Abstract
Background and Purpose
Since the outbreak of the severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) pandemic, several reports indicated neurological involvement in COVID‐19 disease. Muscle involvement has also been reported as evidenced by creatine kinase (CK) elevations and reports of myalgia.
Methods
Creatine kinase, markers of inflammation, pre‐existing diseases and statin use were extracted from records of Austrian hospitalised COVID‐19 patients. Disease severity was classified as severe in case of intensive care unit (ICU) admission or mortality. COVID‐19 patients were additionally compared to an historical group of hospitalised influenza patients.
Results
Three hundred fifty‐one patients with SARS‐CoV‐2 and 258 with influenza were included in the final analysis. CK was elevated in 27% of COVID‐19 and in 28% of influenza patients. CK was higher in severe COVID‐19 as were markers of inflammation. CK correlated significantly with inflammation markers, which had an independent impact on CK when adjusted for demographic variables and disease severity. Compared to influenza patients, COVID‐19 patients were older, more frequently male, had more comorbidities, and more frequently had a severe disease course. Nevertheless, influenza patients had higher baseline CK than COVID‐19, and 35.7% of intensive care unit (ICU)‐admitted patients had CK levels >1,000 U/L compared to only 4.7% of ICU‐admitted COVID‐19 patients.
Conclusions
HyperCKemia occurs in a similar frequency in COVID‐19 and influenza infection. CK levels were lower in COVID‐19 than in influenza in mild and severe disease. CK levels strongly correlate with disease severity and markers of inflammation. To date, it remains unclear whether hyperCKemia is due to a virus‐triggered inflammatory response or direct muscle toxicity.
Keywords: COVID‐19, creatine kinase, hyperCKemia, influenza
Creatine kinase (CK) levels were compared between mild and severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) and influenza cases. CK was higher in severe disease and higher in influenza as compared to COVID‐19. Although the mechanisms are yet unknown, it appears that SARS‐CoV‐2 is less myotoxic than the influenza virus.

INTRODUCTION
In early December 2019, the first cases of atypical pneumonia of unknown origin were observed and reported in China. The novel causative virus was rapidly identified and finally named severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2). COVID‐19 disease quickly spread around the world, and Northern Italy and some areas in Austria became early hotspots in Europe.
Since the outbreak of the disease and the detection of the virus, several reports indicated neurological involvement in SARS‐CoV‐2 infection [1, 2, 3, 4, 5]. Central nervous system manifestations ranged from impaired consciousness, acute cerebrovascular disease and ataxia to headache, seizures and dizziness. Severe disorders of the neuromuscular system, such as cases of peri‐ and postinfectious Guillain‐Barre syndrome [6, 7, 8, 9, 10, 11, 12] and myasthenic crises [13, 14, 15, 16], were reported, even though milder symptoms in the form of peripheral neuropathic pain and impairment of taste and smell [1, 17] were more frequent. Muscle pain and elevated creatine kinase (CK) levels as indications of muscle disorders have been observed in several case series from China [1, 18, 19]. In addition, a few cases of rhabdomyolysis were reported [20, 21], and Dalakas [22] suggested that these patients suffered from COVID‐19–triggered necrotizing autoimmune myositis. It has also been suggested that population‐specific characteristics exist that influence disease severity and presentation. The latter is supported by the higher prevalence of olfactory and gustatory dysfunction in European (85.6%; [17] than in Asian patients (5.1%; [1]). HyperCKemia, on the other hand, seems to occur with a similar frequency of 13.7% and 9.2% in Asian [18] and European [2] patients, respectively.
The present study aimed to investigate muscle involvement in an Austrian cohort of patients with SARS‐CoV‐2 infection by analysing CK values, their evolution over the first 2 weeks of the disease course, the relationship to general markers of inflammation and disease severity. Finally, these results were compared to those of an influenza cohort.
METHODS
SARS‐CoV‐2 cohort
Patient records from three designated COVID‐19 treatment centres, the University Hospital Innsbruck, St. Vincent Hospital Zams and Kaiser Franz Josef Hospital Vienna, were retrospectively analysed. The study is part of a larger study of SARS‐CoV‐2 infection and was approved the Ethics committees of the Medical University Innsbruck (EKNR 1167‐2020) and the Kaiser Franz Josef Hospital (EK 20‐079‐VK).
Values for CK, C‐reactive protein (CRP), white cell blood (WBC) count, procalcitonin, interleukin‐6 (IL‐6) and ferritin were obtained at the day of hospitalisation (baseline), day 7 (±2 days) and day 14 (±2 days), when available. Patients were included into the study when at least one CK value (i.e. at baseline, day 7 or day 14) was available. HyperCKemia was defined as CK exceeding the upper limit of normal (>190 U/L in males and >170 U/L in females).
Known pre‐existing factors affecting disease severity were recorded: diabetes mellitus (DM), coronary heart disease (CHD), arterial hypertension and chronic obstructive lung disease (COPD). Also, the use of statins was recorded as a possible confounder for CK elevations. Disease severity was classified based on admission to an intensive care unit (ICU) and mortality, respectively. Various treatments have been used: hydrochloroquine in 44, favipiravir in 18, ropinavir/ritonavir in 15, and remdesivir in one patient. Muscle biopsies were not performed.
Influenza cohort
For comparison, demographic information, comorbidities, CK and CRP values from patients diagnosed with influenza at the University Hospital Innsbruck were extracted from anonymised patient records [23]. Data on treatment were not available.
Statistics
Statistical analysis was performed using SPSS 26.0 (IBM). Distribution of data was assessed by Kolmogorov‐Smirnov test, and nonparametric data were displayed as median and interquartile range. Spearman coefficient was used for correlation analysis. For group comparisons, the Mann‐Whitney U test and Pearson χ 2 test were applied as appropriate. Paired samples were compared by Friedman test. Linear regression was employed to identify predictors for increased CK levels including age, sex, centre, admission to the ICU, death and various inflammatory markers in peripheral blood (e.g., CRP). The dependent variable (CK) and the covariates CRP, WBC, IL‐6, procalcitonin and ferritin were log‐transformed to achieve normal distribution. A p value <0.05 was considered statistically significant.
RESULTS
From a total of 609 patients, 351 with SARS‐CoV‐2 and 258 with influenza were included in the final analysis. Demographics, the main clinical characteristics and results of laboratory analyses are shown in Table 1. COVID‐19 patients were older, more often male, had more comorbidities and significantly more frequently severe disease than influenza patients (Table 1).
TABLE 1.
Demographic and laboratory findings in COVID‐19 and influenza
| SARS‐CoV‐2 cohort | Influenza cohort | p value | |||
|---|---|---|---|---|---|
| n | n | ||||
| Age, years | 351 | 68 (54–79) | 258 | 51 (32–72) | <0.001a |
| Sex, female, n (%) | 351 | 137 (39) | 258 | 127 (49) | 0.012b |
| ICU admission, n (%) | 349 | 73 (21) | 258 | 19 (7) | <0.001b |
| Death, n (%) | 351 | 53 (15.1) | 258 | 12 (4.7) | <0.001b |
| Concomitant diseases | |||||
| Coronary artery disease, n (%) | 344 | 66 (19) | 258 | 85 (33) | <0.001b |
| Arterial hypertension, n (%) | 346 | 172 (50) | 75 | 22 (29) | 0.001b |
| Diabetes mellitus, n (%) | 346 | 62 (18) | 75 | 11 (15) | 0.500b |
| COPD, n (%) | 348 | 40 (11) | 75 | 4 (5) | 0.113b |
| Others, n (%) | 344 | 109 (32) | 75 | <0.001b | |
| Concomitant medication | |||||
| Statin, n (%) | 344 | 65 (19) | 73 | 8 (11) | 0.105b |
| Laboratory findings | |||||
| CK day 1, U/L | 287 | 99 (54–199) | 257 | 116 (77–199) | 0.006a |
| CK day 7, U/L | 197 | 78 (38–215) | 35 | 90 (50–194) | 0.395a |
| CK day 14, U/L | 107 | 61 (38–123) | 9 | 65 (28–251) | 0.975a |
| CRP day 1, mg/dl | 338 | 5.0 (2.0–10.5) | 256 | 2.3 (1.0–4.3) | <0.001a |
| CRP day 7, mg/dl | 255 | 5.7 (2.0–11.7) | 48 | 1.8 (1.0–6.2) | <0.001a |
| CRP day 14, mg/dl | 123 | 3.7 (0.9–9.6) | 12 | 5.3 (2.0–11.0) | 0.449a |
| Procalcitonin day 1, μg/L | 165 | 0.1 (0.1–0.3) | |||
| Procalcitonin day 7, μg/L | 146 | 0.2 (0.1–0.5) | |||
| Procalcitonin day 14, μg/L | 85 | 0.2 (0.1–0.4) | |||
| IL6 day 1, ng/L | 200 | 41.9 (18.3–105.0) | |||
| IL6 day 7, ng/L | 173 | 41.4 (13.2–117.1) | |||
| IL6 day 14, ng/L | 92 | 41.1 (12.6–99.7) | |||
| WBC day 1, G/L | 343 | 5.9 (4.5–7.7) | |||
| WBC day 7, G/L | 256 | 6.1 (4.7–8.1) | |||
| WBC day 14, G/L | 125 | 7.2 (5.9–9.9) | |||
| Ferritin day 1, μg/L | 280 | 637 (277–1246) | |||
| Ferritin day 7, μg/L | 218 | 899 (422–1580) | |||
| Ferritin day 14, μg/L | 114 | 794 (425–1344) | |||
Data are shown as median (interquartile range) unless otherwise specified. Group comparisons were performed by aMann‐Whitney U test or bPearson χ 2 test.
Abbreviations: CK, creatine kinase; COPD, chronic obstructive pulmonary disease; CRP, C‐reactive protein; G/l, Giga per liter; ICU, intensive care unit; IL, interleukin; IQR, interquartile range; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus‐2; WBC, white blood cells.
At baseline, CK levels were significantly higher in males (p < 0.001) and there was a weak negative correlation with age (r = −0.107, p = 0.069), which became statistically significant in multivariate analyses. CK levels did not differ depending on the presence of CAD, arterial hypertension, DM or COPD, or on the usage of statins. In general, CK and inflammatory markers were significantly higher in severe than in mild disease (Figure 1a for ICU‐admitted and Figure 1b for deceased patients). CK did not change over time in severe disease but decreased significantly from baseline to day 7 in mild disease (Figure 1a). In 27% of patients, baseline CK was elevated with a median of 313 U/L (283–541 U/L), and hyperCKemia was significantly more frequent in the ICU (40%) than in the non‐ICU (23%) group. In the population with elevated baseline CK, it was significantly higher in the ICU (406 U/L; 28–643) than in the non‐ICU group (287; 224–488 U/L).
FIGURE 1.
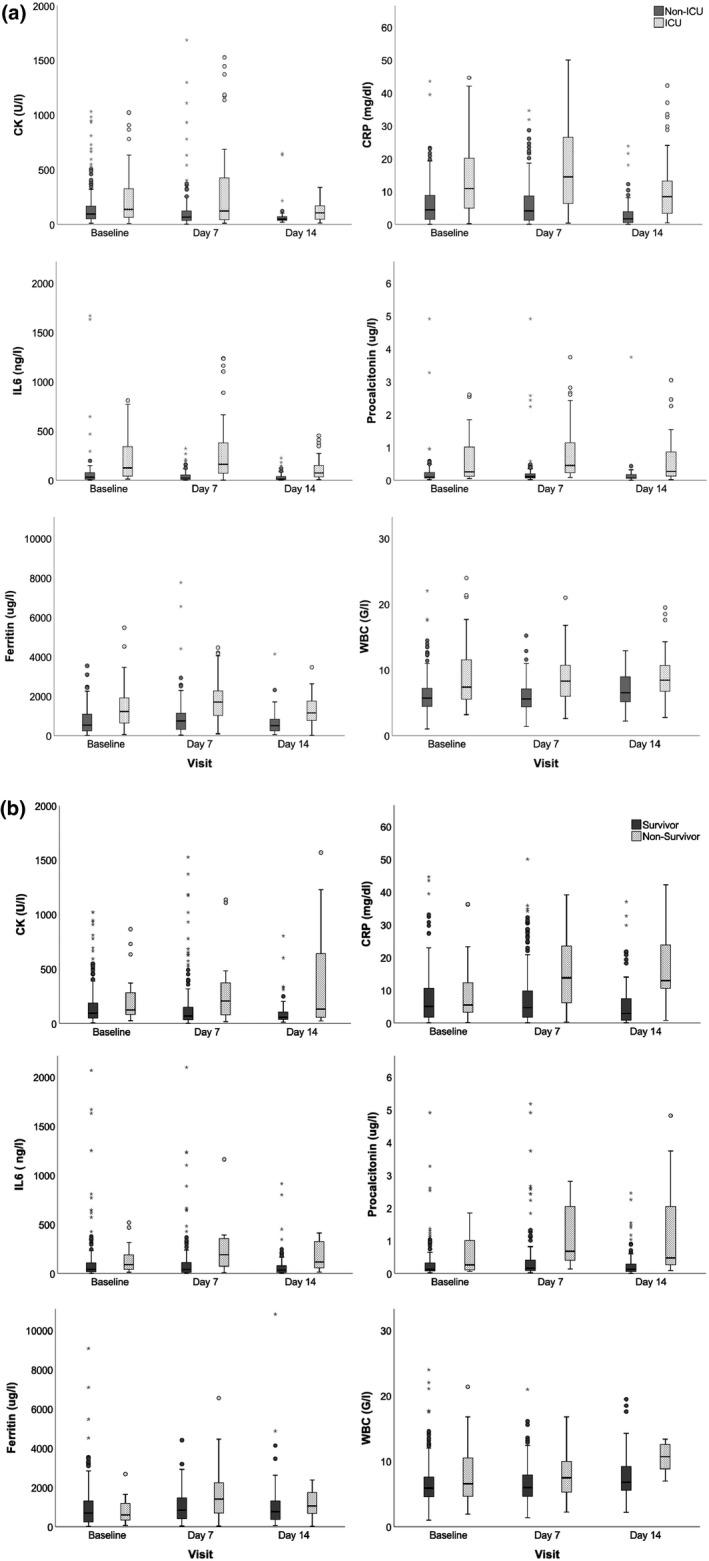
Creatine kinase and inflammatory markers during COVID‐19 disease course. (a) Data for patients with mild versus severe disease. Group comparisons (ICU versus non‐ICU) were performed by Mann‐Whitney U test and revealed statistically significant differences for all biomarkers at every time point (baseline, day 7, day 14): all p ≤ 0.001 except CK at baseline (p = 0.009) and day 7 (p = 0.005). (b) Data of survivors versus nonsurvivors. Group comparisons (survivor versus nonsurvivor) were performed by Mann‐Whitney U test and revealed statistically significant differences (p < 0.05) for all biomarkers at every time point (baseline, day 7, day 14) except CRP at baseline and ferritin at baseline and day 14. CK, creatine kinase; CRP, C‐reactive protein; G/l, Giga per liter; ICU, intensive care unit; IL, interleukin; WBC, white blood cells
Creatine kinase correlated significantly with inflammatory markers (except WBC count) at all time points (Table S1). Linear regression analysis revealed that various inflammatory markers had an independent impact on CK levels, adjusted for demographic variables and disease severity (Table S2).
Baseline CK in the influenza group was significantly higher than in the COVID‐19 group, although CRP was lower (Table 1). At follow‐up, CK did not differ between disease groups, but in contrast to COVID‐19, CK in influenza did not change significantly over time. Similar to COVID‐19, hyperCKemia at baseline was observed in 28% of influenza patients, with a median CK of 300 (251–676 U/L). CK correlated significantly with CRP at baseline (r = 0.204), but not at follow‐up. Although hyperCKemia was more frequent in severe (47%) than in mild (26%) disease, this difference did not reach statistical significance (p = 0.051).
In the hyperCKemia population, CK was significantly higher in the influenza ICU (1,611 U/L; 579–1.776 U/L) than in the COVID‐19 ICU population (406 U/L; 28–643 U/L), whereas CRP did not differ between groups (COVID‐19: 13.8 ± 10.7 mg/dl; influenza: 15.1 ± 12.9 mg/dl). In non‐ICU hyperCKemia patients, CK in influenza patients (357 U/L; 242–586 U/L) did not differ from COVID‐19 patients (287 U/L; 224–488 U/L).
At baseline, CK was >1.000 U/L in 4/287 (1.4%) COVID‐19 and in 14/257 (5.4%) influenza patients (Fisher exact test, p < 0.05). Only 3/63 (4.7%) ICU COVID‐19 patients had a CK > 1.000 U/L, compared to 5/14 (35.7%) ICU influenza patients (Fisher exact test, p < 0.05). The highest CK measured was 6.885 U/L in influenza and 5.077 U/L in COVID‐19.
DISCUSSION
The main findings of the present study are that hyperCKemia is frequent in Austrian COVID‐19 patients, and CK levels correlate with various markers of inflammation in COVID‐19 disease. HyperCKemia is more frequent in severe disease, although in general it is mild. Although the frequency of hyperCKemia is similar in influenza patients, CK levels are higher in influenza patients with severe disease.
HyperCKemia has been consistently reported in COVID‐19 disease [1, 2, 18, 24, 25, 26], for example, in 10.7% [1] and 13.7% [18] of Asian and in 9.2% in southern European [2] COVID‐19 patients. In contrast, we observed CK elevations in 27% in our population. Even if we apply the definition of hyperCKemia (CK >200 U/L) used by other authors [1, 18], there would still be 24% of patients with elevated CK in our population. The higher percentage of hyperCKemia in the present study might be explained by differences in disease severity, as CRP was less in the study by Mao et al. [1], which is also supported by the strong correlation of CRP and other inflammatory markers with CK levels. On the other hand, differences between European and Asian patients might exist, as it has also been suggested for the prevalence of gustatory and olfactory dysfunction [17].
In 40% of severely affected patients (e.g., those admitted to the ICU), hyperCkemia was observed, whereas this was the case in only 24% of mild cases. Also, CK was higher in severely affected patients, which corresponds to findings by others [1, 2, 18, 24, 25, 26]. It has been reported that CK is higher in patients with abnormal findings on lung imaging [27]. However, factors associated with a poor prognosis, such as age, DM, CHD, hypertension and COPD [28], were not associated with elevated CK levels. Taken together, these findings indicate that CK, in addition to traditional markers of inflammation, might serve as an additional maker of disease severity but not as a prognostic marker.
The incidence of hyperCKemia was similar in COVID‐19 and influenza patients. However, baseline CK in ICU‐admitted patients was higher in influenza than in COVID‐19 patients, despite similar CRP levels. Furthermore, 5.4% of influenza patients, compared to 1.4% of COVID‐19 patients, presented CK levels exceeding 1.000. Such a preponderance of very high CK in influenza patients was even more frequent in the ICU population, although the absolute numbers of patients were small. However, others have also shown hyperCKemia is frequent in influenza [29, 30]. Why CK is higher in influenza remains unclear. Studies in cultured human muscle cell indicated that influenza virus infects muscle directly and not via proinflammatory cytokines [31]. On the other hand, a biopsy study from a case of COVID‐19–associated myositis showed perivascular infiltration extending into the endomysium [32]. This suggests that the mechanisms of muscle damage differ between SARS‐CoV‐2 and influenza virus, and that SARS‐CoV‐2 is less myotoxic than influenza virus.
Unfortunately, we were unable to establish the prevalence of myalgia in our patients, but it can be assumed that it is more frequent than hyperCKemia [2, 19, 24, 26]. Despite this, CK is a more robust indicator of muscle damage than myalgia. The pathophysiology of COVID‐19 hyperCKemia is not understood yet. Interaction via the angiotensin‐converting enzyme‐2 receptor, the receptor that binds SARS‐CoV‐2, has been implicated [22]. This is supported by the apparent expression of the receptor on skeletal muscle [33]; however, the receptor was not detected in autopsy studies [34]. Also, the lower muscle involvement in COVID‐19 than in influenza suggests that SARS‐CoV‐2 does not specifically target the muscle. Although we were unable to obtain clinical data on possible ICU‐acquired weakness, critical illness myopathy [35] as the cause of hyperCKemia seems unlikely, as hyperCKemia was already observed in mild cases, CK was already elevated on the first day of ICU admission and CK typically is normal in critical illness myopathy [36]. As many viral infections can cause muscle damage [37], it therefore seems likely that a viral infection–triggered immune response causes muscle damage via T cell expansion or macrophage and proinflammatory cytokine mediated muscle fibre destruction [22, 38]. An immune‐mediated mechanism is also supported by the observation that higher inflammatory biomarkers correlated with higher CK values.
To date, three cases of rhabdomyolysis in COVID‐19 disease have been reported with CK >10.000 U/L [20, 21] or CK >8.000 U/L [39]. Although rhabdomyolysis is, besides clinical criteria, defined by CK >1.000, in cases with severe muscle injury it typically exceeds >10.000 U/L. In the present study, CK >1.000 U/L was only observed in 4/287 (1.4%) COVID‐19 but in 14/257 (5.4%) influenza patients, but none had CK values exceeding 7.000 U/L. This also supports the notion that the SARS‐CoV‐2 virus is less myotoxic than influenza virus.
In conclusion, hyperCKemia is as frequent in COVID‐19 as in influenza infection, but less severe. In both diseases, CK is higher in severe than in mild disease. As morphological data are lacking, it remains unclear whether hyperCKemia is due to a virus‐triggered inflammatory response or direct muscle toxicity.
CONFLICT OF INTEREST
The authors declare no financial or other conflicts of interest.
AUTHOR CONTRIBUTIONS
Lea Pitscheider: Conceptualization (equal); Data curation (equal); Writing‐review & editing (equal). Mario Karoly: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Methodology (equal); Validation (equal); Writing‐review & editing (equal). Francesco Burkert: Data curation (equal); Formal analysis (equal); Methodology (equal); Writing‐review & editing (equal). Raimund Helbok: Data curation (equal); Methodology (equal); Writing‐review & editing (equal). Julia Wanschitz: Conceptualization (equal); Data curation (equal); Methodology (equal); Writing‐review & editing (equal). Corinne GC Horlings: Conceptualization (equal); Methodology (equal); Validation (equal); Writing‐review & editing (equal). Erich Pawelka: Data curation (equal); Methodology (equal); Writing‐review & editing (equal). Sara Omid: Data curation (equal); Methodology (equal); Writing‐review & editing (equal). Marianna Traugott: Data curation (equal); Methodology (equal); Writing‐review & editing (equal). Tamara Seitz: Data curation (equal); Methodology (equal); Writing‐review & editing (equal). Alexander Zoufaly: Data curation (equal); Methodology (equal); Writing‐review & editing (equal). Elisabeth Lindeck‐Pozza: Conceptualization (equal); Data curation (equal); Methodology (equal); Writing‐review & editing (equal). Ewald Wöll: Conceptualization (equal); Data curation (equal); Methodology (equal); Writing‐review & editing (equal). Ronny Beer: Conceptualization (equal); Data curation (equal); Methodology (equal); Supervision (equal); Writing‐review & editing (equal). Stefanie Seiwald: Data curation (equal); Methodology (equal); Writing‐review & editing (equal). Rosa Bellmann: Conceptualization (equal); Data curation (equal); Methodology (equal); Supervision (equal); Writing‐review & editing (equal). Harald Hegen: Conceptualization (equal); Data curation (equal); Formal analysis (lead); Methodology (equal); Visualization (lead); Writing‐review & editing (equal). Wolfgang Loescher: Conceptualization (lead); Data curation (equal); Methodology (lead); Project administration (equal); Supervision (lead); Writing‐original draft (lead); Writing‐review & editing (equal).
Supporting information
Table S1
Table S2
Pitscheider L, Karolyi M, Burkert FR, et al. Muscle involvement in SARS‐CoV‐2 infection. Eur J Neurol. 2021;28:3411–3417. 10.1111/ene.14564
Lea Pitscheider, Mario Karolyi and Francesco R. Burkert contributed equally to this work.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Romero‐Sánchez CM, Díaz‐Maroto I, Fernández‐Díaz E, et al. Neurologic manifestations in hospitalized patients with COVID‐19: the ALBACOVID registry. Neurology. 2020;95:e1060‐e1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Romoli M, Jelcic I, Bernard‐Valnet R, et al. A systematic review of neurological manifestations of SARS‐CoV‐2 infection: the devil is hidden in the details. Eur J Neurol. 2020;27:1712‐1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vacchiano V, Riguzzi P, Volpi L, et al. Early neurological manifestations of hospitalized COVID‐19 patients. Neurol Sci. 2020;41:2029‐2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Whittaker A, Anson M, Harky A. Neurological manifestations of COVID‐19: a systematic review and current update. Acta Neurol Scand. 2020;142:14‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alberti P, Beretta S, Piatti M, et al. Guillain‐Barré syndrome related to COVID‐19 infection. Neurol Neuroimmunol Neuroinflamm. 2020;7:e741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gigli GL, Bax F, Marini A, et al. Guillain‐Barré syndrome in the COVID‐19 era: just an occasional cluster? J Neurol. 2020;36:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ottaviani D, Boso F, Tranquillini E, et al. Early Guillain‐Barré syndrome in coronavirus disease 2019 (COVID‐19): a case report from an Italian COVID‐hospital. Neurol Sci. 2020;41:1351‐1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scheidl E, Canseco DD, Hadji‐Naumov A, et al. Guillain‐Barré syndrome during SARS‐CoV‐2 pandemic: a case report and review of recent literature. J Peripher Nerv Syst. 2020;25:204‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Toscano G, Palmerini F, Ravaglia S, et al. Guillain‐Barré syndrome associated with SARS‐CoV‐2. N Engl J Med. 2020;382:2574‐2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhao H, Shen D, Zhou H, et al. Guillain‐Barré syndrome associated with SARS‐CoV‐2 infection: causality or coincidence? Lancet Neurol. 2020;19:383‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Helbok R, Beer R, Löscher W, et al. Guillain‐Barré syndrome in a patient with antibodies against SARS‐COV‐2. Eur J Neurol. 2020;27:1754‐1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anand P, Slama MCC, Kaku M, et al. COVID‐19 in patients with myasthenia gravis. Muscle Nerve. 2020;62:254‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Delly F, Syed MJ, Lisak RP, et al. Myasthenic crisis in COVID‐19. J Neurol Sci. 2020;414:116888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hübers A, Lascano AM, Lalive PH. Management of patients with generalised myasthenia gravis and COVID‐19: four case reports. J Neurol Neurosurg Psychiatr. 2020;91:1124‐1125. [DOI] [PubMed] [Google Scholar]
- 16. Kushlaf H. COVID‐19 in MuSK myasthenia gravis: a case report. Muscle Nerve. 2020;62:E65‐E66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lechien JR, Chiesa‐Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild‐to‐moderate forms of the coronavirus disease (COVID‐19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277:2251‐2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guan W‐J, Ni Z‐Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang J, Wang X, Jia X, et al. Risk factors for disease severity, unimprovement, and mortality in COVID‐19 patients in Wuhan, China. Clin Microbiol Infect. 2020;26:767‐772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jin M, Tong Q. Rhabdomyolysis as potential late complication associated with COVID‐19. Emerging Infect Dis. 2020;26:1618‐1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Suwanwongse K, Shabarek N. Rhabdomyolysis as a presentation of 2019 novel coronavirus disease. Cureus. 2020;12:e7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dalakas MC. Guillain‐Barré syndrome: the first documented COVID‐19‐triggered autoimmune neurologic disease: more to come with myositis in the offing. Neurol Neuroimmunol Neuroinflamm. 2020;7:e781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pizzini A, Burkert F, Theurl I, et al. Prognostic impact of high sensitive Troponin T in patients with influenza virus infection: a retrospective analysis. Heart Lung. 2020;49:105‐109. [DOI] [PubMed] [Google Scholar]
- 24. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang X, Cai H, Hu J, et al. Epidemiological, clinical characteristics of cases of SARS‐CoV‐2 infection with abnormal imaging findings. Int J Infect Dis. 2020;94:81‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li LQ, Huang T, Wang YQ, et al. COVID‐19 patients' clinical characteristics, discharge rate, and fatality rate of meta‐analysis. J Med Virol. 2020;92:577‐583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang J, Zhao Y, Chen Y. Laboratory findings in patients with avian‐origin influenza A (H7N9) virus infections. J Med Virol. 2013;86:895‐898. [DOI] [PubMed] [Google Scholar]
- 30. Karolyi M, Pawelka E, Daller S, et al. Is there a clinical difference between influenza A and B virus infections in hospitalized patients?: results after routine polymerase chain reaction point‐of‐care testing in the emergency room from 2017/2018. Wien Klin Wochenschr. 2019;131:362‐368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Desdouits M, Munier S, Prevost M‐C, et al. Productive infection of human skeletal muscle cells by pandemic and seasonal influenza A(H1N1) viruses. PLoS ONE. 2013;8:e79628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang H, Charmchi Z, Seidman RJ, et al. COVID‐19‐associated myositis with severe proximal and bulbar weakness. Muscle Nerve. 2020;62:E57‐E60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cabello‐Verrugio C, Morales MG, Rivera JC, et al. Renin‐angiotensin system: an old player with novel functions in skeletal muscle. Med Res Rev. 2015;35:437‐463. [DOI] [PubMed] [Google Scholar]
- 34. Ding Y, He L, Zhang Q, et al. Organ distribution of severe acute respiratory syndrome(SARS) associated coronavirus(SARS‐CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203:622‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Doughty CT, Amato AA. Toxic myopathies. Continuum (Minneap Minn). 2019;25:1712‐1731. [DOI] [PubMed] [Google Scholar]
- 36. Khan J, Burnham EL, Moss M. Acquired weakness in the ICU: critical illness myopathy and polyneuropathy. Minerva Anestesiol. 2006;72:401‐406. [PubMed] [Google Scholar]
- 37. Finsterer J, Löscher WN, Wanschitz J, Quasthoff S, Grisold W. Secondary myopathy due to systemic diseases. Acta Neurol Scand. 2016;134:388‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dalakas MC. Inflammatory muscle diseases. N Engl J Med. 2015;372:1734‐1747. [DOI] [PubMed] [Google Scholar]
- 39. Valente‐Acosta B, Moreno‐Sanchez F, Fueyo‐Rodriguez O, et al. Rhabdomyolysis as an initial presentation in a patient diagnosed with COVID‐19. BMJ Case Rep. 2020;13:e236719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


