Abstract
A new disinfection system utilizing UVC‐LED irradiation was developed. The system was affixed to the toilet seat, and it was challenged by three bacteria strains. Different configurations were tested: 3‐LEDs, 5‐LEDs (two variants), and 8‐LEDs. To determine the arrangement designs of LEDs with the optimum efficacy, two variants of 5‐LEDs configurations were additionally considered—uniform and concentrated (2‐sided) distributions. It was noticed that disinfection efficacy initially increased with the number of LEDs, but with 8‐LEDs, the trend became almost non‐obvious for surface disinfection and just marginally increased for airborne disinfection. The mean efficiencies for the surface disinfection ranged from 55.17 ± 23.89% to 72.80 ± 4.13% for E. coli; 36.65 ± 2.99% to 50.05 ± 13.38% for S. typhimurium; and 8.81 ± 3.23% to 39.43 ± 9.33% for S. epidermidis. Likewise, the mean efficiencies for airborne disinfection ranged from 42.17 ± 8.18% to 70.70 ± 4.80%; 40.40 ± 17.90% to 58.31 ± 13.87%; and 24.16 ± 3.81% to 42.79 ± 10.20% for E. coli; S. typhimurium; and S. epidermidis, respectively. Furthermore, the efficacy of the uniform irradiation was nearly twice that of the concentrated irradiation for surface disinfection and 17.70% higher for airborne disinfection, when tested against E coli. Collectively, these very promising results showcased that this compact, sustainable, and localized disinfection system has a high potential for the next generation of disinfection devices.
Keywords: airborne pathogens, light‐emitting diode, localized disinfection, toilet hygiene, ultraviolet C
Practical Implications.
Other than ventilation, there are no proven engineering solutions for minimizing infectious disease transmission in toilets.
The approach presented in this study (ie, localized elimination approach) can help improve toilet conditions for healthier indoor air quality.
The findings of this study are useful in developing effective engineering methods for controlling infectious diseases.
The study is expected to additionally promote public awareness of the disinfection efficacy of UVC‐LED for inactivating fecal pathogens generated by toilet flushing.
This study calls for effective and urgent design and implementation actions on energy‐efficient and safe interventions for airborne and surface‐borne transmissions.
1. INTRODUCTION
Poor sanitation is one of the leading causative factors of infectious diseases such as cholera, diarrhea, dysentery, hepatitis A, typhoid, and polio. 1 Being an important facility for sanitation, the purpose of a toilet is to provide a sanitation fixture for the storage or disposal of human wastes, including feces and urine, to improve hygienic conditions. However, the toilet and its immediate environment are recognized to be hot‐spots of bio‐contamination 2 , 3 and diverse types of bacteria have been detected in public restrooms. 2 , 4
Toilet plume is a major contributor to the transmission of gastroenteric diseases. 5 Flushing of toilets generates numerous fine droplets. The risk of infection of microorganisms in toilets is primarily related to infective doses of pathogens. For certain enteric pathogens, such as norovirus and enterohemorrhagic Escherichia coli (EHEC) in which low doses (<50 cells) suffice in infection, the likelihood of transmission is high. 6 , 7 Depending on the toilet design and other environmental factors such as flushing pressure, a single toilet flushing generates a large number of potentially infectious aerosols ranging between hundreds of thousands and millions. 8 , 9 In the literature, a high fecal viral load of >109 viral particles copies per g of feces has been detected in patients infected with norovirus. 10 , 11 If feces containing pathogenic organisms are shed into the toilet bowl and the toilet is flushed, water will be atomized and copious pathogen‐laden aerosol droplets are produced. 2 , 3 , 12 , 13
Small droplets would usually become airborne and could travel very long distances while coarse droplets would settle near the toilet bowl. Also, these pathogen‐laden droplets would result in two routes of exposure or transmission of infectious pathogens due to toilet flushing, namely airborne and contact modes. 2 , 3 , 5 , 14 Through airborne pathway, infections occur by direct inhalation of pathogenic airborne droplets. 15 , 16 In extant studies, bioaerosols were detected even at a few tens of centimeters above the toilet seat persisting up to an hour after flushing. 2 , 5 , 12 In light of this observation, it is not unreasonable to imply that enteric viruses and bacteria in the air present a potential risk of infection via inhalation among toilet users. 2 , 5 , 17
For the contact mode of infection, the fine and coarse pathogen‐laden droplets would lead to surface or fomite contamination. A toilet user will inevitably touch various surfaces inside the cubicle. Surface contamination studies have identified microbial contamination of washroom surfaces, including doors, toilet seats, sinks, and floors. 2 , 4 , 13 , 18 Contact exposures are no doubt an important risk, as toilet users may become infected whenever they touch surfaces already contaminated by rapidly falling fecal microbes. This source of contamination is also a major public health concern because hand contact with contaminated surfaces can result in self‐inoculation through touching of the eyes, nose, or mouth. 13
One matter further complicating the issue is that the microorganisms from the fecal waste may remain on the bowl surfaces even after multiple flushing. Studies have observed that residual microorganisms on the bowl might be aerosolized later. 13 , 19 A recent field measurement 12 in a hospital also confirmed the collection of airborne microorganisms, even by simply flushing without fecal waste. The observation has important implications for public hygiene control. Besides, recent studies have found SARS‐CoV‐2, the causative pathogen of COVID‐19 in toilet areas. 20 , 21 This evidence suggests that COVID‐19 can be potentially generated through toilet flushing.
To reduce the risk of airborne transmission of pathogens due to toilet flushing, the control cannot only be effective for episodic events. The use of mechanical ventilation to remove odor and pollutants is a conventional approach and can be very costly. Recently, some disinfection units, which can be mounted on walls or ceilings are becoming more popular for commercial buildings. Most of them utilize UV for the disinfection of pathogens. More importantly, the devices are always mounted at the washing basins, which means that the “disinfection action” would not take place until the pathogens are well‐mixed within the restroom. To date, no studies have reported the quantitative disinfection performance of those devices in field settings.
It is always reasonable and effective to control exposure at the spot of emission if condition permits. That is the concept of localized disinfection. Very recently, the authors have tested the concept of localized disinfection by utilizing low‐pressure mercury UVC lamps. It might be the first study that systematically tested the disinfection performance of UVC for toilet‐flushing‐generated pathogens. The results were compared with measurements from a conventional upper‐room ultraviolet germicidal irradiation (UR‐UVGI) device and higher disinfection efficacy was observed for the localized UV disinfection device. For airborne tests involving Escherichia coli (E. coli), the disinfection efficacy was 51% for the UR‐UVGI device, and 97% for the localized UV disinfection device. As the irradiation level for the UR‐UVGI device, when measured at the toilet seat level, was virtually zero, it was not surprising to observe that the surface disinfection efficacy was also very low, approximately 17% while for the localized device, it was about 60%. 22
Even though the mercury‐type localized UVC device has been shown to reduce the bioburden of toilets more significantly, the prototype is bulky and cannot be used in practice. In this study, we utilized light‐emitting diodes (LEDs) to design a localized disinfection device for the toilet seat. The use of LEDs to produce UV radiation presents many advantages. LEDs require no warm‐up time, less energy to operate, exhibit a very long operational lifespan up to 100 000 hours. 23 Also, LEDs are environmentally friendly as they do not contain toxic materials or pollutants. Furthermore, another strength of LEDs over lamps is that their disposal treatment is very easy. Most importantly, they are very compact.
The authors have utilized UVC‐LED for upper‐room installation. 24 A very high disinfection performance was observed. However, the indoor environment and the toilet bowl have very different emission characteristics. Flushing‐generated airborne pathogens are intense, flowing around for a short period (~ seconds) in the vicinity of the toilet, followed by mixing inside the toilet cubicle and entire restroom. From the pollutant control perspective, it is recognized that for very high efficacy, it is better to remove pathogens in the vicinity of the source. However, this approach has not been applied to commercial products. The major challenge of the localized device is how to ensure that sufficient UV dose is absorbed by the pathogens since the emission period during flushing and the exposure time are very short.
This study is the first to investigate the use of UVC‐LED to disinfect the toilet bowl. The results would not only be useful for developed countries but also third world counties because toilet hygiene is still extremely poor in many countries. Therefore, the development of a compact, portable toilet disinfection technology can be of significant benefits to global public hygiene. In this work, we aim to test the efficacy of the novel toilet disinfection system for airborne and settled infectious pathogens; factors affecting the efficacy, that is, number of LEDs and different bacteria species.
2. SYSTEM DESIGN
The experimental chamber consists of a custom‐made pre‐existing toilet rig. The facility was equipped with an American standard wash‐down type water closet (WC), one 50‐L volume water tank, and a flushometer. Also, the toilet facility was equipped with a clean water supply. The UVC‐LED has a UVC output of <20 mW (www.cisuvc.com) with a rated current of 400 mA. Each LED was soldered on a printed circuit board (PCB) and each PCB was fixed onto a small aluminum plate—12 mm × 18 mm—for mounting on a tailor‐made aluminum ring (see Figure 1). In this study, the ring, fitted with LEDs, was put on the top of the WC for disinfection of airborne pathogens generated by toilet flushing.
FIGURE 1.
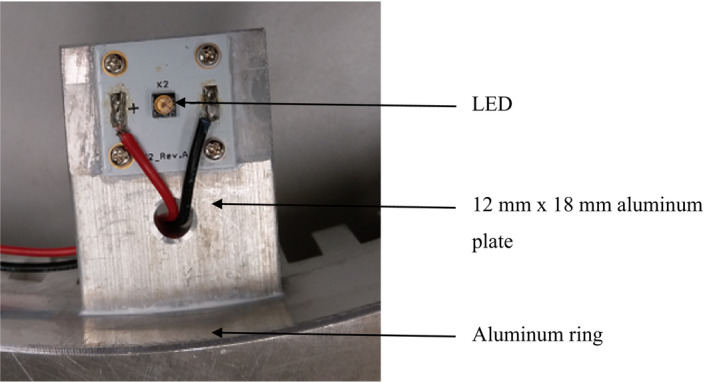
Components of the localized toilet disinfection system
3. METHODOLOGY
3.1. Different configurations of LED studied
Different configurations of LEDs were designed and tested in this study: (a) Evenly distributed configuration involving 3 LEDs, 5 LEDs, and 8 LEDs (see Figure 2A‐C) (b) Concentrated configuration involving two‐sided 5‐LEDs (Figure 2D). The purpose of the first arrangement was to achieve uniform irradiance distribution in the toilet bowl. On the contrary, the second arrangement was designed to mimic a non‐uniform irradiance distribution scenario in the toilet bowl.
FIGURE 2.

Different LED arrangements. (A) 3‐LEDs, evenly distributed configuration on the ring. (B) 5‐LEDs, evenly distributed configuration on the ring. (C) 8‐LEDs, evenly distributed configuration on the ring. (D) 2‐sided 5‐LEDs, concentrated configuration on the ring
Depending on the number of the LEDs used for the particular set of experiments, the small aluminum plate (with LED) was mounted to the aluminum ring (see Figure 2A‐D). During toilet flushing, water splashing was anticipated, and to prevent the LEDs or the PCB from the ingress of water, a thin transparent film was used to wrap the PCB.
3.2. Measurement of UV irradiance
To correlate the UV irradiance level and disinfection performance, the measurement of UV irradiance was required. A UV‐VIS fiber‐optic spectrometer (AvaSpec‐ULS3648) was used to measure the irradiance. Two sets of measurements were taken, one for individual LEDs at different distances, and the other for different configurations inside the toilet bowl. For individual LED measurement, a UVC‐LED irradiance was measured at different distances from the source up to seven centimeters. Five different LEDs were measured in this set.
The disinfection of pathogens depends on the UV dose absorbed. The latter set of measurement was to measure the total irradiance for each configuration inside the toilet bowl. It is important to measure the total irradiance by different configurations for relative comparison, thus in this regard, the selection of the depth of the sensing probe was arbitrary. The water in the toilet bowl was drained, and the probe was put at a preset depth below the seating level in the middle of the bowl for measuring the total irradiance. The setup illustrated in Figure 3 was used to measure the incident irradiance distribution. The actual design of the aluminum ring could allow the mounting of ten LEDs, labeled “A” to “J” as shown in Figure 4. For instance, for 3 LEDs configuration, “A”, “E”, and “F” LEDs were mounted on the ring and the intensity was measured. The mounting positions of LEDs for other configurations are reported in section 5.3.
FIGURE 3.
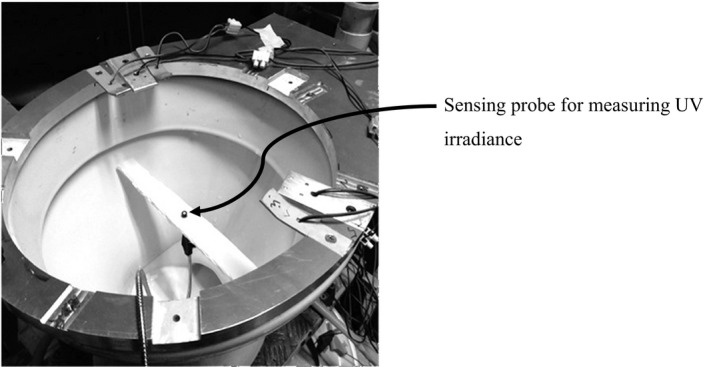
The position of the probe for measuring UV irradiance in the toilet bowl for different LED configurations
FIGURE 4.
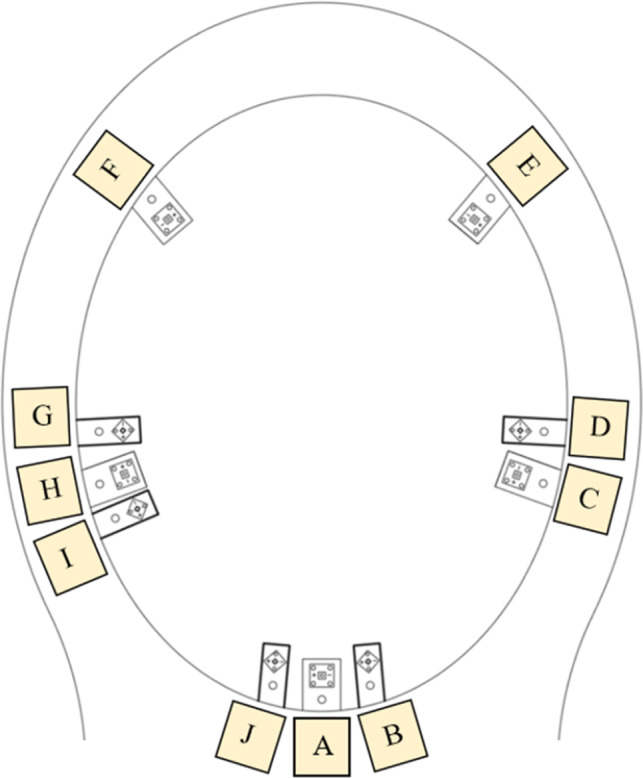
The mounting positions of different LEDs
3.3. Microorganism selection
The criteria for the selection of microorganisms include biosafety issues and pathogenic properties. In this study, three species of pathogenic bacteria were selected including Escherichia coli (E. coli) (ATCC #10536); Salmonella typhimurium (S. typhimurium) (ATCC #53648), and Staphylococcus epidermidis (S. epidermidis) (ATCC #12228). These bacteria have been previously used as nonpathogenic surrogate species in other bioaerosol and surface contamination studies. 17 , 22 , 25 The procedures for the preparation of these bacteria have been extensively documented in our previous publication. 25
3.4. Experimental procedure
Before seeding the toilet bowl with bacteria, the toilet bowl, and the cistern were thoroughly cleaned with a measure of 100 mL of commercially available Clorox (chlorine) bleach and a toilet brush and then flushed three times, to completely remove residues of the cleaning compound and any microorganisms present in the flushing water. A solution of 12 mL of sodium thiosulphate was then added to inactivate any bleach chemicals present in the water. Finally, water was again used to wash the bowl and cistern in the same manner as previously described. The water used for this study was public utility water and had been filtered to remove suspended solids or microbes. This cleaning process was repeated before each experiment. After thoroughly cleaning the system, the tank was filled with water. During the cleaning process, the air in the chamber was simultaneously disinfected using an upper‐room ultraviolet germicidal irradiation system which was installed at the upper part of one of the chamber walls. The UR‐UVGI device was turned off when the cleaning of the toilet bowl and cistern was completed. It is worth noting that for safety reasons, appropriate protective kits were worn.
At the end of the cleaning task, the LEDs were fixed to the rim of the WC. Subsequently, three air sampling components were installed at carefully selected locations to mimic the inhalation of different toilet users and categorized as low‐level air samples (ASL) for seat level initial upsurge of aerosol from the flushed toilet bowl, middle‐level air samples (ASM) for children's breathing zone, and high‐level air samples (ASH) for adults’ breathing zone (Figure 5). The vertical distance from the ground floor level to the ASL, ASM, and ASH levels was 0.4 m, 0.9 m, 1.3 m, respectively. To simplify the collection of air samples at the three levels mentioned, three copper sampling tubes were used. Each copper tube, 0.012 m (diameter) and 1 m (length), was connected to a cast acrylic sheet squared box (0.15 m × 0.15 m × 0.15 m) at one end. The cast acrylic sheet squared box was then connected to the impactor and the three air sampling manifolds were carefully adjusted to align the other ends of the copper tubes to the center of the toilet bowl. The air samples were transferred to the agar plates through the copper tubes and the cast acrylic sheet square box. The schematic of the copper tube‐acrylic box sampling manifold is shown in Figure 6.
FIGURE 5.
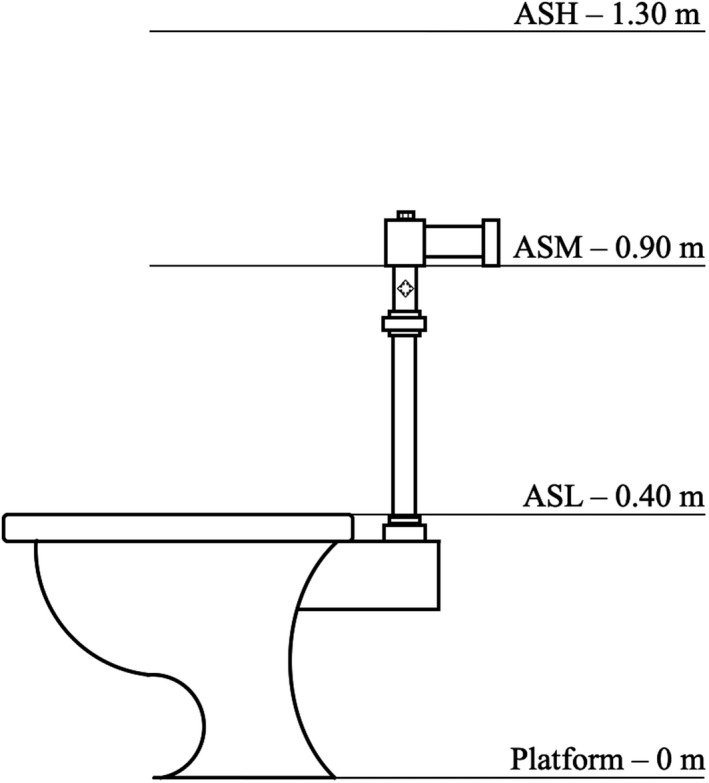
Different levels of air sample collection
FIGURE 6.

The copper tube‐acrylic box sampling manifold
Also, to measure bioaerosols deposited onto the toilet seat, four nutrient agar‐filled plates with lids were set out in predetermined positions near the edges of the toilet seat. Further information about the selected locations for the collection of surface samples has been systematically discussed elsewhere. 22
Thereafter, a 250 mL solution of bacteria was poured from a vial into the toilet bowl (seeding). Having seeded the toilet bowl, lids of the agar plates for surface sample collection were opened and the LEDs were activated. The door of the toilet chamber was closed, and the flush was triggered. Since a high priority was given to safety, no one was allowed inside the chamber during the experiments. To activate the flushing, a long string was attached to the flush lever, so that the toilet could be flushed from outside the test room. At the time the toilet was flushed to generate airborne microorganism emission, the three single‐stage Anderson biological impactors were also run for 1 minute with calibrated vacuum pumps operated at a flow rate of 28.3 L/min. The vacuum pump drew air samples from the experimental toilet facility into the inlet of the impactor and then aimed the particle‐laden airstreams at the nutrient‐filled medium spread on the agar plate. After allowing additional 15 minutes for droplets emitted from the one‐time toilet flush to settle onto the agar plates, the door was unsealed to collect all plates. After a collection cycle, all of the seven agar plates (three air and four surface samples) were then immediately incubated at 37°C overnight and colony‐forming units (CFUs) were counted.
Control experiments were also conducted in the same environmental conditions without exposure to UVC‐LED irradiation for equal time points and on the same day as the treatment experiment. During the experiments, the environmental conditions such as relative humidity and temperature were closely monitored and kept the same between control and treatment.
4. DATA INTERPRETATION
4.1. Determination of disinfection efficiency
Each inactivation experiment was repeated in triplicate. Each data bar represents the arithmetic mean of the three replicates and the standard deviation of the three trials was used as the error bar. The disinfection efficiency () was estimated using Equation (1).
| (1) |
where and are the colony‐forming units with and without LED exposure, respectively at the same time point, n represents the number of samples taken (in this study, 3 for airborne and 4 for surface samples). The p‐value was used to determine the statistical significance of the observed differences. A positive hole correction factor was applied to the raw CFU counts on the Petri dish after appropriate incubation. 26
5. RESULTS
5.1. Spectra distribution of UVC‐LED
Figure 7 shows the emission spectrum of UV light produced by a typical LED. The spectrum was measured at 8 V and a distance of 1 cm. The LED exhibited a peak emission wavelength at 269.60 nm with a corresponding intensity of 98.63 μWcm−2 and full widths at half maximum (FWHM) spectral bandwidth (SBW) of 12.14 nm.
FIGURE 7.
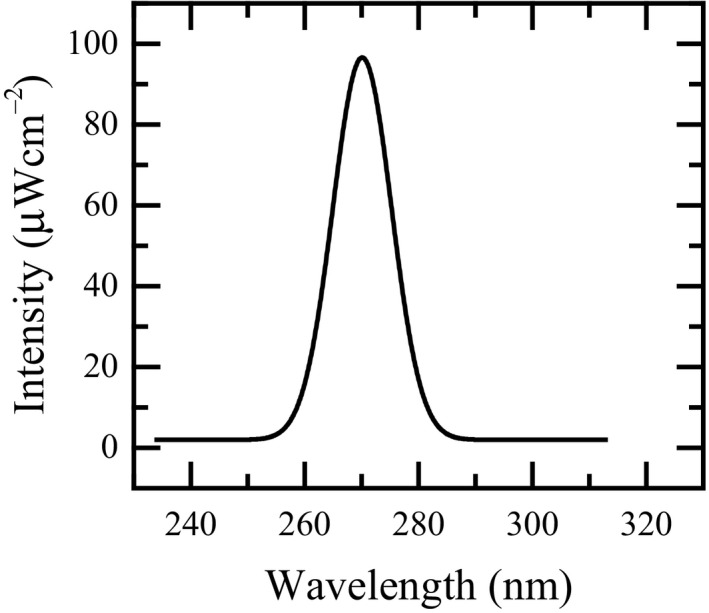
A typical emission spectrum curve of a LED
5.2. Individual UVC‐LED irradiance measurements at different distances
Figure 8 shows the averaged irradiance and standard deviations of the sum of 5 individual LEDs versus distance. The UVC‐LED irradiance was measured from the source up to seven centimeters, to estimate the effect of distance on irradiance changes, specifically for future modeling and estimating UV dose for the prototype unit. The mean LED irradiance varied from 98.63 ± 14.72 to 0 µWcm−2 when the distance increased from 1 to 7 cm. High uncertainly was found for the measurements nearest to the UV source. At the location with such a high intensity, even a very small deviation may cause a large difference in the reading. Also, it was observed that the UV intensity dropped very rapidly and reached close to zero when the distance was just 4 cm away from the LED.
FIGURE 8.
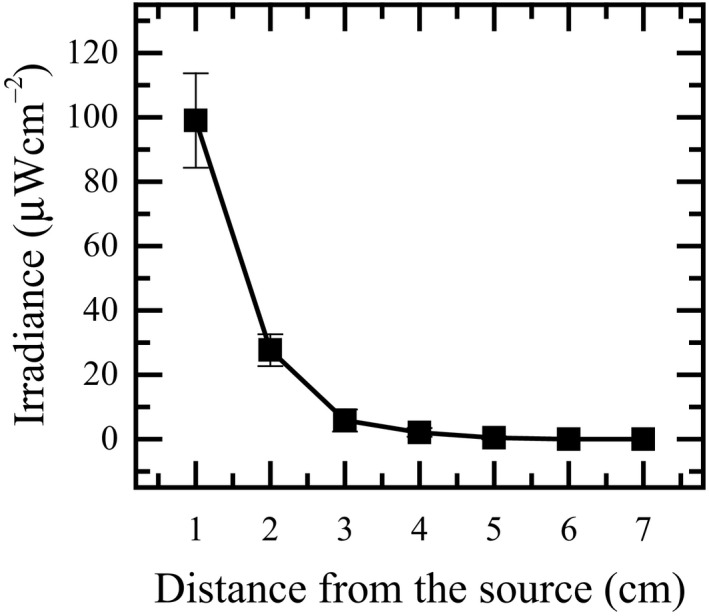
Averaged LED irradiance as a function of distance from the source
The decrease in irradiance with such a small distance could have implications for system designs. It suggests a potential scenario. It could indicate that the bacteria disinfection reported in this study occurred at a short emission distance within the locality of the UVC‐LEDs, that is, almost immediately the flushing was activated.
5.3. Total irradiance measurement in the toilet bowl
The results for the total irradiance measured inside the toilet bowl for different LED configurations are reported in Table 1. The total UV irradiance increased with the numbers of the LEDs tested. For the 3‐LEDs, 5‐LEDs, and 8‐LEDs well‐distributed configurations, the measured irradiances were 0.86, 1.15, and 3.07 μWcm−2, respectively. Likewise, the irradiance of the 5‐LEDs two‐sided nonuniformly‐distributed configuration was 1.57 μWcm−2. Due to the arrangement of the LEDs, it can be observed that the total irradiance was not linearly proportional to the number of LEDs used. Besides, the two‐sided 5‐LEDs gave a higher irradiance than the well‐distributed 5‐LEDs.
TABLE 1.
Different LEDs configurations tested
| Configurations | Position arrangements | Total irradiance (μWcm−2) |
|---|---|---|
| 3 LEDs | A, E, F | 0.86 |
| 5 LEDs | A, C, E, F, H | 1.15 |
| 5 LEDs (two‐sided) | C, D, G, H, I | 1.57 |
| 8 LEDs | B, C, D, E, F, H, I, J | 3.07 |
5.4. Surface disinfection
The assessment of the disinfection efficiency on the surface of a toilet seat for the different LED configurations and the bacteria under evaluation is shown collectively in Figure 9. The estimated mean efficiencies (mean ± SD) with 3‐LEDs, 5‐LEDs and 8‐LEDs were 55.17 ± 23.89% (range 23.09‐73.28%), 72.03 ± 9.02% (range 62.86‐80.89%), and 72.80 ± 4.13% (range 69.63‐77.47%) for E. coli; 36.65 ± 2.99% (range 33.33‐39.13%), 46.04 ± 10.69% (range 35.29‐56.67%), and 50.05 ± 13.38% (range 41.30‐65.45%) for S. typhimurium; 8.81 ± 3.23% (range 5.62‐12.07%), 39.63 ± 2.72% (range 36.65‐41.98%), and 39.43 ± 9.33% (Range 30.61‐49.19%) for S. epidermidis, respectively. It is clear from these results that the maximum achieved surface disinfection efficacy was obtained for E. coli with 8‐LEDs operational configurations. It is also noted that among the three tested bacteria, UV irradiance has minimum effects against S. epidermidis.
FIGURE 9.
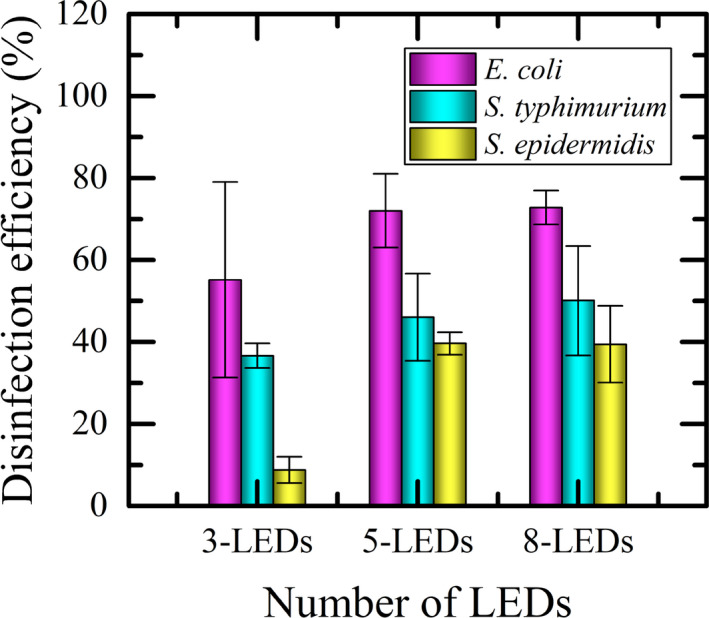
Efficacy of different configurations of localized UVC‐LEDs for surface disinfection
Another salient observation is that the disinfection efficacy seems to reach a plateau. It is evident for all the surface disinfection experiments we conducted that there was a modest but no statistically significant difference (P > .05) between the disinfection efficiency of 5‐LEDs and 8‐LEDs operational configurations, both having nearly the same impact. This has an important energy and design implication. Firstly, this result suggests that disinfection efficacy may reach a plateau and, in this work, perhaps 5‐LEDs are adequate for achieving the same performance as employing 8‐LEDs. Secondly, an avoidable higher energy cost may be incurred by using 8‐LEDs instead.
5.5. Airborne disinfection
The numbers of CFUs at different levels (ie, ASH, ASM, and ASL) were counted and summed up. The results of the efficacy of airborne disinfection by the localized UVC‐LED system are shown in Figure 10.
FIGURE 10.
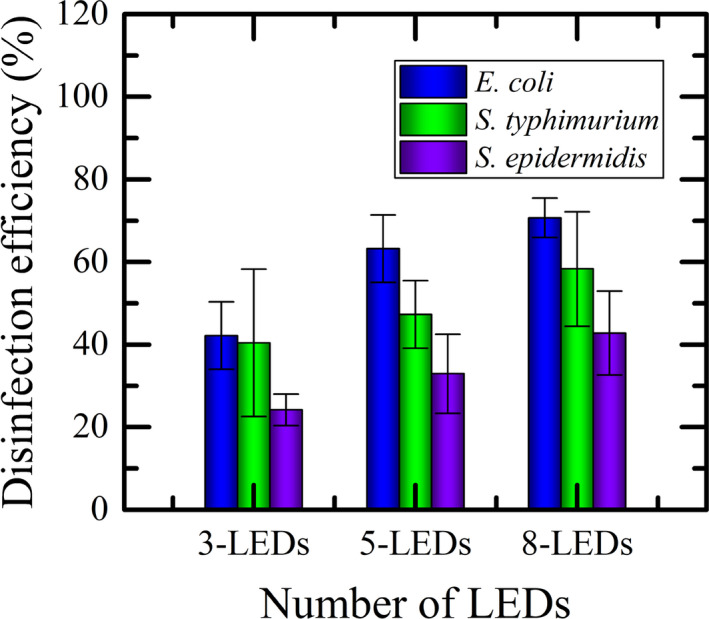
Efficacy of different configurations of localized UVC‐LEDs for airborne disinfection
The estimated disinfection efficiencies were 42.17 ± 8.18%, 63.25 ± 8.17% and 70.70 ± 4.80% for E. coli; 40.40 ± 17.90%, 47.31 ± 8.20%, and 58.31 ± 13.87% for S. typhimurium; 24.16 ± 3.81%, 32.92 ± 9.59, and 42.79 ± 10.20% for S. epidermidis with 3‐LEDs, 5‐LEDs, and 8‐LEDs, respectively. These results appear to be in almost the same order of efficiency as with the mercury vapor UV lamps reported in our previous study 22 without having any of the problems associated with this UV source (eg, toxicity, fragility, high energy consumption, etc).
In Figure 10, disinfection efficacy increased with the number of LEDs used as clearly shown. However, a distinct point is observed. It seems that the intensity did not affect S. epidermidis much, nonetheless, E. coli was affected significantly. In the case of S. epidermidis, the cell structure is thick, resilient, and counteracts, thereby making its inactivation more difficult compared to the other two bacteria tested. This is, nevertheless, not unexpected and consistent with the observed behavior in the literature related to other disinfection approaches such as mercury vapor UV lamps 22 and ionizers. 25 Similarly, to further support this claim, it is worth noting that all published values of UVGI rate constants for all of the three test organisms have also indicated that the susceptibility of E. coli and S. typhimurium to UV is considerably higher than that of S. epidermidis for the air medium. 27 To further indirectly confirm the accuracy of the present results, the order of UVC susceptibility of these bacteria, as observed in our current study, is the same for both surface and air disinfections: Higher for E. coli, followed by S. typhimurium, and then S. epidermidis.
It is also worth noting that a higher number of LEDs configuration, specifically 10‐LEDs, was tried but no significant increase in the irradiance and disinfection efficacy was observed (data not shown). Therefore, the 8‐LEDs configuration is considered optimum for the current airborne disinfection application.
5.6. Performance of uniform versus concentrated UV irradiance distribution
Here, we further tested the influence of UVC‐LEDs configurations on the efficacy of the localized disinfection system. We compared the germicidal results of two cases of UVC‐LED configuration, both of which had 5‐LEDs but one was evenly distributed and the other was concentrated at two opposing sides, against E. coli—being the most susceptible to UVC‐LED among the three tested bacteria.
With the evenly distributed and concentrated (two‐sided) irradiations, the mean disinfection efficiencies for airborne disinfection were 63.25 ± 8.17% and 53.74 ± 4.47%, respectively. Similarly, the estimated mean efficiency under surface disinfection was 72.03 ± 9.02% for the evenly distributed irradiation and 36.83 ± 7.47% for the concentrated (two‐sided) irradiation (Figure 11). The results implied that the performance of the localized disinfection system with the evenly distributed irradiation was about 95.57% (surface) and 17.70% (airborne) higher than the concentrated (two‐sided) irradiation.
FIGURE 11.
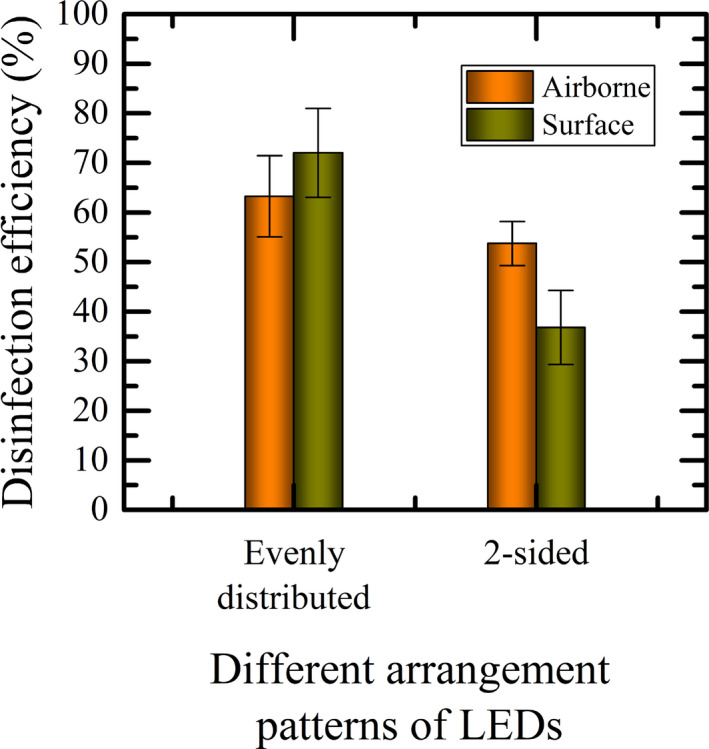
Comparison between the disinfection efficacy of evenly distributed and two‐sided UVC‐LEDs arrangements
An independent sample t test (two‐tail, α = .05) was conducted and the result showed that the difference between the efficacy of the evenly distributed and the concentrated (two‐sided) UVC‐LED arrangements is statistically significant (P = .006) for surface disinfection. However, there is no statistically significant difference between the performance of these two cases for airborne disinfection (P = .15).
It is interesting to note that even though the numbers of the LEDs used were the same (the same UV output), the measured irradiance was different. For evenly distributed pattern, the irradiance was 1.15 µWcm−2 while for the 2‐sided pattern, it was 1.57 µWcm−2 as noted earlier in Section 5.3. Although the irradiance was higher for the 2‐sided configuration, the disinfection efficacy was lower. The rationale for this is that the aerosolization process occurs randomly in the bowl surface, hence the uniform irradiance might give better results unless the design of the bowl is special to spread the water nozzles unevenly around the rim of the bowl.
6. DISCUSSIONS
Toilets are potential sources for the transmission of fecal‐borne diseases, being unique locations in buildings for defecation and urination. The size of flushing‐generated pathogens influences their role in airborne and contact transmissions. The total and size‐resolved airborne droplet emissions per flush under different flushing pressure conditions had been reported in our previously published study. 17 In that study, we showed statistically significant differences in the generation of different sizes of airborne droplets, with the average airborne concentrations decreasing as the particle size increased. Inferring from the results, the ratio of droplet concentration of 0.3 to 0.6 µm to >2‐10 µm can be as high as 124 times. These findings further provide justification for concerns that flushing toilets could play a role in both airborne and surface transmission of infectious diseases through droplet bioaerosols. Once aerosols become airborne, they can settle near the toilet bowl, and the small aerosols can stay airborne for up to hours and may lead to surface contamination, which is believed to be a major route for transmission of infective diseases. 2 , 4 , 14 The risk of contracting diseases is even much higher where toilets are shared with many other users. Unfortunately, almost all of the well‐known cleaning and disinfection approaches are done after a period or cycle of multiple uses, as they are sometimes considered highly labor‐intensive. The fact that pathogens would not be removed after each toilet use creates a critical microbiological problem within the toilet environment. Besides the detrimental effects of inhaling contaminated toilet air on the wellbeing of users, contacts of the intact human body with toilet contaminated surfaces may lead to possible fomites infection. 28 Recent studies have identified SARS‐CoV‐2 in toilet areas used by infected patients 20 , 21 which suggests that transmitting COVID‐19 by contacting fomites in toilets is possible. Another major concern is the formation of biofilms under favorable conditions following the adhesion of pathogens to toilet surfaces. Besides, the water content of air is a major factor in the transmission of airborne microbes in toilets. Droplets emitted during toilet flushing could rapidly lose their water content and shrink to smaller sizes by evaporation in dry toilet environments. The occurrence of evaporation produces more droplet that are capable of remaining airborne and increases the overall time they can be suspended within the toilet microenvironment. Moreover, fecal bacteria get preserved in suspended droplet capsules, where they remain infectious and have greater potential to infect toilet users. Although this dynamic and complex phenomenon is also possible in toilet environments with saturated humidity, albeit slower than in dry environments, it is important to also note that some of the flushing‐generated droplets would maintain their water content, be denser, and less likely to stay airborne, increasing the risk of surface transmission.
Since air‐ and surface‐mediated transmissions are common for toilet‐acquired infections, good infection control methods must attempt to keep toilet microenvironments free from pathogens. In this regards, control of toilet infections must, therefore, involve the disinfection of both air and surfaces in the toilet microenvironment. Also, it is crucial to acknowledge here that fecal pathogens can persist and survive from hours to weeks on inanimate surfaces including the toilet seat. 29 , 30
To reduce infection risk, engineering control strategies should be implemented. A conventional approach for reducing infection risk for toilets is through dilution of pathogen concentration. It can be realized by increasing the ventilation rate, however, this approach is not sustainable. Another potential issue of increasing the ventilation rate is that enhancing mixing inside toilets can increase the spatial spread of the airborne pathogens, which may lead to long range transport of pathogens induced by toilet flushing. Besides dilution, another active control strategy is inactivation.
It is worth noting that the inactivation of airborne indoor pathogens has generally been limited to the use of UR‐UVGI systems. Although the UR‐UVGI has been proven to be an excellent intervention technology for airborne infection, the use of such an approach is limited to the upper room, due to exposure concerns when used in real‐time and does not perform source control function for reducing whole room dispersal of pathogens generated from toilet flushing. 22
In this study, we addressed these issues through the use of a novel localized UVC‐LED disinfection system. We designed the study's toilet facility to model what is typically done in actual homes. It is important to mention here that the increased potency of LEDs for surface and air disinfections presented herein is congruous with earlier studies with fecal related pathogens. The results demonstrate the effectiveness of our novel disinfection technology to inactivate gram‐negative (E. coli and S. typhimurium) and gram‐positive (S. epidermidis) bacteria despite the shorter exposure time of these toilet‐emitted pathogens to LED irradiation.
E. coli, S. typhimurium, and S. epidermidis have been identified as some of the most significant fecal pathogens. They belong to the family of gastroenteric pathogens. It can be implied from our results that controlling and curtailing infectious pathogens at the source of generation can eliminate or reduce at the least their presence both in air and on indoor surfaces. However, the degree of susceptibility of fecal bacteria in the face of UV irradiation differs. Even with the general toughness of S. epidermidis to disinfectants, 22 , 25 the results in this study have proven that LED source control is an effective way for inactivating them, overcoming any resistance from their structural mechanism. Unlike the gram‐negative bacteria, all gram‐positive bacteria notably share a characteristically unique cell wall, which consists mainly of a thick peptidoglycan layer and the cytoplasmic lipid membrane. The thick nature of their cell wall plays a significant role in their survivability. Also, it is accountable for the resistance of gram‐positive bacteria to many inactivation methods and commercially available disinfectants.
Apart from those explored in this study, there are other pathogenic toilet bacteria species. Consequently, to translate the localized UVC‐LED disinfection method into practical use, it is necessary to consider its disinfection efficacy not only for those evaluated in this study but also for other bacteria that are transmitted through contaminated surfaces and air and become a burden in toilet infections such as Shigella sp., Listeria monocytogenes, Clostridium difficile, Candida, and Cryptosporidium, et cetera.
This study is one of the first to demonstrate an intervention technology for inactivating enteropathogenic bacteria. To conclude, this study proposed an innovative design of utilizing very low levels of UVC‐LED irradiance as an alternative intervention strategy to achieve a significant reduction in the concentration levels of deposited and airborne pathogens (ie, air and surface disinfections) in high‐risk microenvironments such as toilet facilities. It was shown that the potential of LEDs to remove bacteria and prevent dispersal into the whole room during toilet flushing depends on the quantity of UVC‐LED irradiance in the toilet bowl. It was observed that for higher UV irradiance, the disinfection efficiency increased, although in a non‐linearly manner as expected. While disinfection performance increases with the numbers of LEDs, the plateau stage seems to occur consistently. Thus, it is necessary to test the irradiance output before fixing the device input current to avoid wasting energy.
One of the interesting features of LEDs is that they are environmentally friendly. This property confers a significant advantage of this source control approach described in our study over other traditional approaches using chemical disinfectants (eg, chlorine‐based bleach), which have been linked to significant environmental implications. Moreover, other unique characteristics, which give this hazard‐free novel method advantage over competitive disinfection technologies include a longer lifetime, very low‐cost requirement (ie, they are very cheap), and very low energy consumption.
Finally, we believe that the point‐source nature of the localized UVC‐LED emission paves the way toward the instantaneous removal of deadly fecal pathogens during toilet flushing before spreading.
7. CONCLUSIONS
A novel UVC‐LED disinfection system was designed, and its disinfection efficacy was tested. We presented the concept of a source control method using UVC‐LEDs to inactivate toilet‐flushing‐generated bacteria thereby hampering their spread onto surfaces and in the air within the toilet space. The design was very compact and has the potential to make it portable.
The LED‐based technology for the inactivation of fecal bacteria demonstrated promising results. The LEDs were effective in inactivating three differentmicrobes specifically important to the toilet microenvironment: E. coli, S. typhimurium, and S. epidermidis. The delivery of the UV irradiance produced by LEDs was enhanced by increasing the number of LEDs. More importantly, it was clearly shown that exposure to increased UV irradiance resulted in higher disinfection potential. Also, higher efficacy was observed for the uniform irradiance compared to the concentrated irradiance when tested against E. coli, reaching up to 95.57% for surface disinfection and 17.70% for airborne disinfection. Finally, the very encouraging microbial disinfection results as well as the absence of any potential hazardous and/or toxic element indoors, render this method a promising intervention technology, which can be used in the battle against toilet‐acquired diseases.
CONFLICT OF INTEREST
The authors declare that they have no known or expected conflict of interest related to the research, authorship, or publication of this article.
AUTHOR CONTRIBUTIONS
Alvin Lai: Conceptualization (lead); Formal analysis (equal); Funding acquisition (lead); Investigation (equal); Methodology (lead); Project administration (lead); Supervision (lead); Validation (lead); Visualization (equal); Writing‐original draft (equal); Writing‐review & editing (lead). Sunday Nunayon: Data curation (lead); Formal analysis (equal); Investigation (equal); Methodology (supporting); Validation (supporting); Visualization (equal); Writing‐original draft (equal); Writing‐review & editing (supporting).
ACKNOWLEDGEMENT
This research was fully supported by General Research Funds from the Research Grants Council of the Hong Kong Special Administrative Region of China [CityU 11273116 and CityU 11204217].
Lai ACK, Nunayon SS. A new UVC‐LED system for disinfection of pathogens generated by toilet flushing. Indoor Air.2021;31:324–334. 10.1111/ina.12752
DATA AVAILABILITY STATEMENT
The datasets supporting the findings of this study are available from the corresponding author on reasonable requests.
REFERENCES
- 1. WHO . Fact sheet on sanitation, 2019. https://www.who.int/news-room/fact-sheets/detail/sanitation. Accessed February 24, 2020
- 2. Barker J, Jones MV. The potential spread of infection caused by aerosol contamination of surfaces after flushing a domestic toilet. J Appl Microbiol. 2005;99(2):339‐347. [DOI] [PubMed] [Google Scholar]
- 3. Verani M, Bigazzi R, Carducci A. Viral contamination of aerosol and surfaces through toilet use in health care and other settings. Am J Infect Control. 2014;42:758‐762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Flores GE, Bates ST, Knights D, et al. Microbial biogeography of public restroom surfaces. PLoS One. 2011;6:e28132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Best EL, Sandoe JA, Wilcox MH. Potential for aerosolization of Clostridium difficile after flushing toilets: the role of toilet lids in reducing environmental contamination risk. J Hosp Infect. 2012;80(1):1‐5. [DOI] [PubMed] [Google Scholar]
- 6. Willshaw GA, Thirlwell J, Jones AP, Parry S, Salmon RL, Hickey M. Vero cytotoxin‐producing Escherichia coli O157 in beefburgers linked to an outbreak of diarrhoea, haemorrhagic colitis and haemolytic uraemic syndrome in Britain. Lett Appl Microbiol. 1994;19:304‐307. [DOI] [PubMed] [Google Scholar]
- 7. Gerhardts A, Hammer TR, Balluff C, Mucha H, Hoefer D. A model of the transmission of micro‐organisms in a public setting and its correlation to pathogen infection risks. J Appl Microbiol. 2012;112(3):614‐621. [DOI] [PubMed] [Google Scholar]
- 8. Johnson D, Lynch R, Marshall C, Mead K, Hirst D. Aerosol generation by modern flush toilets. Aerosol Sci Technol. 2013;47:1047‐1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lin K, Marr LC. Aerosolization of EBOLA virus surrogates in wastewater systems. Environ Sci Technol. 2017;51(5):2669‐2675. [DOI] [PubMed] [Google Scholar]
- 10. Chan MCW, Sung JJY, Lam RKY, et al. Fecal viral load and norovirus‐associated gastroenteritis. Emerg Infect Dis. 2006;12:1278‐1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Atmar RL, Opekun AR, Gilger MA, et al. Norwalk virus shedding after experimental human infection. Emerg Infect Dis. 2008;14(10):1553‐1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Knowlton SD, Boles CL, Perencevich EN, Diekema DJ, Nonnenmann MW. Bioaerosol concentrations generated from toilet flushing in a hospital‐based patient care setting. Antimicrob Resist Infect Control. 2018;7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gerba CP, Wallis C, Melnick JL. Microbiological hazards of household toilets: droplet production and the fate of residual organisms. Appl Environ Microbiol. 1975;30(2):229‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Otter JA, Yezli S, French GL. The role played by contaminated surfaces in the transmission of nosocomial pathogens. Infect Control Hosp Epidemiol. 2011;32:687‐699. [DOI] [PubMed] [Google Scholar]
- 15. Yu ITS, Li Y, Wong TW, et al. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N Engl J Med. 2004;350(17):1731‐1739. [DOI] [PubMed] [Google Scholar]
- 16. Nicas M, Jones RM. Relative contributions of four exposure pathways to influenza infection risk. Risk Anal. 2009;29:1292‐1303. [DOI] [PubMed] [Google Scholar]
- 17. Lai ACK, Tan TF, Li WS, Ip DKM. Emission strength of airborne pathogens during toilet flushing. Indoor Air. 2018;28(1):73‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barker J, Bloomfield SF. Survival of salmonella in bathrooms and toilets in domestic homes following salmonellosis. J Appl Microbiol. 2000;89(1):137‐144. [DOI] [PubMed] [Google Scholar]
- 19. Darlow HM, Bale WR. Infective hazards of water‐closets. Lancet. 1959;273:1196‐1200. [DOI] [PubMed] [Google Scholar]
- 20. Ong SWX, Tan YK, Chia PY, et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) from a symptomatic patient. J Am Med Assoc. 2020;323(16):1610‐1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu Y, Ning Z, Chen Y, et al. Aerodynamic analysis of SARS‐CoV‐2 in two Wuhan hospitals. Nature. 2020;582:557‐560. [DOI] [PubMed] [Google Scholar]
- 22. Lai ACK, Nunayon SS, Tan TF, Li WS. A pilot study on the disinfection efficacy of localized UV on the flushing‐generated spread of pathogens. J Hazard Mater. 2018;358:389‐396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Crawford MH, Banas MA, Ross MP, et al. Final LRDR report: ultraviolet water purification systems for rural environments and mobile applications. Sandia Rep. 2005;7:20‐33. [Google Scholar]
- 24. Nunayon SS, Zhang HH, Lai ACK. A novel upper‐room UVC‐LED irradiation system for disinfection of indoor bioaerosols under different operating and airflow conditions. J Hazard Mater. 2020;396:122715. [DOI] [PubMed] [Google Scholar]
- 25. Nunayon SS, Zhang HH, Jin X, Lai ACK. Experimental evaluation of positive and negative air ions disinfection efficacy under different ventilation duct conditions. Build Environ. 2019;158:295‐301. [Google Scholar]
- 26. Macher JM. Positive‐hole correction of multiple‐jet impactors for collection viable microorganisms. Am Indust Hyg Assoc J. 1989;50:561‐568. [DOI] [PubMed] [Google Scholar]
- 27. Kowalski W. Ultraviolet germicidal irradiation handbook: UVGI for air and surface disinfection. Heidelberg, Dordrecht, London, New York: Springer; 2009. [Google Scholar]
- 28. Xiao S, Li Y, Wong TW, Hui DSC. Role of fomites in SARS transmission during the largest hospital outbreak in Hong Kong. PLoS One. 2017;12(7):e0181558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kramer A, Schwebke I, Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis. 2006;6:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Otter JA, Donskey C, Yezli S, Douthwaite S, Goldenberg SD, Weber DJ. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J Hosp Infect. 2016;92(3):235‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the findings of this study are available from the corresponding author on reasonable requests.


