Abstract
Background
Dental procedures often produce aerosol and splatter which have the potential to transmit pathogens such as SARS‐CoV‐2. The existing literature is limited.
Objective(s)
To develop a robust, reliable and valid methodology to evaluate distribution and persistence of dental aerosol and splatter, including the evaluation of clinical procedures.
Methods
Fluorescein was introduced into the irrigation reservoirs of a high‐speed air‐turbine, ultrasonic scaler and 3‐in‐1 spray, and procedures were performed on a mannequin in triplicate. Filter papers were placed in the immediate environment. The impact of dental suction and assistant presence were also evaluated. Samples were analysed using photographic image analysis and spectrofluorometric analysis. Descriptive statistics were calculated and Pearson's correlation for comparison of analytic methods.
Results
All procedures were aerosol and splatter generating. Contamination was highest closest to the source, remaining high to 1‐1.5 m. Contamination was detectable at the maximum distance measured (4 m) for high‐speed air‐turbine with maximum relative fluorescence units (RFU) being: 46,091 at 0.5 m, 3,541 at 1.0 m and 1,695 at 4 m. There was uneven spatial distribution with highest levels of contamination opposite the operator. Very low levels of contamination (≤0.1% of original) were detected at 30 and 60 minutes post‐procedure. Suction reduced contamination by 67‐75% at 0.5‐1.5 m. Mannequin and operator were heavily contaminated. The two analytic methods showed good correlation (r = 0.930, n = 244, P < .001).
Conclusion
Dental procedures have potential to deposit aerosol and splatter at some distance from the source, being effectively cleared by 30 minutes in our setting.
Keywords: aerosols, COVID‐19, dental high‐speed equipment, dental infection control, dental scaling, suction
1. BACKGROUND
The coronavirus disease 2019 (COVID‐19) pandemic has had significant impact upon the provision of medical and dental care globally. In the United Kingdom, routine dental treatment was suspended in late March 2020, 1 , 2 , 3 , 4 with care instead being provided through a network of urgent dental care centres. 5 During this period, it was advised that aerosol‐generating procedures (AGPs) were avoided unless absolutely necessary, leading to altered treatment planning and a negative impact on patient care. 6 As more routine dental services start to resume worldwide, the guidance in the UK and elsewhere is still to avoid or defer AGPs where possible. 7 , 8 , 9 , 10 , 11 , 12 , 13 This will have an effect both on patients attending for urgent and emergency care, as well as those requiring routine dental treatment for oral rehabilitation. Standard operating procedures (SOPs) have been published by a number of organisations to inform practice; however, many of these acknowledge a limited evidence base. 14 , 15 , 16 , 17 , 18 Additionally, all face‐to‐face undergraduate and postgraduate clinical dental teaching in the UK is suspended at the time of writing. 19
Various definitions exist for the terms ‘aerosol’ and ‘splatter’. For the purposes of this study, we define particles which make up aerosols as having a diameter of less than 10 µm, 20 and splatter as comprising of particles larger than this; in reality, aerosols and splatter are made up of a spectrum of droplet sizes and this distinction is somewhat arbitrary. Many dental procedures produce both aerosol and splatter contaminated with saliva and/or blood. 21 , 22 Saliva has been shown to contain severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) in infected individuals, 23 , 24 many of whom may be asymptomatic, 25 with the salivary gland potentially being an early reservoir of infection. 26 , 27 Equally, however, preliminary data suggest that in asymptomatic carriers, the viral load may be low in saliva and these individuals may have faster viral clearance. 28 , 29 Early data suggest that SARS‐CoV‐2 can remain viable and infectious in aerosol for hours, and on surfaces for days. 30 Hence, dental aerosols and splatter are likely to be a high‐risk mode of transmission for SARS‐CoV‐2, and it is highly likely that international clinical protocols across the spectrum of dental practice will need to be significantly modified to allow a safe return to routine care.
A review of the impact of AGPs generally across health care (including dentistry) concluded that the existing evidence is limited. 31 The current literature regarding the risks posed by aerosols and splatter in dental settings is particularly limited. A number of authors have used microbiological methods to study bacterial contamination from aerosol and splatter following dental procedures, either by air sampling, 21 , 32 , 33 swabbing of contaminated surfaces, 34 , 35 or most commonly, by collection directly onto culture media. 36 , 37 , 38 , 39 These studies are limited in that they only detect culturable bacteria as a marker of aerosol and splatter distribution. A smaller number of studies have used various fluorescent 40 , 41 , 42 , 43 , 44 and non‐fluorescent tracers 45 , 46 to measure aerosol and splatter distribution, although some of these have significant methodological flaws and major limitations. Many studies are small and report only one repetition of a single procedure, and some have only examined contamination of the operator and assistant; a number of studies which have measured spatial distribution of aerosol and splatter have only done so to a limited distance from the source. Few studies have considered the temporal persistence of aerosol and splatter with sufficient granularity to inform clinical practice.
Open plan clinical environments such as those common in dental (teaching) hospitals with multiple patients and operators in close proximity are problematic. The current lack of robust evidence about dental aerosol and splatter distribution and persistence will be a barrier to the reintroduction of routine dental services and dental education, which is likely to have a negative impact on the availability of care for patients, and on the future dental workforce if not addressed expediently. 19 Patients’ oral health care will also suffer if routine care cannot be re‐established, especially for those with high dental needs and active dental disease.
The aim of the present study was to establish a robust, reliable and valid methodology to evaluate the distribution and persistence of aerosol and splatter following dental procedures. This can then be used in future work linking specifically to transmission of SARS‐CoV‐2, as well as other pathogens, in a dental environment. We present initial data on three dental procedures (high‐speed air‐turbine, ultrasonic scaler, and 3‐in‐1 spray use) and examine the effect of dental suction and the presence of an assistant on aerosol and splatter distribution.
2. METHODS
Experiments were conducted in the Clinical Simulation Unit (CSU) at the School of Dental Sciences, Newcastle University (Newcastle upon Tyne, United Kingdom). This is a 308 m2 dental clinical teaching laboratory situated within a large dental teaching hospital. The CSU is supplied by a standard hospital ventilation system with ventilation openings arranged as shown in Figure 1; this provides 6.5 air changes per hour and all windows and doors remained closed during experiments. The temperature remained constant at 21.5°C.
Figure 1.
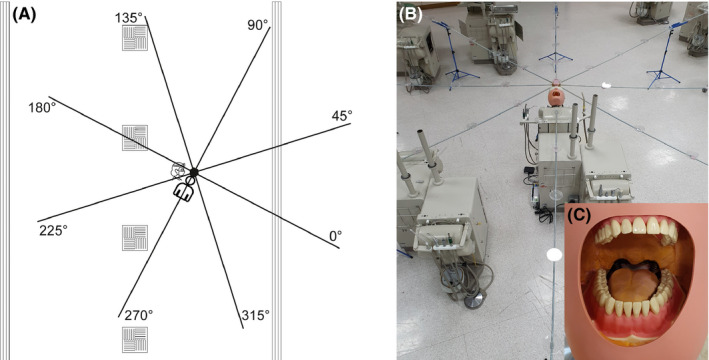
A, Schematic diagram of experimental set up. Position of air vents shown; square vents = air intake; long vents = air output. Experimental set up shown with collection positions labelled (note: degrees are relative to facing the mannequin). B, Photograph of experimental set up showing platforms spaced at 0.5 m intervals to support filter papers. C, Demonstration of polyvinyl siloxane addition to mouth of mannequin [Colour figure can be viewed at wileyonlinelibrary.com]
Dental procedures were conducted on a dental simulator unit (Model 4820, A‐dec; OR, USA) with a mannequin containing model teeth (Frasaco GmbH; Tettnang, Germany). Polyvinyl siloxane putty (Lab‐putty, Coltene/Whaledent; Altstätten, Switzerland) was added to the mouth of the mannequin to recreate the normal dimensions of the oral cavity as described by Dahlke et al 42 (Figure 1). Fluorescein solution (2.65 mmol L−1) was made by dissolving fluorescein sodium salt (Sigma‐Aldrich; MO, USA) in deionised water, and this was then introduced to the irrigation reservoirs of the dental unit and ultrasonic scaler. The procedures investigated were as follows: anterior crown preparation—preparation of the upper right central incisor tooth for a full coverage crown using a high‐speed air‐turbine (Synea TA‐98, W&H (UK) Ltd.; St Albans, UK); full mouth scaling using a magnetostrictive ultrasonic scaler (Cavitron Select SPS with 30K FSI‐1000‐94 insert, Dentsply Sirona; PA, USA); 3‐in‐1 spray (air/water syringe) use—washing of mesial‐occlusal cavity in upper right first premolar tooth with air and water from 3‐in‐1 spray. Procedure durations were 10 minutes for anterior crown preparation and ultrasonic scaling, and 30 seconds for the 3‐in‐1 spray use with air and water (to represent removing acid etchant). Irrigant flow rate was measured at 29.3 mL/min for the air‐turbine, 38.6 mL/min for the ultrasonic scaler and 140.6 mL/min for the 3‐in‐1 spray. We also investigated dental suction (measured at 6.3 L of water per minute) and the presence of an assistant.
Having developed the methods reported by other investigators, 37 , 42 , 44 the present study used a reproducible, height adjustable rig. This rig was constructed to support cotton‐cellulose filter papers spaced at known distances from the mannequin (Figure 1). 30 mm diameter grade 1 qualitative filter papers (Whatman; Cytiva, MA, USA) were used to collect aerosol and splatter. These were supported on platforms spaced at 0.5 m intervals along eight, 4 m, rigid rods, laid out at 45° intervals and supported by a central hub, thus creating an 8 m diameter circle around the mannequin; the centre of this circle was located 25 cm superior to the mouth of the mannequin, and in the same horizontal plane as the mouth of the mannequin (73 cm above the floor). Four filter papers were also placed on the body of the mannequin: two at 40 cm from the hub and two at 80 cm. In addition, filter papers were placed on the arms (upper mid‐forearm), body (upper chest) and legs (upper mid‐thigh) of the operator and assistant as well as on their full‐face visor (width: 28.0, height: 27.5 cm) and the vertex of the head. For one condition (anterior crown prep with suction and assistant), we also placed three filter papers on the mask of the operator/assistant (beneath a full‐face visor). Two operators conducted the procedures: RH conducted the high‐speed air‐turbine and ultrasonic scaler procedures (operator height = 170 cm); JRA conducted the 3‐in‐1 spray procedure (operator height = 175 cm). There was a single assistant (KP) with a height of 164 cm.
Before each procedure, the mannequin, rig and filter paper platforms were cleaned with 70% ethanol and left to fully air dry. A period of 120 minutes was left between each procedure to allow for clearance of aerosol and splatter. Following each procedure, the filter papers were left in position for 10 minutes to allow for settling and drying of aerosol and splatter, before being collected with clean tweezers and placed into a single‐use, sealable polyethylene bag. For the anterior crown preparation without suction, additional filter papers were placed at 30 minutes and again at 60 minutes to examine persistence of aerosol and splatter. At both of these time points, the risk of fluorescein transfer was minimised by placing the new filter papers on new platforms, and filter papers were then left for 10 minutes before collection. All experimental conditions were repeated three times.
2.1. Image analysis
Filter papers were placed on a glass slide on a black background, covered by a second glass slide, and illuminated by two halogen dental curing lights (QHL75 model 503; Dentsply, NC, USA) with 45 mW/cm2 output at 400‐500 nm; these were positioned at 0 and 180 degrees, 5 cm from the centre of the sample horizontally and 9 cm vertically, with both beams of light focussed on the centre of the sample. Images were captured with a digital single‐lens reflex (DSLR) camera (EOS 1000D, Canon; Tokyo, Japan) at 90 mm focal length (SP AF 90mm F/2.8 Di Macro, Tamron; Saitama, Japan) with an orange lens filter, positioned 43 cm directly above the sample (sample to sensor). Exposure parameters were f/10, 1/80 seconds and ISO 400. Image analysis was performed using ImageJ 47 (version 1.53b, US National Institutes of Health; MD, USA) in a darkened room by one of four examiners blind to experimental conditions and sample position (JRA, CCC, DE, RH). Images were converted into 8‐bit images and the pixel scale set across the maximum diameter of the sample at 30mm. A manual threshold was used to create a mask selecting all high‐intensity areas. The ‘analyse particles’ function was used to identify particles from 0 to infinity mm2 in area and 0 to 1 in circularity. The number of particles, total surface area and average particle size were calculated. Total surface area was selected as the primary outcome measure, representing contamination levels of the samples and most likely representing the larger splatter produced from the procedures. Examiners underwent calibration prior to formal analysis by independently analysing 10 images and then discussing to reach consensus. Following this, examiners then independently analysed 30 images to assess inter‐examiner agreement. Examiners re‐examined the same 30 images one week later to assess intra‐examiner agreement.
2.2. Spectrofluorometric analysis
For one experimental condition (anterior crown preparation without suction, samples from the initial, 30‐, and 60‐minute time points), we completed spectrofluorometric analysis to allow validation of the image analysis technique, and to also capture aerosol produced which may not be easily detected in image analysis. Building on the methods reported by Steiner et al, 48 fluorescein was recovered from filter papers by addition of 350 µL deionised water. Immersed samples were shaken for 5 minutes at 300 rpm using an orbital shaker at room temperature. The fluorescein was then eluted by centrifugation at 15,890 g for 3 min using a microcentrifuge. 100 µL of the supernatant was transferred to a black 96‐well microtitre plate with a micro‐clear bottom (Greiner Bio‐One; NC, USA) in triplicate in order to measure fluorescence. Fluorescence measurements were performed using a Synergy HT Microplate Reader (BioTek; VT, USA) at an excitation wavelength of 485 ± 20 nm and an emission wavelength of 528 ± 20 nm with the top optical probe. For background correction, negative controls (n = 26) were included in the measurements for all runs. These included fresh filter papers out of the box and filter papers that had been placed on platforms in CSU for 10 minutes exposed to air. The negative control filter papers were processed for imaging and fluorescent measurements in the same manner as the remainder of samples. The negative control mean + 3SD (164 RFU; relative fluorescence units) was used as the limit of detection; hence, a zero reading was assigned to values below 164 RFU. For readings above the detection limit of the instrument (>100 000 RFU), a value of 100 000 RFU was assigned.
2.3. Statistical methods
Data were collected using Excel (2016, Microsoft; WA, USA) and analysed using SPSS (version24, IBM Corp.; NY, USA) using basic descriptive statistics and Pearson's correlation (to compare analytical techniques). Heatmaps demonstrating aerosol and splatter distribution were generated using Python 3. 49 A two‐way mixed effects model was used to assess inter‐ and intra‐examiner agreement by calculating interclass correlation coefficient (ICC) using STATA release 13 (StataCorp; TX, USA).
3. RESULTS
3.1. Examiner calibration for image analysis
Inter‐examiner ICC for 30 images showed excellent agreement for total surface area (ICC 0.98; 95% CI 0.97‐0.99), good agreement for total number of particles (ICC 0.88; 95% CI 0.80‐0.93) and moderate agreement for average particle size (ICC 0.63; 95% CI 0.47‐0.78). Intra‐examiner agreement at one week for the same 30 images was excellent for total surface area (ICC 0.97‐0.99), good to excellent for total number of particles (ICC 0.82‐0.97) and good for average particle size (ICC 0.75‐0.97). 50
3.2. Aerosol and splatter distribution
Aerosol and/or splatter deposition (assessed by surface area outcome) was highest at the centre of the rig and decreased with increasing distance from the centre (Table 1). Most contamination was within 1.5 m, but there were smaller readings up to 4 m for some conditions. The spatial distribution is shown in Figures 2 and 3.
Table 1.
Dental aerosol and splatter as measured by contaminated surface area using image analysis or by spectrofluorometric analysis. For each experimental condition, the data from an average of three repetitions for all samples at each distance are included together. A total is also given for all samples for each condition, which also includes data from samples placed on the mannequin
|
Min Mean (SD) Max Sum [n] |
Distance from centre (m) | Total e | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.5 | 1 | 1.5 | 2 | 2.5 | 3 | 3.5 | 4 | ||
| Surface area (mm2) | ||||||||||
| Anterior crown prep (no suction) a | 668.54 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 690.07 (19.60) | 77.81 (110.30) | 1.47 (4.33) |
0.03 (0.09) |
0.00 (0.00) |
0.00 (0.00) |
0.00 (0.00) |
0.00 (0.00) |
0.00 (0.00) |
25.32 (101.04) |
|
| 706.86 | 386.87 | 21.00 | 0.42 | 0.08 | 0.00 | 0.00 | 0.00 | 0.00 | 706.86 | |
| 2070.20 | 1867.32 | 35.40 | 0.75 | 0.11 | 0.00 | 0.00 | 0.00 | 0.00 | 5241.05 | |
| [3] | [24] | [24] | [24] | [24] | [24] | [24] | [24] | [24] | [207] | |
| Anterior crown prep with suction b | 656.46 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 671.48 (20.10) | 19.58 (36.58) | 0.48 (1.57) | 0.01 (0.02) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) |
0.00 (0.00) |
15.14 (83.54) | |
| 694.38 | 145.02 | 7.65 | 0.06 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 694.38 | |
| 2,014.44 | 470.01 | 11.60 | 0.13 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 3,133.33 | |
| [3] | [24] | [24] | [24] | [24] | [24] | [24] | [24] | [24] | [207] | |
| Anterior crown prep with suction and assistant c | 204.55 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 460.21 (227.75) | 10.19 (21.87) | 0.04 (0.11) | 0.15 (0.73) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) |
0.00 (0.00) |
0.00 (0.00) |
14.26 (76.13) | |
| 641.29 | 100.54 | 0.47 | 3.58 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 641.29 | |
| 1380.64 | 244.51 | 1.07 | 3.60 | 0.06 | 0.01 | 0.03 | 0.02 | 0.03 | 2952.17 | |
| [3] | [24] | [24] | [24] | [24] | [24] | [24] | [24] | [24] | [207] | |
| Ultrasonic scaling with suction d | 2.71 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 129.11 (191.14) | 3.15 (7.99) | 0.00 (0.01) | 0.00 (0.01) | 0.00 (0.01) | 0.00 (0.01) | 0.00 (0.01) | 0.00 (0.01) | 0.00 (0.01) | 7.76 (42.67) | |
| 349.00 | 30.04 | 0.02 | 0.03 | 0.02 | 0.03 | 0.02 | 0.03 | 0.02 | 349.00 | |
| 387.32 | 75.59 | 0.06 | 0.06 | 0.05 | 0.07 | 0.06 | 0.08 | 0.05 | 1,605.91 | |
| [3] | [24] | [24] | [24] | [24] | [24] | [24] | [24] | [24] | [207] | |
| 3‐in‐1 spray with suction e | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 0.78 (0.13) | 20.47 (47.32) | 0.02 (0.05) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 10.30 (53.19) | |
| 2.30 | 220.14 | 0.20 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 490.77 | |
| 2.34 | 491.29 | 0.37 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2,131.64 | |
| [3] | [24] | [24] | [24] | [24] | [24] | [24] | [24] | [24] | [207] | |
| Fluorescence (RFU) | ||||||||||
| Anterior crown prep (no suction) a | 82,812 | 89 | 103 | 48 | 70 | 71 | 56 | 47 | 55 | 0 |
| 91 406 (12 153) | 11 438 (14 907) | 889 (932) | 319 (390) | 381 (600) | 239 (330) | 388 (555) | 243 (342) | 242 (437) | 4056 (14 997) | |
| 100 000 | 46 091 | 3541 | 1545 | 2097 | 1506 | 2739 | 1106 | 1695 | 100 000 | |
| 182 812 | 274 529 | 21 355 | 7661 | 9141 | 5738 | 9309 | 5842 | 5826 | 835 741 | |
| [2] | [24] | [24] | [24] | [24] | [24] | [24] | [24] | [24] | [206] | |
| 30‐40 min post‐procedure collection | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 8 (39) | 0 (0) | 1 (13) | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 191 | 0 | 191 | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 191 | 0 | 191 | |
| [3] | [24] | [24] | [24] | [24] | [24] | [24] | [24] | [24] | [207] | |
| 60‐70 min post‐procedure collection | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0 (0) | 0 (0) | 14 (49) | 12 (60) | 0 (0) | 8 (38) | 0 (0) | 0 (0) | 0 (0) | 4 (29) | |
| 0 | 0 | 177 | 294 | 0 | 184 | 0 | 0 | 0 | 294 | |
| 0 | 0 | 344 | 294 | 0 | 184 | 0 | 0 | 0 | 822 | |
| [3] | [24] | [24] | [24] | [24] | [24] | [24] | [24] | [24] | [205] | |
Anterior crown preparation on upper right central incisor without suction or assistant. 10 minute duration.
Anterior crown preparation on upper right central incisor with suction. 10 minute duration.
Anterior crown preparation on upper right central incisor with suction and assistant. 10 minute duration.
Full mouth ultrasonic scaling with suction. 10 minute duration.
3‐in‐1 spray with suction of a MO cavity in upper right first premolar tooth. 30 second duration to replicate washing acid etchant.
All measurements from the rig with the addition of readings from the mannequin, representing an 8 m diameter experimental area.
Figure 2.
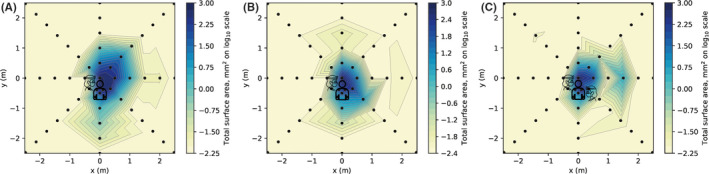
Heatmap showing surface area outcome measure for three clinical procedures. A, anterior crown preparation (without suction). B, anterior crown preparation with suction. C, anterior crown preparation with suction and assistant. For each coordinate, the maximum value recorded from three repetitions of each clinical procedure was used as this was deemed most clinically relevant. Logarithmic transformation was performed on the data (Log10). Note the scale is reduced to remove areas showing zero readings [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 3.
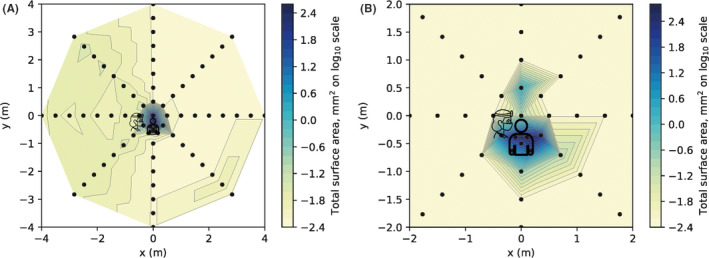
Heatmap showing surface area outcome measure for two clinical procedures. A, ultrasonic scaling. B, 3‐in‐1 spray. For each coordinate, the maximum value recorded from three repetitions of each clinical procedure was used as this was deemed most clinically relevant. Logarithmic transformation was performed on the data (Log10). Note the scale is reduced to remove areas showing zero readings in panel B only [Colour figure can be viewed at wileyonlinelibrary.com]
For one experimental condition (anterior crown prep with no suction, representing a presumed worst‐case scenario), at three time points, we also completed spectrofluorometric analysis (Table 1). The particle count was weakly correlated with spectrofluorometric measurements (r = 0.344, n = 244, P < .001), average particle size was moderately correlated (r = 0.555, n = 244, P < .001) and total surface area was very strongly correlated (r = 0.930, n = 244, P < .001), supporting our use of surface area as the main outcome measure from image analysis (Figure S1). Data from one time point are presented in Figure 4. Using serial dilution of fluorescein, we derived a standard curve covering the range 50 nM to 102 µM. The equation y = 700.42 x − 1449.5 was derived from the standard curve (y = fluorescence, RFU; x = fluorescein concentration, µM). For illustrative purposes, we detail these for the 270 degree axis (mean values across three repetitions from the initial time point): 0 m = 132.6 µmol L−1; 0.5 m = 26.3 µ mol L−1; 1 m = 5.25 µ mol L−1; 1.5 m = 3.02 µ mol L−1; 2 m = 3.44 µ mol L−1; 2.5 m = 3.30 µ mol L−1; 3 m = 2.79 µ mol L−1; 3.5 m = 2.86 µ mol L−1; 4 m = 3.09 µ mol L−1.
Figure 4.
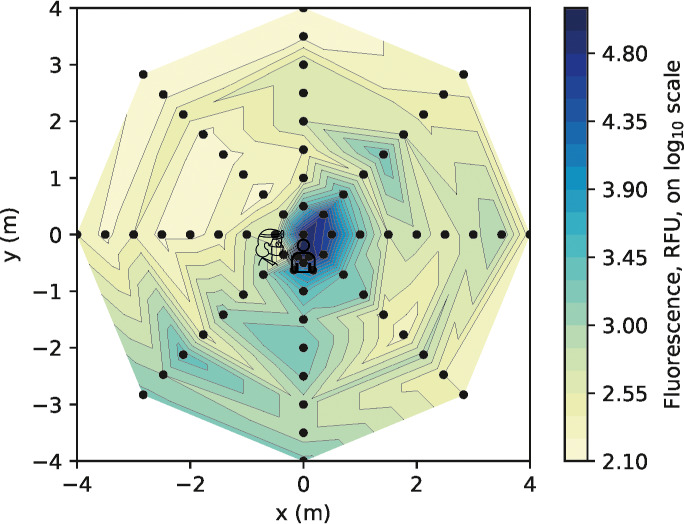
Heatmap presenting spectrofluorometric analysis of the samples from the anterior crown preparation (without suction) clinical procedure at 0 ‐ 10 minutes (surface area data shown in Figure 2A). For each coordinate, the maximum value recorded from three repetitions of each clinical procedure was used. Logarithmic transformation was performed on the data (Log10). Note the scale includes the full dimensions of the experimental rig. RFU: relative fluorescence units [Colour figure can be viewed at wileyonlinelibrary.com]
The mannequin, operator and assistant were all heavily contaminated (Table S1). The operator's left (non‐dominant) arm, left body and lower visor were the most contaminated sites. Generally, levels of contamination were much lower for the assistant, being highest on the left arm and left chest (the assistant used their left hand to hold the suction tip). All areas of the mannequin were heavily contaminated. The operator and assistant's masks (only assessed in one condition) showed low but measurable contamination, usually at the lateral edges.
3.3. Effect of dental suction (with and without assistant)
The use of dental suction, held by the operator, reduced the contamination of filter papers at each distance (Table 1), although image analysis still detected contamination up to 2 m. Between 0.5 and 1.5 m, there was a 67%‐75% reduction (central site contamination was unaffected). The spatial distribution was altered as demonstrated in Figure 2. When an assistant was present and held the dental suction, this further reduced contamination readings within the first 1 m; however, we noted a marked increase at the 1.5 m reading behind the assistant (0°).
3.4. Procedure type
Three clinical procedures (anterior crown preparation, ultrasonic scaling and 3‐in‐1 spray use) were assessed while the operator held dental suction. The highest readings were obtained from the anterior crown preparation, but each procedure gave a unique distribution (Table 1, Table S1, Figures 2 and 3). The ultrasonic scaler produced high levels of contamination at the centre, reducing markedly at 0.5 m, but with low levels of contamination detectable up to the 4 m limit of measurement. The 3‐in‐1 spray procedure produced high levels of contamination at 0.5 m but little beyond 1 m.
3.5. Effect of time
Image analysis demonstrated no detectable fluorescein contamination of the filter papers at 30‐40 and 60‐70 minutes post‐procedure (for the anterior crown preparation without suction condition). Additionally, spectrofluorometric analysis of these samples demonstrated very low levels of contamination. The overall contamination across the 8 m diameter experimental area at 30‐40 minutes was 0.02% of the original level, and at 60‐70 minutes, it was 0.10% of the original level (Table 1).
3.6. Particle size
Average particle size measurements (from photographic analysis, likely to represent splatter particles) were combined for the 0, 0.5, 1 and 1.5 m readings for each condition to give an indication of the nature of the particles in this area. The anterior crown preparation without suction produced the largest particles (mean ± SD: 0.49 ± 2.98 mm2) which were similar to when suction was added by the operator (0.56 ± 3.34 mm2). There was a size reduction when an assistant provided suction (0.11 ± 0.69 mm2). The ultrasonic scaling produced the smallest particles (0.05 ± 0.24 mm2) followed by the 3‐in‐1 spray (0.08 ± 0.25 mm2). Figure 5 presents images of all samples for one repetition of a single experimental condition to demonstrate the distribution of particles and size.
Figure 5.
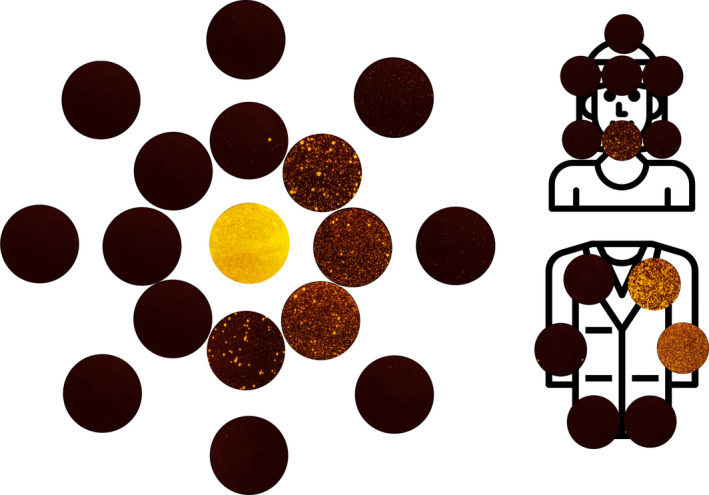
Composite image comprised of all filter paper samples images used for image analysis within the first 1 m from the one repetition of the anterior crown preparation (no suction) condition. Colour balance and contrast adjusted to aid visualisation. Samples are arranged with the central sample in the centre, and samples from 0.5 m and 1 m arranged concentrically moving outwards. The axis is the same as demonstrated in Figures 1, 2, 3, 4 [Colour figure can be viewed at wileyonlinelibrary.com]
When looking at all the experimental conditions combined, the average particle size was largest closest to the source, decreasing with distance (mean ± SD): 0 m = 5.52 ± 8.88 mm2; 0.5 m = 0.11 ± 0.28 mm2; 1 m = 0.01 ± 0.00 mm2; 1.5 ‐ 4 m = 0.00 ± 0.00 mm2. Figure S2 presents the average particle size by distance for each separate experimental condition.
4. DISCUSSION
Dental aerosol and splatter are an important potential mode of transmission for many pathogens, including SARS‐CoV‐2. Understanding the risk these phenomena pose is vitally important in the reintroduction of dental services in the current COVID‐19 pandemic. Our study is novel in that we are the first to measure aerosol and splatter distribution at distances up to 4 m from the source, and the first to apply image and spectrofluorometric analysis to the study of dental aerosol and splatter. This has allowed us to gather urgently needed data relevant to the provision of dental services during the COVID‐19 pandemic, and more widely. Specifically, we have demonstrated the relative distribution of aerosol and splatter following different dental procedures, the effect of suction and assistant presence, and the persistence of aerosol and splatter over time.
Previous investigators have used various tracer dyes and visual examination techniques to evaluate ‘dental aerosol’ and have demonstrated positive readings at up to 1.2 m. 42 , 44 Our study further optimises these methods and we have demonstrated positive readings at up to 2 m (and low levels at up to 4 m in the case of ultrasonic scaling). This is consistent with the findings of other investigators using bacterial culture methods to detect contamination at up to 2 m. 37 , 38 , 51 Importantly, our spectrofluorometric analysis demonstrates that some fluorescein contamination may occur beyond this on filter papers that appear clean by image analysis, representing aerosol which cannot be detected with image analysis alone. In addition, a DSLR camera with a complementary metal‐oxide‐semiconductor sensor is likely to be limited to the detection of larger particles (i.e. splatter) using the methods we report in the present study. We therefore propose that studies which use dye tracers assessed by visual examination or image analysis techniques alone are assessing primarily splatter rather than aerosol; this is because in order for deposits to be visible to the eye or camera, it has to be relatively large in size. Previous research using these methods should therefore be interpreted in this context. It is, however, worth noting that larger particles are likely to contain a greater viral load, and given the risk of SARS‐CoV‐2 transmission through contact with mucosal surfaces, 52 from a cross‐infection perspective splatter is likely to be highly significant. Reassuringly, in our study splatter was greatly reduced using suction.
Findings from both analytical techniques demonstrate contamination at a distance from the source although contamination was lower at greater distances; this shows the potential for pathogens to travel a similar distance, although our methods replicate a worst‐case scenario. Within closed surgery environments, this reinforces the need for minimal clutter and strict cross‐infection control measures. Within open clinic environments, further research is required to investigate parameters such as the impact of partitions on aerosol and splatter.
We demonstrated significant contamination of the operator, assistant and mannequin for all procedures, which is consistent with the findings of other investigators. 34 , 38 , 41 , 44 This is unsurprising and underscores the need for adequate personal protective equipment (PPE), for the operator and assistant. Of particular note is the importance of the full‐face visor which was heavily contaminated in our study. This also highlights the importance of enhanced PPE 53 during the peak of a pandemic for AGPs, because of the likelihood of treating an asymptomatic carriers. Coverage of the operator and assistant's exposed arms with a waterproof covering would protect against contamination, although scrupulous hygiene with an effective antiseptic (povidone‐iodine or 70% alcohol 54 , 55 ) would be a minimum requirement if this was not used. PPE for patients’ clothes does not feature in dental guidelines relating to COVID‐19, and our findings would suggest significant contamination of the patient is likely during AGPs, presenting a risk of onward cross‐contamination by contact with surroundings; it is therefore important to provide waterproof protection for patients’ clothes.
Our findings demonstrate that use of a high‐speed air‐turbine, ultrasonic scaler and 3‐in‐1 spray are all AGPs. 3‐in‐1 use is not currently included in the list of defined healthcare‐related AGPs recently updated by Health Protection Scotland, 31 which only details ‘high‐speed devices such as ultrasonic scalers and high‐speed drills’. The highest levels of contamination were from the high‐speed air‐turbine, although the ultrasonic scaler demonstrated contamination at greater distances, in keeping with the findings of Bennett et al. 21 Dental suction was effective at reducing fluorescein contamination, with reduction of 67%‐75% between 0.5 and 1.5 m. This is consistent with the effect of suction demonstrated by other investigators. 37 , 56
When dental suction was provided by an assistant, this was more effective in reducing contamination, although increased readings were seen at 1.5 m, potentially indicating that an additional barrier in the form of an assistant may have a more complex aerodynamic effect. High‐volume dental suction is recommended in most dental guidelines and SOPs relating to COVID‐19, as an essential mitigation procedure when conducting AGPs. However, we are not aware of any that provide a definition or basic minimal requirements for effective high‐volume dental suction. National guidelines 57 classify suction systems based on air flow rate (high‐volume systems: 250 L/min at the widest bore size of the operating hose). We did not have a suitable device available to measure air flow rate of the system used in the present study and hence we chose to use the term ‘dental suction’ as we were unable to confirm whether it met this definition. We did, however, measure water flow rate (6.3 L/min) which we found to be similar to that reported by other investigators. 39 Our findings highlight the importance of suction as a mitigation factor in splatter and aerosol distribution following dental procedures, and future research should examine the impact of this effect in relation to different levels of suction based on air flow rate.
Safe times following procedures, after which contamination becomes negligible, have rarely been investigated robustly. In studies using tracer dyes, we are only aware of a single paper reporting contamination at 30 minutes. 44 This conflicts with our findings of no contamination by image analysis at 30 and 60 minutes, and only very low levels by spectrofluorometric analysis (≤ 0.10% of original levels). It is unclear from the methods of Veena et al 44 whether new filter papers were placed immediately following the procedure and collected at 30 minutes, or placed at 30 minutes and collected thereafter; in the prior case, any contamination found on the samples could have arisen at any time from the end of the procedure up to 30 minutes, and it cannot therefore be determined when contamination actually occurred. In addition, the authors do not report whether the tape they used to support filter papers was replaced following the initial exposure, and if not, it is possible that existing contamination was transferred to filter papers placed subsequently. Finally, the investigation reported by Veena et al 44 was a single experiment and did not use multiple repetitions. It is important to note that our findings relate to the environmental setting studied, with 6.5 air changes per hour. Air exchange rates in dental surgeries are likely to vary which may affect translation.
Our study has several limitations, and our results need to be interpreted in the context of these. Our methods serve as a model for aerosol and splatter contamination, and further work is required to confirm their biological validity. As our knowledge of the infective dose of SARS‐CoV‐2 required to cause COVID‐19 develops, the clinical relevance of our findings needs to be put into context; our understanding of this is still too basic to be able to draw definitive conclusions as to the risks posed by dental aerosol and splatter. The particle size analysis is likely to overestimate the size of the particles for two reasons: first, when fluorescein droplets are absorbed into the filter paper, they will spread out creating an area with a diameter greater than that of the original droplet; second, when samples are heavily contaminated the droplets coalesce on the filter paper to produce larger areas of contamination and the software measures the total surface area of the fused droplets. Our experimental set up incorporated the tracer dye within the irrigation system of the dental units and represents a worst‐case scenario for distribution of biological material.
In reality, a small amount of blood and saliva will mix with large volume of water irrigant creating aerosol and splatter with diluted pathogen concentration compared to blood or saliva, and a likely reduced infective potential. 19 It has been estimated that over a 15 minute exposure during dental treatment with high‐speed instruments, an operator may be exposed to 0.014‐0.12 µL of saliva. 21 Early data suggest a median SARS‐CoV‐2 viral load of 3.3 × 106 copies per mL in the saliva of infected patients 23 , 24 ; taken together, this suggests that an operator without PPE at around 0.5 m from the source may be exposed to an estimated 46‐396 viral copies during a 15‐minute procedure. These data were collected from hospital inpatients, and recent data suggest that asymptomatic carriers may have lower salivary viral loads 28 , 29 ; similarly the average concentration of fluorescein detected by spectrofluorometric analysis past 2 m in the present study was almost two orders of magnitude lower than at 0.5 m, and so at distances beyond 0.5 m, this risk is likely to be lower. Importantly, we still do not yet know what the infective dose of SARS‐CoV‐2 required to cause COVID‐19 is.
5. CONCLUSIONS
Within the limitations of this study, dental aerosol and splatter have the potential to be a cross‐infection risk even at a distance from the source. The high‐speed air‐turbine generated the most aerosol and splatter, even with assistant‐held suction. Our findings suggest that it may be safe to reduce fallow times between dental AGPs in settings with 6.5 air changes per hour to 30 minutes. Future research should evaluate further procedures, mitigation strategies, time periods and aim to assess the biological relevance of this model.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
AUTHOR CONTRIBUTIONS
J. R. Allison, C. C. Currie, D. Edwards, J. Durham, C. J. Nile, N. Jakubovics and R. Holliday contributed to the conception and design of the study. J. R. Allison, C. C. Currie, D. Edwards, C. Bowes, J. Coulter, K. Pickering, E. Kozhevnikova, C. J. Nile, N. Jakubovics, N. Rostami and R. Holliday contributed to the acquisition, analysis and interpretation of data. All authors were involved in drafting and critically revising the manuscript and have given final approval for publication. All authors agree to be accountable for all aspects of the work.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1111/joor.13098.
Supporting information
Fig S1
Fig S2
Table S1
ACKNOWLEDGEMENTS
Charlotte Currie is funded by a National Institute for Health Research (NIHR) Doctoral Research Fellowship. David Edwards is funded by a NIHR Academic Clinical Fellowship. Jamie Coulter is funded by a NIHR BRC Doctoral Research Fellowship. Richard Holliday is funded by a NIHR Clinical Lectureship. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. Nadia Rostami has been funded during this period by the Dunhill Medical Trust (RPGF1810/101) who kindly extended her funding to support this urgent COVID‐19‐related research. We would like to thank Ashling Dolan for advice on displaying the heat maps, Colm Kelleher (Department of Molecular and Cellular Biology, Harvard University, USA) for advice and expertise on Python programming, and to the engineers and tool curators at Newcastle Dental Hospital for their assistance with some practical aspects of this project. Consumables for this project were supported by internal funding from the School of Dental Sciences, Newcastle University.
Allison JR, Currie CC, Edwards DC, et al. Evaluating aerosol and splatter following dental procedures: Addressing new challenges for oral health care and rehabilitation. J Oral Rehabil 2020;48:61–72. 10.1111/joor.13098
DATA AVAILABILITY STATEMENT
Data available from the authors on reasonable request.
REFERENCES
- 1. Bridgman C.Letter to all primary care dental teams in Wales, 23rd March 2020 [Accessed: 23/06/2020]. Available from: https://www.fgdp.org.uk/sites/fgdp.org.uk/files/editors/2020.03.23%20CDO%20Wales%20COVID‐19%20advice%20letter.pdf.
- 2. Donaldson M. COVID‐19. Outline strategic plan for General Dental Services and updated guidance for General Dental Practice, 23rd March 2020 [Accessed: 23/06/2020]. Available from: http://www.hscbusiness.hscni.net/pdf/HSCB_COVID‐19_GDS‐Strategic‐Plan.pdf.
- 3. Ferris T.Letter to NHS Dental Services (ref: POL/33888), 23rd March 2020 [Accessed: 23/06/2020]. Available from: https://www.fgdp.org.uk/sites/fgdp.org.uk/files/editors/2020.03.23%20CDO%20Scotland%20COVID‐19%20advice%20letter.pdf.
- 4. Hurley S, Neligan M.Letter to general dental practices and community dental services (ref: 001559), 25th March 2020 [Accessed: 06/06/2020]. Available from: https://www.england.nhs.uk/coronavirus/wp‐content/uploads/sites/52/2020/03/issue‐3‐preparedness‐letter‐for‐primary‐dental‐care‐25‐march‐2020.pdf.
- 5. Carter E, Currie CC, Asuni A, et al. The first six weeks – setting up a UK urgent dental care centre during the COVID‐19 pandemic. Br Dent J. 2020;228(11):842‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Long L, Corsar K. The COVID‐19 effect: number of patients presenting to The Mid Yorkshire Hospitals OMFS team with dental infections before and during The COVID‐19 outbreak. Br J Oral Maxillofac Surg. 2020;58(6):713–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bridgman C.Letter to All Primary Care Dental Teams and Health Boards, 22nd May 2020 [Accessed: 23/06/2020]. Available from: https://www.gdc‐uk.org/docs/default‐source/covid‐19/2020‐05‐22–‐cdo‐letter–‐restoration‐of‐dental‐services–‐22‐05‐20.pdf?sfvrsn=3f9d26a4_2.
- 8. Donaldson M.Plans for the Restoration of General Dental Services, 2nd June 2020 [Accessed: 23/06/2020]. Available from: https://www.fgdp.org.uk/sites/fgdp.org.uk/files/editors/2020.06.02%20CDO%20NI%20Plans%20for%20the%20Restoration%20of%20General%20Dental%20Services.pdf.
- 9. Ferris T.Remobilisation of NHS Dental Services in Scotland, 20th May 2020 [Accessed: 23/06/2020]. Available from: https://www.gdc‐uk.org/docs/default‐source/covid‐19/cdo‐letter–‐remobilisation‐of‐nhs‐dental‐services–‐20‐may‐2020.pdf?sfvrsn=2ab26ee_2.
- 10. Hurley S, Neligan M.Resumption of dental services in England, 28th May 2020 [Accessed: 23/06/2020]. Available from: https://www.fgdp.org.uk/sites/fgdp.org.uk/files/editors/2020.05.28%20CDO%20England%20resumption%20of%20dental%20services.pdf.
- 11. Danish Health Authority . COVID‐19 management: adaptation and progressively increased dental activity. Copenhagen, Denmark: Danish Health Authority; 2020. [Google Scholar]
- 12. Kenya Dental Association . Update of guidelines to prevent spread of COVID‐19 in the management of patient requiring dental and oralmaxillofacial services. Nairobi, Kenya: Kenya Dental Association; 2020. [Google Scholar]
- 13. Centres for Disease Control and Prevention . Guidance for Dental Settings: Centres for Disease Control and Prevention; 2020. [Accessed: 25/06/2020]. Available from: https://www.cdc.gov/coronavirus/2019‐ncov/hcp/dental‐settings.html
- 14. British Endodontic Society . BES COVID‐19 Return to Work SOP. St Albans, Herts: British Endodontic Society; 2020. [Google Scholar]
- 15. College of General Dentistry, Faculty of General Dental Practice . Implications of COVID‐19 for the safe management of general dental practice: A practical guide. London: College of Genreal Dentistry, Faculty of General Dental Practice. [Google Scholar]
- 16. NHS England . Urgent dental care guidance and standard operating procedure. London: NHS England; 2020. [Google Scholar]
- 17. Office of The Chief Dental Officer England . Standard Operating Procedure Transition to Recovery. London: Office of The Chief Dental Officer England; 2020. [Google Scholar]
- 18. Scottish Dental Clinical Effectiveness Programme . Resuming General Dental Services Following COVID‐19 Shutdown. Dundee: Scottish Dental Clinical Effectiveness Programme. [Google Scholar]
- 19. Durham J, Bagg J, Bain S, Bissell V, Burgden D, Chadwick B, et al.COVID‐19 – returning to student‐led dental clinical treatments 2020 [22/06/2020]. Available from: https://www.dentalschoolscouncil.ac.uk/wp‐content/uploads/2020/06/COVID‐19‐report‐on‐returning‐to‐student‐leddental‐clinical‐treatments.pdf.
- 20. Kumar PS, Subramanian K. Demystifying the mist: Sources of microbial bioload in dental aerosols. J Periodontol. 2020;91(9):1113–1122. 10.1002/JPER.20-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bennett AM, Fulford MR, Walker JT, Bradshaw DJ, Martin MV, Marsh PD. Microbial aerosols in general dental practice. Brit Dent J. 2000;189(12):664‐667. [DOI] [PubMed] [Google Scholar]
- 22. Miller RL. Characteristics of blood‐containing aerosols generated by common powered dental instruments. Am Ind Hyg Assoc J. 1995;56(7):670‐676. [DOI] [PubMed] [Google Scholar]
- 23. To KK‐W, Tsang OT‐Y, Yip CC‐Y, et al. Novel Coronavirus in Saliva. Clin Infect Dis. 2019;2020: 10.1093/cid/ciaa149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wyllie AL, Fournier J, Casanovas‐Massana A, et al. Saliva is more sensitive for SARS‐CoV‐2 detection in COVID‐19 patients than nasopharyngeal swabs. medRxiv.. 2020;2020(04):pp. 16.20067835. [Google Scholar]
- 25. Gudbjartsson DF, Helgason A, Jonsson H, et al. Spread of SARS‐CoV‐2 in the Icelandic Population. N Engl J Med. 2020;382(24):2302‐2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pascolo L, Zupin L, Melato M, Tricarico PM, Crovella S. TMPRSS2 and ACE2 coexpression in SARS‐CoV‐2 salivary glands infection. J Dent Res. 2020;0022034520933589. [DOI] [PubMed] [Google Scholar]
- 27. Xu J, Li Y, Gan F, Du Y, Yao Y. Salivary Glands: Potential reservoirs for COVID‐19 asymptomatic infection. J Dent Res. 2020;0022034520918518. [DOI] [PubMed] [Google Scholar]
- 28. Chau NVV, Thanh Lam V, Thanh Dung N, et al. The natural history and transmission potential of asymptomatic SARS‐CoV‐2 infection. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kawasuji H, Takegoshi Y, Kaneda M, et al. Viral load dynamics in transmissible symptomatic patients with COVID‐19. medRxiv.. 2020;2020(06):pp. 02.20120014. [Google Scholar]
- 30. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS‐CoV‐2 as compared with SARS‐CoV‐1. N Engl J Med. 2020;382(16):1564‐1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Health Protection Scotland . Transmission based precautions literature review: aerosol generating procedures. Glasgow: Health Protection Scotland; 2020. [Google Scholar]
- 32. Dutil S, Meriaux A, de Latremoille MC, Lazure L, Barbeau J, Duchaine C. Measurement of airborne bacteria and endotoxin generated during dental cleaning. J Occup Environ Hyg. 2009;6(2):121‐130. [DOI] [PubMed] [Google Scholar]
- 33. Zemouri C, Volgenant CMC, Buijs MJ, et al. Dental aerosols: microbial composition and spatial distribution. J Oral Microbiol. 2020;12(1):1762040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Al‐Amad SH, Awad MA, Edher FM, Shahramian K, Omran TA. The effect of rubber dam on atmospheric bacterial aerosols during restorative dentistry. J Infect Public Health. 2017;10(2):195‐200. [DOI] [PubMed] [Google Scholar]
- 35. Watanabe A, Tamaki N, Yokota K, Matsuyama M, Kokeguchi S. Use of ATP bioluminescence to survey the spread of aerosol and splatter during dental treatments. J Hosp Infect. 2018;99(3):303‐305. [DOI] [PubMed] [Google Scholar]
- 36. Holloman JL, Mauriello SM, Pimenta L, Arnold RR. Comparison of suction device with saliva ejector for aerosol and spatter reduction during ultrasonic scaling. J Am Dent Assoc. 2015;146(1):27‐33. [DOI] [PubMed] [Google Scholar]
- 37. Miller RL, Micik RE, Abel C, Ryge G. Studies on dental aerobiology: II. Microbial splatter discharged from the oral cavity of dental patients. J Dent Res. 1971;50(3):621‐625. [DOI] [PubMed] [Google Scholar]
- 38. Rautemaa R, Nordberg A, Wuolijoki‐Saaristo K, Meurman JH. Bacterial aerosols in dental practice – a potential hospital infection problem? J Hosp Infect. 2006;64(1):76‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Timmerman MF, Menso L, Steinfort J, van Winkelhoff AJ, van der Weijden GA. Atmospheric contamination during ultrasonic scaling. J Clin Periodontol. 2004;31(6):458‐462. [DOI] [PubMed] [Google Scholar]
- 40. Bentley CD, Burkhart NW, Crawford JJ. Evaluating spatter and aerosol contamination during dental procedures. The Journal of the American Dental Association. 1994;125(5):579‐584. [DOI] [PubMed] [Google Scholar]
- 41. Chanpong B, Tang M, Rosenczweig A, Lok P, Tang R. Aerosol‐generating procedures and simulated cough in dental anesthesia. Anesth Prog. 2020;. 10.2344/anpr-67-03-04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dahlke WO, Cottam MR, Herring MC, Leavitt JM, Ditmyer MM, Walker RS. Evaluation of the spatter‐reduction effectiveness of two dry‐field isolation techniques. J Am Dent Assoc. 2012;143(11):1199‐1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Harrel SK, Barnes JB, Rivera‐Hidalgo F. Aerosol and splatter contamination from the operative site during ultrasonic scaling. The Journal of the American Dental Association. 1998;129(9):1241‐1249. [DOI] [PubMed] [Google Scholar]
- 44. Veena HR, Mahantesha S, Joseph PA, Patil SR, Patil SH. Dissemination of aerosol and splatter during ultrasonic scaling: a pilot study. J Infect Public Health. 2015;8(3):260‐265. [DOI] [PubMed] [Google Scholar]
- 45. Chiramana S, Hima Bindu OS, Kishore Kadiyala K, Prakash M, Durga Prasad T, Chaitanya SK. Evaluation of minimum required safe distance between two consecutive dental chairs for optimal asepsis. Journal of Orofacial. Research. 2013;3:12‐15. [Google Scholar]
- 46. Harrel SK, Barnes JB, Rivera‐Hidalgo F. Reduction of aerosols produced by ultrasonic sealers. J Periodontol. 1996;67(1):28‐32. [DOI] [PubMed] [Google Scholar]
- 47. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671‐675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Steiner S, Majeed S, Kratzer G, Hoeng J, Frentzel S. A new fluorescence‐based method for characterizing in vitro aerosol exposure systems. Toxicol In Vitro. 2017;38:150‐158. [DOI] [PubMed] [Google Scholar]
- 49. Van Rossum G, Drake FL. Python 3 Reference manual. Scotts Valley, CA: CreateSpace; 2009. [Google Scholar]
- 50. Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. Journal of chiropractic medicine. 2016;15(2):155‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tag El Din AM, Ghoname NAH. Efficacy of rubber dam isolation as an infection control procedure in paediatric dentistry. La Revue de Santé de la Mediterranée Orientale. 1997;3:530‐539. [Google Scholar]
- 52. Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019‐nCoV on the epithelial cells of oral mucosa. International journal of oral science. 2020;12(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Verbeek JH, Rajamaki B, Ijaz S, et al. Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff. Cochrane Database Syst Rev. 2020;4:CD011621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kratzel A, Todt D,V’kovski P, Steiner S, Gultom M, Thao TTN, et al. Inactivation of severe acute respiratory syndrome coronavirus 2 by WHO‐recommended hand rub formulations and alcohols. Emerg Infect Dis J. 2020;26(7):1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bidra AS, Pelletier JS, Westover JB, Frank S, Brown SM, Tessema B. Rapid In‐VITRO inactivation of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) using povidone‐iodine oral antiseptic rinse. Journal of Prosthodontics. 2020;. 10.1111/jopr.13209(n/a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Micik RE, Miller RL, Mazzarella MA, Ryge G. Studies on dental aerobiology: I. Bacterial aerosols generated during dental procedures. J Dent Res. 1969;48(1):49‐56. [DOI] [PubMed] [Google Scholar]
- 57. Estates NHS. HTM 2022 ‐ Supplement 1: Dental compressed air and vacuum systems. London: The Stationary Office; 2003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Table S1
Data Availability Statement
Data available from the authors on reasonable request.


