injury (ALI). Given that changes in aspartate aminotransferase (AST), bilirubin, or albumin can be attributable to multiple nonhepatic factors, we used alanine aminotransferase (ALT), a more specific marker for ALI, to define liver injury. ALT values at the peak of COVID‐19 disease were used to define ALI as follows: no liver injury = ALT values <2× the upper limit of normal (ULN); moderate liver injury 2‐5× ULN; or severe liver injury >5× ULN.( 10 ) Patients with both moderate and severe liver injury were classified as having ALI. Cutoffs for normal values of ALT were 19 U/L for women and 30 U/L for men.( 12 ) The secondary outcome was all‐cause mortality. Severe COVID‐19 was defined as admission to the intensive care unit (ICU), receipt of vasopressors, or mechanical ventilation. To determine independent risk factors for the primary outcome, we performed multinomial logistic regression analyses. After considering the number of outcome events, the multivariable‐adjusted models were confined to variables that were based on clinical plausibility, statistical significance in the univariate model, and had <10% missingness (a) Informed consent was waived upon IRB approval since this was a minimal risk study. (b) the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the appropriate institutional review committee.
Results
Demographic and Clinical Characteristics of LT Recipients with COVID‐19
We identified 112 LT recipients who were diagnosed with COVID‐19 before May 30, 2020 from 15 U.S. medical centers. Median age of the cohort was 61 years (IQR, 20), and 54.5% (n = 61) were male. This racially and ethnically diverse study cohort was 39.3% (n = 44) Hispanic, 27.7% (n = 31) non‐Hispanic White, and 25.9% (n = 29) non‐Hispanic African American. The median follow‐up period for the cohort was 20.0 (IQR, 19) days. The most common comorbidities were hypertension (HTN; 53.2% [n = 59]), diabetes mellitus (DM; 45.5% [n = 51]), obesity (23.4% [n = 26]), and hyperlipidemia (20.7% [n = 23]). The most common indication for LT was hepatitis C–related cirrhosis (28.6% [n = 32]), followed by nonalcoholic fatty liver disease (NAFLD; 14.3% [n = 16]) and alcohol‐associated liver disease (ALD; 14.3% [n = 16]). Median time from LT to diagnosis of COVID‐19 was 4.0 (IQR, 11) years; 12.5% (n = 14) had been transplanted within 1 year preceding COVID‐19 diagnosis.
Clinical Outcomes of COVID‐19 in LT Recipients
All‐cause mortality in our cohort was 22.3% (n = 25). Overall, 72.3% (n = 81) were hospitalized, and median length of hospital stay was 6.5 days (IQR, 10). Among the hospitalized patients, 37.0% (n = 30) were admitted to the ICU, and 29.6% (n = 24) received vasopressors. Supplemental oxygen was given to 52.7% (n = 59), and 23.2% (n = 26) were placed on mechanical ventilation. The most common medications used for treatment of COVID‐19 were hydroxychloroquine (37.5%) or azithromycin alone (27.7%). Table 1 shows the clinical and demographic features of the cohort stratified by clinical outcomes.
TABLE 1.
Demographics and Clinical Outcomes in LT Recipients With COVID‐19
| Variable | Subcategory | All n = 112 (100%) | Death n = 25 (22.3%) | Hospitalization n = 81 (72.3%) | ICU Admission n = 30 (26.8%) |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, years | <65 | 64 (57.1%) | 10 (15.6%) | 43 (67.2%) | 13 (20.3%) |
| >/=65 | 48 (42.9%) | 15 (31.3%) | 38 (79.2%) | 17 (35.4%) | |
| Sex | Male | 61 (54.5%) | 15 (29.4%) | 40 (65.6%) | 13 (21.3%) |
| Female | 51 (45.5%) | 10 (16.4%) | 41 (80.4%) | 17 (33.3%) | |
| Race/ethnicity | NH White | 31 (27.7%) | 6 (19.4%) | 22 (71.0%) | 7 (22.6%) |
| NH Black | 29 (25.8%) | 6 (20.7%) | 20 (69.0%) | 8 (27.6%) | |
| NH Asian | 5 (4.5%) | 2 (40.0%) | 4 (80.0%) | 2 (40.0%) | |
| NH other | 3 (2.7%) | 1 (33.3%) | 1 (33.3%) | 1 (33.3%) | |
| Hispanic or Latino | 44 (39.3%) | 10 (22.7%) | 34 (77.3%) | 12 (27.3%) | |
| Indication for LT | |||||
| Hepatitis C | 32 (28.6%) | 3 (18.8%) | 11 (68.8%) | 6 (18.8%) | |
| Hepatitis B | 8 (7.1%) | 1 (12.5%) | 7 (87.5%) | 1 (12.5%) | |
| ALD | 16 (14.3%) | 6 (18.8%) | 22 (68.8%) | 5 (31.3%) | |
| NAFLD | 16 (14.3%) | 9 (56.3%) | 15 (93.8%) | 10 (62.5%) | |
| HCC | 17 (15.2%) | 6 (35.3%) | 14 (82.1%) | 7 (41.2%) | |
| Comorbidities | |||||
| DM | 51 (45.5%) | 18 (35.3%) | 40 (78.4%) | 18 (35.3%) | |
| HTN | 59 (52.6%) | 20 (33.3%) | 45 (75.0%) | 20 (33.3%) | |
| Hyperlipidemia | 23 (20.5%) | 8 (34.8%) | 21 (91.3%) | 9 (39.1%) | |
| Obesity | 26 (23.2%) | 6 (23.1%) | 19 (73.1%) | 9 (34.6%) | |
| Coronary artery disease | 9 (8.0%) | 4 (44.4%) | 8 (88.9%) | 3 (33.3%) | |
| Congestive heart failure | 6 (5.4%) | 2 (33.3%) | 5 (83.3%) | 2 (33.3%) | |
| HIV | 1 (0.9%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| COPD | 4 (3.6%) | 1 (25.0%) | 4 (100%) | 2 (50.0%) | |
| Asthma | 7 (6.3%) | 2 (28.6%) | 6 (85.7%) | 2 (28.6%) | |
| Other cancer | 7 (6.3%) | 5 (71.4%) | 7 (100%) | 4 (57.1%) | |
| Obstructive sleep apnea | 7 (6.3%) | 2 (28.6%) | 3 (42.9%) | 2 (28.6%) | |
| Metabolic syndrome | 22 (19.6%) | 8 (36.4%) | 17 (77.3%) | 9 (40.9%) | |
| Substance use | |||||
| Alcohol use | Current daily drinking | 1 (0.9%) | 0 (0.0%) | 1 (100.0%) | 0 (0.0%) |
| Social drinking | 1 (0.9%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Do not drink currently | 108 (96.4%) | 5 (4.6%) | 78 (72.2%) | 30 (27.8%) | |
| Tobacco | Current smoker | 5 (4.5%) | 0 (0.0%) | 5 (100.0%) | 1 (20.0%) |
| Former smoker | 39 (34.8%) | 10 (25.6%) | 26 (66.7%) | 12 (30.8%) | |
| Never smoker | 68 (60.7%) | 15 (22.1%) | 50 (73.5%) | 17 (25.0%) | |
| Other | Opioid use | 1 (0.9%) | 1 (100.0%) | 1 (100.0%) | 1 (100.0%) |
| Treatment for COVID‐19 | |||||
| Remedesivir | 3 (2.7%) | 1 (33.3%) | 3 (100.0%) | 2 (66.7%) | |
| Steroids | 4 (3.6%) | 2 (50.0%) | 4 (100.0%) | 3 (75.0%) | |
| HCQ+ Azithromycin | 26 (23.2%) | 12 (46.2%) | 26 (100.0%) | 16 (61.5%) | |
| HCQ alone | 42 (37.5%) | 18 (42.9%) | 42 (100.0%) | 23 (54.8%0 | |
| Azithromycin alone | 31 (27.7%) | 12 (38.7%) | 23 (90.3%) | 17 (54.8%) | |
| Immunosuppression | |||||
| Tacrolimus | 103 (91.9%) | 24 (23.3%) | 73 (70.9%) | 28 (27.2%) | |
| Cyclosporine | 7 (6.3%) | 0 (0.0%) | 6 (85.7%) | 1 (14.3%) | |
| MMF | 56 (50.0%) | 15 (26.8%) | 41 (73.2%) | 21 (37.5%) | |
| Azathioprine | 1 (0.9%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Prednisone, low dose* | 27 (24.1%) | 8 (29.6%) | 25 (92.6%) | 11 (40.7%) | |
| Prednisone, high dose † | 7 (6.3%) | 1 (14.3%) | 6 (85.7%) | 2 (28.6%) | |
| mTOR inhibitors | 4 (3.6%) | 2 (50.0%) | 4 (100.0%) | 2 (50.0%) | |
| Other | 3 (2.7%) | 1 (33.3%) | 3 (100.0%) | 1 (33.3%) |
Low dose, <20 mg/d.
High dose, >20 mg/d.
Abbreviations: COPD, chronic obstructive pulmonary disease; HCQ, hydroxychloroquine; HIV, human immunodeficiency virus; MMF, mycophenolate; mTOR, mammalian target of rapamycin; NH, non‐Hispanic; HCC, hepatocellular carcinoma.
Patterns of Liver Injury in LT Recipients with COVID‐19
Our objective was to study the patterns and predictors of ALI in transplant recipients, so we included 82 (73.2%) patients who had the following three longitudinal values of liver enzymes: (1) values before the diagnosis of COVID‐19; (2) values at the time of diagnosis of COVID‐19; and (3) peak values during COVID‐19. One patient had a documented diagnosis of acute cellular rejection and, hence, was excluded from further analysis of etiology of liver injury. We compared the incidence of liver injury with a control group of 375 nontransplant patients with CLD. The LT cohort and the control group were matched for median age (63 [IQR, 14] vs. 60 [IQR, 19]; P = 0.10), sex (males, 52.4% vs. 56.9%; P = 0.669), and incidence of comorbidities like DM (47.6% vs. 47.1%; P = 1.000), HTN (57.3% vs. 59.3%; P = 0.804), and obesity (26.8% vs. 37.5%; P = 0.075). At baseline, before the diagnosis of COVID‐19, LT recipients and nontransplant patients with CLD had similar median ALT (23 [IQR, 22] vs. 25 [IQR, 24]; P = 0.380). Peak ALT during COVID‐19 was higher in nontransplant patients with CLD than LT recipients than (41 [IQR, 60] vs. 32.5 [IQR, 44]); P = 0.043). Correspondingly, incidence of ALI (peak ALT >2× ULN) was higher in nontransplant patients with CLD than LT recipients (47.5% vs. 34.6%; P = 0.037).
Among LT recipients, all three liver enzymes (AST, ALT, and alkaline phosphatase [ALP]) showed significant increases from baseline during COVID‐19 (Fig. 1A). The pattern of liver injury was predominantly hepatocellular, with the highest increases between baseline and peak values observed in AST (median, 19 vs. 41 IU/L; P < 0.001) and ALT (median, 23 vs. 32 IU/L; P < 0.001), followed by ALP (median, 100 vs. 120 IU/L; P = 0.007). AST and ALT values closely correlated at all three time points (P < 0.0001 all three). Bilirubin also increased slightly (median, 0.6 vs. 0.7; P = 0.03) during COVID‐19, whereas albumin significantly decreased (3.7 vs. 2.8; P < 0.001; Fig. 1A). Furthermore, we evaluated a subgroup of 33 patients who had clinical resolution of COVID‐19. A significant decrease in AST (44.9 vs. 29.0; P = 0.005), ALT (40.3 vs. 29.8; P = 0.06), and ALP (212.8 vs. 156.3; P = 0.04) was noted upon COVID‐19 resolution in this cohort. On review of other parameters, a decrease in both leukocytes (6.00 vs. 5.18; P = 0.03) and platelets (198 vs. 158; P < 0.001) was observed during COVID‐19.
FIG. 1.
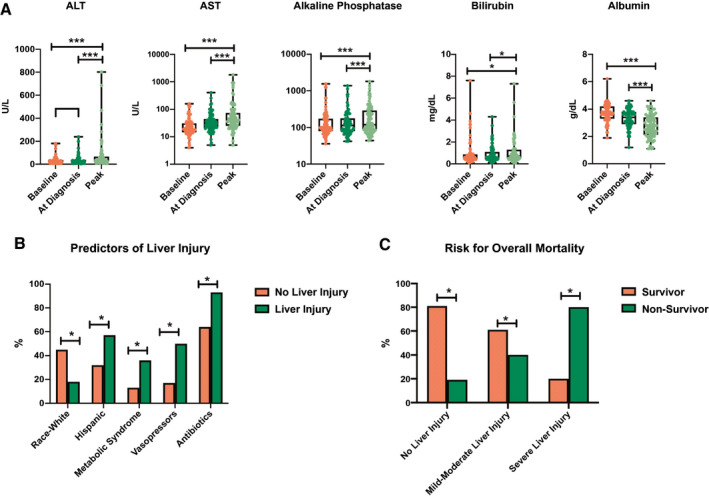
Liver injury in LT recipients with COVID‐19. (A) Pattern of liver test elevations comparing baseline values with values at diagnosis of COVID‐19 and peak values during COVID‐19. (B) Predictors of liver injury in LT recipients with COVID‐19. (C) Risk for overall mortality in LT recipients with COVID‐19 with patients stratified by presence of liver injury. *P < 0.05; ***P < 0.001.
Predictors of Liver Injury in Transplant Recipients during COVID‐19
We wanted to determine predictors of liver injury during COVID‐19 in LT recipients. The majority of LT recipients had peak ALT <2× ULN (65.4% [n = 53]), whereas moderate elevation in ALT 2‐5× ULN was noted in 22.2% (n = 18) and severe elevation >5× ULN in 12.3% (n = 10). Table 2 shows the proportion of liver injury observed in the different clinical and demographic subgroups. On univariate analysis, patients with more severe COVID‐19, that is, patients who were admitted to the ICU (P = 0.002), received vasopressors (P < 0.001), or were mechanically ventilated (P < 0.001), were more likely to have ALI (ALT <2× ULN). We obtained a detailed list of several classes of potentially hepatotoxic medications the patients had received (Supporting Table S1). Among them, receipt of antibiotics was associated with risk for liver injury (P = 0.016), whereas use of statins, proton pump inhibitors (PPIs), or acetaminophen was not. None of the medications used to treat COVID‐19 were associated with liver injury.
TABLE 2.
Liver Injury During COVID‐19 in LT Recipients
| Variable | Covariate | All Patients (n = 81) | ALT <2× ULN (n = 53; 65.4%) | ALT 2‐5× ULN (n = 18; 22.2%) | ALT >5× ULN (n = 10, 12.3%) | P Value |
|---|---|---|---|---|---|---|
| Age, years | Median (IQR) | 63 (14) | 65.0 (13) | 57.5 (23) | 62.0 (11) | 0.406 |
| Sex | Male | 38 (46.9%) | 29 (54.7) | 8 (44.4) | 5 (50.0) | 0.747 |
| Female | 42 (51.9%) | 24 (45.3) | 10 (55.6) | 5 (50.0) | ||
| Race/ethnicity | NH White | 39 (48.1%) | 18 (34.0) | 2 (11.0) | 1 (10.0) | 0.038 |
| NH Black | 21 (25.9%) | 15 (28.3) | 6 (33.3) | 1 (10.0) | ||
| NH Asian | 22 (27.2%) | 3 (5.7) | 1 (5.6) | 0 (0) | ||
| Hispanic or Latino | 33 (40.7%) | 17 (32.1) | 9 (50.0) | 7 (70.0) | ||
| Comorbidities | DM | 38 (46.9%) | 20 (37.7) | 12 (66.7) | 6 (60.0) | 0.071 |
| HTN | 46 (56.8%) | 26 (49.1) | 11 (61.1) | 9 (90.0) | 0.050 | |
| Hyperlipidemia | 20 (24.7%) | 9 (17.0) | 7 (38.9) | 4 (40.0) | 0.086 | |
| Obesity | 21 (25.9%) | 11 (20.8) | 6 (33.3) | 4 (40.0) | 0.319 | |
| Metabolic syndrome | 17 (21.0%) | 7 (13.2) | 6 (33.3) | 4 (40.0) | 0.056 | |
| Coronary artery disease | 9 (11.1%) | 5 (9.4) | 1 (5.6) | 3 (30.0) | 0.115 | |
| Severity of COVID‐19 | Hospitalization | 71 (87.7%) | 44 (83.0) | 17 (94.4) | 10 (100.0) | 0.199 |
| ICU admission | 28 (34.6%) | 12 (22.6) | 7 (38.9) | 9 (90.0) | 0.002 | |
| Mechanical ventilation | 24 (29.6%) | 9 (17.0) | 6 (33.3) | 9 (90.0) | <0.001 | |
| Vasopressor use | 23 (28.4%) | 9 (17.0) | 5 (27.8) | 9 (90.0) | <0.001 | |
| Supplemental oxygen | 54 (66.7%) | 33 (62.3) | 11 (61.1) | 10 (100.0) | 0.057 | |
| Death | 25 (30.9%) | 10 (18.6) | 6 (33.3) | 8 (80.0) | 0.003 | |
| Hepatotoxic medication | Yes | 65 (80.2%) | 40 (75.4) | 16 (88.8) | 9 (90.0) | 0.331 |
| Any antibiotics | 60 (74.1%) | 34 (64.15) | 16 (88.8) | 10 (100.0) | 0.016 | |
| Cephalosporin | 40 (49.4%) | 21 (40.4) | 10 (55.6) | 9 (90.0) | 0.012 | |
| APAP | 29 (35.8%) | 16 (49.1) | 8 (44.4) | 5 (50.0) | 0.335 | |
| Statins | 16 (19.8%) | 9 (17.0) | 4 (22.2) | 3 (30.0) | 0.610 | |
| PPI | 33 (40.7%) | 21 (39.6) | 8 (44.4) | 4 (40.0) | 0.936 | |
| COVID‐19 treatment | Hydroxychloroquine + azithromycin | 23 (28.4%) | 13 (24.5) | 6 (33.3) | 4 (40.0) | 0.246 |
| Hydroxychloroquine | 16 (19.8%) | 8 (15.1) | 5 (27.8) | 3 (30.0) | 0.347 | |
| Immunosuppression | Tacrolimus | 73 (90.1%) | 46 (86.7) | 17 (94.4) | 10 (100.0) | 0.344 |
| Cyclosporine | 6 (7.4%) | 6 (11.3) | 0 (0) | 0 (0) | 0.181 | |
| MMF | 41 (50.6%) | 28 (52.8) | 6 (33.3) | 7 (70.0) | 0.153 | |
| Prednisone, low dose | 22 (27.2%) | 14 (45.3) | 4 (18.2) | 4 (40.0) | 0.586 | |
| Change in immunosuppression | Decreased tacrolimus | 21 (25.9%) | 12 (22.6) | 4 (22.2) | 5 (50.0) | 0.735 |
| Held MMF | 27 (33.3%) | 18 (34.0) | 4 (22.2) | 5 (50.0) | 0.617 | |
| Labs (peak COVID‐19) | Creatinine | 1.9 (2.3) | 1.6 (2.3) | 1.8 (1.7) | 4.1 (2.3) | 0.026 |
| WBC | 5.3 (4.6) | 5.0 (3.8) | 5.5 (7.4) | 14.1 (20.6) | 0.237 | |
| Neutrophil | 3.4 (4.1) | 3.3 (3.8) | 4.2 (5.0) | 2.7 (10.5) | 0.792 | |
| Lymphocyte | 0.8 (0.7) | 0.8 (0.6) | 0.9 (1.4) | 0.6 (2.1) | 0.145 |
Bold signifies statistically significant.
Abbreviations: APAP, acetaminophen; MMF, mycophenolate; NH, non‐Hispanic; WBC, white blood cell count.
Multivariate logistic regression was performed to identify independent predictors of liver injury (ALT >2× ULN). Non‐Hispanic White transplant recipients had a lower risk for liver injury during COVID‐19 (P = 0.016; odds ratio [OR], 0.13; 95% confidence interval [CI], 0.02‐0.68). Younger age (P = 0.009; OR, 2.06; 95% CI, 1.20‐3.54), Hispanic ethnicity (P = 0.011; OR, 6.01; 95% CI, 1.51‐23.9), metabolic syndrome (P = 0.016; OR, 5.87; 95% CI, 1.38‐24.99), receipt of vasopressors (P = 0.018; OR, 7.34; 95% CI, 1.39‐38.52), and antibiotic use (P = 0.046; OR, 6.93; 95% CI, 1.04‐46.26) were associated with independent risk for liver injury (Fig. 1B).
Changes in Immunosuppression were not associated with Liver Injury during COVID‐19
Tacrolimus (90.1% [n = 73]) was the most common immunosuppressant used, followed by mycophenolate (50.6% [n = 41]) and low‐dose (<20 g/d) prednisone (27.2% [n = 22]; Supporting Fig. S2A). Immunosuppression was modified in approximately half the patients during COVID‐19 (49.4% [n = 40]). Immunosuppression was mostly changed in patients who had more severe COVID‐19, with a higher likelihood of modification in those who were admitted to the ICU (P = 0.020), received vasopressors (P = 0.008), or were on mechanical ventilation (P = 0.012; Supporting Fig. S2B). The most common change was holding mycophenolate (33.3% [n = 27]), followed by decrease in tacrolimus dose (25.9% [n = 21]) or holding tacrolimus (4.9% [n = 4]). Acute cellular rejection was reported only in 1 patient in our cohort, and that patient’s tacrolimus dose had been reduced during COVID‐19. Reducing tacrolimus (P = 0.735) or holding mycophenolate (P = 0.617) were not associated with liver injury (Table 2). Overall, reduction in immunosuppression during COVID‐19 was not associated with liver injury (P = 0.156) or risk for mortality (P = 0.084; Table 3).
TABLE 3.
Multivariate Analysis of Predictors of ALI in LT Recipients With COVID‐19
| Covariate | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| P Value | OR | 95% CI | P Value | OR | 95% CI | |
| Age (years) | 0.286 | 1.21 | 0.85‐1.71 | 0.009 | 2.06 | 1.20‐3.54 |
| Female sex | 0.494 | 0.72 | 0.28‐1.79 | 0.333 | 1.93 | 0.51‐7.33 |
| Race, White | 0.016 | 0.26 | 0.08‐0.79 | 0.016 | 0.13 | 0.024‐0.680 |
| Ethnicity, Hispanic | 0.035 | 2.82 | 1.09‐7.26 | 0.011 | 6.01 | 1.51‐23.90 |
| Metabolic syndrome | 0.024 | 3.65 | 1.20‐11.07 | 0.016 | 5.87 | 1.38‐24.99 |
| Antibiotics | 0.007 | 7.27 | 1.55‐34.02 | 0.046 | 6.93 | 1.04‐46.26 |
| Vasopressors | 0.004 | 4.88 | 1.74‐13.71 | 0.018 | 7.34 | 1.39‐38.52 |
| Oxygen requirement | 0.324 | 1.81 | 0.65‐5.04 | |||
| Immunosuppression modified | 0.156 | 2.07 | 0.81‐5.37 | |||
| Hydroxychloroquine | 0.157 | 2.250 | 0.74‐6.85 | |||
| Azithromycin | 0.312 | 1.8 | 0.68‐4.77 | |||
| Any hepatotoxic | 0.240 | 2.71 | 0.70‐10.46 | |||
| APAP | 0.223 | 2.000 | 0.78‐5.16 | |||
| Statins | 0.396 | 1.630 | 0.53‐4.98 | |||
| Creatinine (peak during COVID‐19) | 0.799 | 0.971 | 0.77‐1.22 | |||
| PPI | 0.815 | 1.140 | 0.45‐2.89 | |||
Bold signifies statistically significant.
Abbreviation: APAP, acetaminophen.
ALI is Associated with Mortality in Transplant Recipients and COVID‐19
We evaluated predictors of overall mortality in LT recipients with COVID‐19 (Table 4). After adjusting for age, sex, race, ethnicity, comorbidities, time posttransplantation, and immunosuppression, presence of liver injury was significantly and independently associated with higher overall mortality (P = 0.007; OR = 6.91; 95% CI, 1.68‐28.48; Fig. 1B). The other factor independently associated with overall mortality was DM (P = 0.04; OR = 3.73; 95% CI, 1.04‐13.45). Furthermore, we confirmed that baseline ALT before COVID‐19 (nonsurvivors 33.8% vs. survivors 29.2%; P = 0.530) or ALT at diagnosis (nonsurvivors 38.4% vs. survivors 34.0%; P = 0.641) of COVID‐19 did not predict higher mortality. However, peak ALT was significantly higher in nonsurvivors than survivors (149.8 vs. 43.5; P = 0.001).
TABLE 4.
Multivariate Analysis of Predictors of Mortality in LT Recipients With COVID‐19
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| P Value | OR | 95% CI | P Value | Hazard Ratio | 95% CI | |
| Liver injury | 0.002 | 4.96 | 1.80‐13.65 | 0.007 | 6.91 | 1.67‐28.48 |
| Age (>65 years) | 0.150 | 2.15 | 0.82‐5.63 | 0.130 | 2.93 | 0.73‐11.81 |
| Sex (female) | 0.229 | 0.5 | 0.19‐1.30 | 0.066 | 3.69 | 1.11‐12.21 |
| Race, non‐Hispanic White | 0.803 | 0.78 | 0.28‐2.13 | 0.224 | 1.17 | 0.35‐3.86 |
| Ethnicity, Hispanic | 1.000 | 1 | 0.36‐2.50 | 0.153 | 2.01 | 0.67‐13.38 |
| DM | 0.004 | 4.630 | 1.65‐12.96 | 0.044 | 3.7 | 1.04‐13.09 |
| HTN | 0.028 | 3.41 | 1.18‐9.78 | 0.763 | 2.04 | 0.61‐6.94 |
| Active cancer | 0.027 | 6.75 | 1.21‐37.63 | 0.127 | 10.86 | 1.04‐113.47 |
| Hyperlipidemia | 0.404 | 1.72 | 0.60‐4.92 | |||
| Obesity | 1.000 | 0.86 | 0.29‐2.57 | |||
| Metabolic syndrome | 0.140 | 2.45 | 0.82‐7.44 | |||
| Time transplant (<1 year) | 1.000 | 0.91 | 0.35‐2.40 | |||
| Immunosuppression Modified | 0.084 | 2.51 | 0.90‐6.95 | |||
| Tacrolimus | 0.424 | 3.45 | 0.39‐29.47 | |||
| Cyclosporine | 0.332 | 0.75 | 0.67‐0.84 | |||
| MMF | 0.337 | 1.73 | 0.66‐4.51 | |||
| Prednisone, low dose | 0.592 | 1.41 | 0.50‐3.96 | |||
Bold signifies statistically significant.
Abbreviations: HLD, hyperlipidemia; MMF, mycophenolate.
We analyzed predictors of ICU admission among LT recipients with COVID‐19. Incidence of liver injury was associated with significant and independent risk for ICU admission (P = 0.007; OR, 7.93; 95% CI, 1.75‐35.69; Supporting Table S2).
Discussion
Liver injury has been reported in a significant proportion of nontransplant patients with COVID‐19, but data on transplant recipients are scarce. LT recipients are at particular risk for graft injury given their immunocompromised state, high prevalence of metabolic comorbidities, and risk for drug‐drug interactions leading to hepatotoxicity. Our multicenter, observational study of 112 patients explores clinical outcomes and patterns of liver injury in LT recipients with COVID‐19. We found that 34.6% of LT recipients had liver injury during COVID‐19, with liver enzyme elevations predominantly in a hepatocellular pattern. Age, Hispanic ethnicity, metabolic syndrome, and vasopressor and antibiotic use predicted liver injury, highlighting the multifactorial nature of this process. Moreover, liver injury was independently associated with risk for mortality and ICU admission. Hence, following liver enzymes closely can help in the early identification of LT recipients at risk for adverse outcomes with COVID‐19. Type of immunosuppression did not have an impact on mortality or liver injury. Real‐world data from our study shows that immunosuppression was modified in 50% of patients during COVID‐19, but only 1 patient experienced acute rejection and none of the patients experienced graft failure. These data are reassuring and will hopefully guide physicians taking care of LT recipients with COVID‐19.
Recent studies in the nontransplant general population have shown that liver injury is relatively common during COVID‐19, with rates ranging from 15% to 53%.( 8 , 10 ) In our study, we show that around one third of LT recipients sustained ALI during COVID‐19, but despite being immunocompromised, this rate was lower than that of our nontransplant cohort with CLD (47.5%). There has been significant concern that SARS‐CoV‐2 may cause cholestatic liver injury given that angiotensin‐converting enzyme 2, the host cell receptor for the virus, has been reported to be expressed on cholangiocytes.( 13 ) But data, including results from our study, consistently show a predominantly hepatocellular pattern of injury.( 13 , 14 , 15 ) This raises the possibility that SARS‐CoV‐2 may cause direct hepatocellular damage, as recently reported by Wang et al.( 16 ) Other nonhepatotropic viruses have also been shown to be associated with a similar pattern of liver injury. In fact, rates of liver injury in viral infections from severe acute respiratory syndrome (SARS; 50.3%) and other human coronaviruses (36.0%) were higher than COVID‐19 (22.5%).( 8 ) However, most of these data are from nontransplant patients. In transplant recipients, even though SARS‐associated coronavirus and Middle East respiratory syndrome coronavirus have rarely been reported to cause transaminitis,( 17 , 18 ) our study clearly shows that SARS‐CoV‐2 is associated with liver injury in a substantial proportion of LT recipients.
Drug‐induced liver injury (DILI) is a major cause for abnormal liver tests. We collected an extensive history of various classes of potentially hepatotoxic medications used during the clinical course of COVID‐19. We show that patients in the ICU who received vasopressors were more likely to have liver injury, highlighting the association of liver injury with hypotension in severe COVID‐19. We identified use of antibiotics, several of which are known to cause DILI, to be another risk factor for liver injury. However, use of acetaminophen, PPIs, and statins were not associated with liver injury, and these medications can be safely continued during COVID‐19. Moreover, medications commonly used to treat COVID‐19 were not associated with significant liver injury, and this should encourage physicians to continue to offer such treatment in this patient population even in the presence of mild‐to‐moderate ALT elevations.
Overall mortality in our study was 22.3% (n = 25). A similar mortality rate of 20.5% has been reported from 482 solid organ transplant recipients in the United States.( 19 ) The Centers for Disease Control and Prevention reports that the general U.S. population has a COVID‐19 mortality rate of 5%.( 20 ) However, the median age of LT recipients who acquire COVID‐19 is higher at 60.1 years compared to the reported median age of 48 years in the general population.( 20 ) Furthermore, metabolic comorbidities like DM and HTN, which are known to increase COVID‐19 severity, were present in almost 50% of the LT recipients in our cohort whereas it was present in less than one third of the general population with COVID‐19.( 20 ) A recent study does report that mortality among COVID‐19 SOT recipients is similar to the general population, after controlling for age and other comorbidities.( 21 ) Another study from Spain, of 111 LT recipients, showed that mortality rates were actually lower in LT recipients than the matched general population.( 22 ) Based on these studies, it appears that transplant itself is not a risk factor of higher COVID‐19 mortality, but coexisting comorbidities are. In our study, we report that liver injury is associated with COVID‐19 severity. Thus, closely monitoring liver tests can enable earlier identification of patients at risk for adverse outcomes. A promising treatment directed against SARS‐CoV‐2 that reduces the severity of COVID‐19 can potentially also decrease the incidence of liver injury.
During the initial phases of the COVID‐19 pandemic, it was not clear how, or whether, immunosuppression should be reduced in LT recipients with COVID‐19. Following evaluation of early reports, several societies published guidelines recommending continuation of immunosuppression at stable doses for most patients.( 7 , 23 , 24 ) Real‐world data from our study show that immunosuppression was actually modified in 50% of patients, but only 1 patient experienced acute rejection. As expected, immunosuppression was more likely to have been reduced in patients with more severe COVID‐19 (i.e., those who were in the ICU on vasopressors). Nonetheless, decreases in immunosuppression were not associated with liver injury or mortality. These data are reassuring and will hopefully guide physicians taking care of LT recipients with COVID‐19.
To our knowledge, our study represents one of the largest multicenter cohorts of LT recipients from the United States. Another strength of our study is that we evaluated serial changes in ALT to more accurately identify those with liver injury. Different studies have used varying definitions of liver injury; we used standard definitions for ALT cutoffs( 12 ) that have also been used by other groups.( 8 , 10 ) We believe that ALT is a more specific marker of liver injury in this context given that AST can originate from the heart, which is also known to be affected during COVID‐19.( 25 ) One limitation of our study is potential referral bias, given that most of the centers contributing patients to our cohort are tertiary referral centers, but we did include both out‐ and inpatients in this cohort and have captured patients with mild and severe COVID‐19. Another limitation is the lack of a control group of the general population with COVID‐19, which prevents us from analyzing whether transplant recipients are at higher risk for mortality. Nonetheless, our study furthers our understanding of patterns and incidence of liver injury in LT recipients when compared to a control group of nontransplant patients with CLD. Last, longer‐term follow‐up will be needed to study the course of liver injury after COVID‐19 resolution, but we do present reassuring data from a subgroup of patients who have already recovered.
In conclusion, we show that liver injury is common and independently associated with mortality with COVID‐19. We recommend that liver enzymes should be closely monitored in LT recipients with COVID‐19, given that they can serve as predictors of outcome. We performed a detailed analysis of various potentially overlapping causes of liver injury and determined that it is mostly driven by hepatotoxic medications and severity of COVID‐19. Although antibiotics have been commonly utilized in the direct management of COVID‐19, as well as secondary infections, judicious antibiotic stewardship remains a target in infected LT recipients and may reduce risk of liver injury. Furthermore, avoiding hypotension in patients admitted to ICU can potentially mitigate liver injury. Decisions regarding modifications to immunosuppression regimens in LT recipients with COVID‐19 need to be made on a case‐by‐case basis, but our study shows that immunosuppression can be safely reduced, if necessary. Lastly, Although our subgroup analysis shows that liver injury appears to be transient and mostly resolves following recovery from COVID‐19, extended follow‐up will be needed to understand long‐term effects.
Author Contributions
Manuscript writing, Data contribution, A.R., B.S. Data Analysis, Data contribution, N.A. Data contribution, Study Design, Manuscript editing, N.L., S.K., P.P.B., E.S.A., P.V.P., A.M.C., K.W., R.M.C., C.A., V.L.C., V.N., W.D., K.C., A.M. Manuscript writing, Data Analysis, Data coordination, Study Planning, R.D.
Supporting information
Supplementary Material
Acknowledgment
We thank the following persons for their expertise and assistance throughout all aspects of our study:
Zoe Reinus, Michael Daidone, Julia Sjoquist, Faruq Pradhan, Mohanad Al‐Qaisi, Nael Haddad, Nicholas Blackstone, Katherine Marx, Susan McDermott, Alyson Kaplan, Mallori Ianelli, Julia Speiser, Angela Wong, Dhuha Alhankawi, Sunny Sandhu, Sameeha Khalid, Aalam Sohal, and Christina Gainey
Jennifer Smart, Neil Marimoto for their administrative support.
We also thank all of the first‐line responders who are working tirelessly and with dedication to care for patients and their families during the COVID‐19 crisis.
Members of the COLD consortium
Donghee Kim MD PhD, Stanford University, CA; Paul Kwo MD, Stanford University, CA. Marina Roytman MD, Univeristy of San Francisco, Fresno, CA; Kathleen Viveiros MD, Brigham and Women's Hospital, MA. Walter Chan MD MPH, Brigham and Women's Hospital, MA. Michel Li MD, Brigham and Women's Hospital, MA. Alexander Vogel MD, Brigham and Women's Hospital, MA. Kara Wegerman MD, Duke University, NC. Tzu‐Hao Lee MD, Duke University, NC. Kali Zhou MD, University of Southern California, LA. Blanca Lizaola‐Mayo MD, David Chascsa, M.D, Mayo Clinic, AZ.
Potential conflict of interest: Dr. Bloom consults for Synlogic. Dr. Carr advises for and received grants from Intercept. Dr. Latt received grants from Gilead.
Contributor Information
Renumathy Dhanasekaran, Email: dhanaser@stanford.edu.
the COLD Consortium:
Donghee Kim, Marina Roytman, Kathleen Viveiros, Walter Chan, Michael Li, Alexander Vogel, Kara Wegerman, Tzu‐Hao Lee, and Kali Zhou
References
- 1. Johns Hopkins University & Medicine . COVID‐19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). 2020. https://coronavirus.jhu.edu/map.html. Accessed on November 9, 2020.
- 2. Lee BT, Perumalswami PV, Im GY, Florman S, Schiano TD. COVID‐19 in liver transplant recipients: an initial experience from the U.S. epicenter. Gastroenterology 2020;159:1176‐1178.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Becchetti C, Zambelli MF, Pasulo L, Donato MF, Invernizzi F, Detry O, et al. COVID‐19 in an international European liver transplant recipient cohort. Gut 2020. Jun 22. 10.1136/gutjnl-2020-321923. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Belli LS, Duvoux C, Karam V, Adam R, Cuervas‐Mons V, Pasulo L, et al. COVID‐19 in liver transplant recipients: preliminary data from the ELITA/ELTR registry. Lancet Gastroenterol Hepatol 2020;5:724‐725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bhoori S, Rossi RE, Citterio D, Mazzaferro V. COVID‐19 in long‐term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy. Lancet Gastroenterol Hepatol 2020;5:532‐533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Webb GJ, Moon AM, Barnes E, Barritt AS, Marjot T. Determining risk factors for mortality in liver transplant patients with COVID‐19. Lancet Gastroenterol Hepatol 2020;5:643‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bollipo S, Kapuria D, Rabiee A, Ben‐Yakov G, Lui RN, Lee HW, et al. One world, one pandemic, many guidelines: management of liver diseases during COVID‐19. Gut 2020;69:1369‐1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yip TC, Lui GC, Wong VW, Chow VC, Ho TH, Li TC, et al. Liver injury is independently associated with adverse clinical outcomes in patients with COVID‐19. Gut 2020. Jul 8. 10.1136/gutjnl-2020-321726. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9. Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, et al. COVID-19: Abnormal liver function tests. J Hepatol. [Internet]. 2020;73:566‐574. 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Phipps MM, Barraza LH, LaSota ED, Sobieszczyk ME, Pereira MR, Zheng EX, et al. Acute liver injury in COVID‐19: prevalence and association with clinical outcomes in a large US cohort. Hepatology 2020. May 30. 10.1002/hep.31404. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. National Center for Health Statistics . Guidance for certifying deaths due to coronavirus disease 2019 (COVID‐19). Report No. 3. April 2020. www.cdc.gov/nchs/data/nvss/vsrg/vsrg03-508.pdf. Accessed October 15, 2020.
- 12. Kasarala G, Tillmann HL. Standard liver tests. Clin Liver Dis 2016;8:13‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019‐nCoV infection. bioRxiv 2020.02.03.931766; 10.1101/2020.02.03.931766. [DOI] [Google Scholar]
- 14. Alqahtani SA, Schattenberg JM. Liver injury in COVID‐19: the current evidence. United European Gastroenterol J 2020;8:509‐519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, et al. Clinical features of COVID‐19‐related liver functional abnormality. Clin Gastroenterol Hepatol 2020;18:1561‐1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, et al. SARS‐CoV‐2 infection of the liver directly contributes to hepatic impairment in patients with COVID‐19. J Hepatol 2020;73:807‐816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kumar D, Tellier R, Draker R, Levy G, Humar A. Severe Acute Respiratory Syndrome (SARS) in a liver transplant recipient and guidelines for donor SARS screening. Am J Transplant 2003;3:977‐981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. AlGhamdi M, Mushtaq F, Awn N, Shalhoub S. MERS CoV infection in two renal transplant recipients: case report. Am J Transplant 2015;15:1101‐1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kates OS, Haydel BM, Florman SS, Rana MM, Chaudhry ZS, Ramesh MS, et al. COVID‐19 in solid organ transplant: a multi‐center cohort study. Clin Infect Dis 2020. Aug 7. 10.1093/cid/ciaa1097. [Epub ahead of print] [DOI] [Google Scholar]
- 20. Stokes EK, Zambrano LD, Anderson KN, Marder EP, Raz KM, El Burai FS, et al. Coronavirus Disease 2019 Case Surveillance—United States, January 22–May 30, 2020. MMWR Morb Mortal Wkly Rep 2020;69:759‐765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rinaldi M, Bartoletti M, Bussini L, Pancaldi L, Pascale R, Comai G, et al. COVID‐19 in solid organ transplant recipients: no difference in survival compared to general population. Transpl Infect Dis 2020. Aug 2. 10.1111/tid.13421. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Colmenero J, Rodríguez‐Perálvarez M, Salcedo M, Arias‐Milla A, Muñoz‐Serrano A, Graus J, et al. Epidemiological pattern, incidence and outcomes of COVID‐19 in liver transplant patients. J Hepatol 2020. Aug 1. 10.1016/j.jhep.2020.07.040. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fix OK, Hameed B, Fontana RJ, Kwok RM, McGuire BM, Mulligan DC, et al. Clinical best practice advice for hepatology and liver transplant providers during the COVID‐19 pandemic: AASLD Expert Panel Consensus Statement. Hepatology 2020;72:287‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boettler T, Newsome PN, Mondelli MU, Maticic M, Cordero E, Cornberg M, et al. Care of patients with liver disease during the COVID‐19 pandemic: EASL‐ESCMID position paper. JHEP Rep 2020;2:100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meyer P, Ghadri JR, Templin C. Coronavirus disease 2019 (COVID‐19) and cardiac injury. JAMA Cardiol 2020. Jul 8. 10.1001/jamacardio.2020.2453. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


