Dear Editor,
Coronavirus disease 2019 (COVID‐19), caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), is continuously expanding in morbidity and mortality, with 22 million confirmed cases and 0.77 million deaths worldwide as of August 20, 2020 (WHO Coronavirus Disease (COVID‐19) Dashboard). Major clinical manifestations of SARS‐CoV‐2 involve respiratory failure caused by virus‐induced pulmonary injury and/or a hyperactive host immune response involving an inflammatory response and secondary “cytokine storm,” leading to acute respiratory distress syndrome (ARDS) in patients (von der Thüsen & van der Eerden, 2020; Xu et al., 2020).
In addition to antiviral therapy, immunomodulatory drugs are also being used as treatment strategies to suppress hyperinflammation and to reduce death among COVID‐19 patients (Mehta et al., 2020). Corticosteroids have a long history of use in severe cases of coronavirus disease, including SARS, MERS, and COVID‐19, as immunomodulatory drugs (Arabi et al., 2018; Huang et al., 2020; So et al., 2003). Recently, dexamethasone (a synthetic adrenal corticosteroid), whose demand has surged suddenly after publication of the preprint paper of the RECOVERY trial (https://www.medrxiv.org/content/10.1101/2020.06.22.20137273v1.full.pdf), was found to be the first drug, which successfully reduced deaths in approximately one‐third of critically ill COVID‐19 patients, who were on invasive mechanical ventilation support and by one‐fifth in noninvasive ventilated patients. Interestingly, no positive outcome was reported from less severe COVID‐19 patients, who were without any oxygen support. Likewise, in another randomized controlled trial (RCT), dexamethasone reduced mortality in ARDS patients (Villar et al., 2020). The findings of Selvaraj, Dapaah‐Afriyie, Finn, and Flanigan (2020) also support the use of dexamethasone in COVID‐19 treatment, where the short‐term course of dexamethasone prevented disease severity and in turn reduced the length of ICU stay of patients with hypoxic respiratory failure.
Dexamethasone, by suppressing the immune response, helps to overcome serious manifestations caused by the hyperactive immune response in COVID‐19 patients. However, in the absence of adequate RCTs, there has always been controversy regarding the use of corticosteroids. The limiting element is the lack of standardization of various aspects, such as drug dose, route of administration, bioavailability, side effects and exact therapeutic impacts in patients. It has been observed that corticosteroid treatment prolonged the viral presence in SARS‐CoV‐1 patients (Lee et al., 2004), and their administration in low doses had no impact on virus clearance in COVID‐19 patients (Fang et al., 2020). Because of several contradictory outcomes, it seems difficult to support the routine use of corticosteroids in COVID‐19 disease management. Even existing guidelines of the World Health Organization (WHO) are not in favor of the routine use of corticoseroides in SARS‐CoV‐2 patients. In such a scenario, we searched for a suitable alternate option that can either augment or substitute dexamethasone action in dexamethasone‐nonresponsive severely ill Covid‐19 patients and for mild and moderate Covid‐19 cases. At this point, it is imperative to discuss an interesting fact that Bhutto et al. (2018) identified molecular as well as functional similarity between dexamethasone and quercetin, [permeability‐glycoprotein (P‐gp) inducers]. P‐gp, a membrane transporter, helps in the retention of various drugs, but pro‐inflammatory cytokines released during infections have been observed to inhibit its expression and activity (Iqbal et al., 2012). As P‐gp inducers, both dexamethasone and quercetin, by interfering with p‐gp expression, can inhibit the “cytokine storm”‐like consequences in COVID‐19 patients.
Quercetin, a ubiquitous natural flavonoid, has been approved by the U.S. Food and Drug Administration (FDA) because of its beneficial medicinal/therapeutic properties, such as antiinflammatory, antiviral, antiproliferative, antioxidative, antibacterial and anti‐cancerous properties [comprehensively reviewed in Cushnie & Lamb, 2005 and Batiha et al., 2020]. However, the misleading indications and health claims made by online available quercetin containing dietary supplements, along with the risk of potential drug interactions is a matter of severe concern for health care experts and customers (Vida, Fittler, Somogyi‐Végh, & Poór, 2019).
Due to pleiotropic properties and capability to synergize with conventional drugs, it is assumed that flavonoids can reduce both transmembrane peptidase serine 2 (TMPRSS2) and Furin, which cleave the SARS‐CoV‐2 Spike protein enabling SARS‐CoV‐2 infection (Russo, Moccia, Spagnuolo, Tedesco, & Russo, 2020). Yi et al. (2004) specifically revealed that quercetin and another related flavonoid, luteolin, can inhibit the entry of SARS virus inside the host cell. Quercetin seems to impede the entry of SARS virus by targeting angiotensin‐converting enzyme 2 (ACE2) expression (ACE2 gene expression is required for SARS‐CoV‐2 entry into human cells) (Figure 1) (Glinsky, 2020). Quercetin modulates the cellular unfolded protein response, which is utilized by viruses to complete its life cycle (Nabirotchkin, Peluffo, Bouaziz, & Cohen, 2020). Study of Abian et al. (2020) is a direct evidence of quercetin's efficacy to target SARS‐CoV‐2 3C‐like protease (3CLpro) on solid experimental bases. In vitro studies have revealed the immunomodulatory effects of quercetin, which prevents the production of tumor necrosis factor‐α in macrophages (Manjeet & Ghosh, 1999) and interleukin (IL)‐8 production in lung cells (Geraets et al., 2007). It is pertinent here to note that production of interferon‐gamma (Th‐1‐derived cytokine) and the inhibition of IL‐4 (Th‐2‐derived cytokine) may be responsible for the beneficial immunostimulatory effects of quercetin (Figure 1) (Nair et al., 2002). Moreover, it is tempting to speculate that due to quercetin's zinc ionophore property, it along with zinc can be a potential therapeutic/prophylactic option for Covid 19 subjects. Notably, zinc has been shown to inhibit the RNA‐dependent RNA polymerase activity of SARS virus under in vitro conditions in a dose‐dependent manner [Figure 2; reviewed in Read, Obeid, Ahlenstiel, & Ahlenstiel, 2019]. The significance of quercetin in COVID‐19 is amply evident by the fact that currently (as of August 20, 2020), two open label interventional clinical trial (NCT04377789 and NCT04468139; source: https://clinicaltrials.gov/; Table 1) are under way for evaluation of quercetin efficacy as prophylaxis and treatment option against Covid‐19.
FIGURE 1.
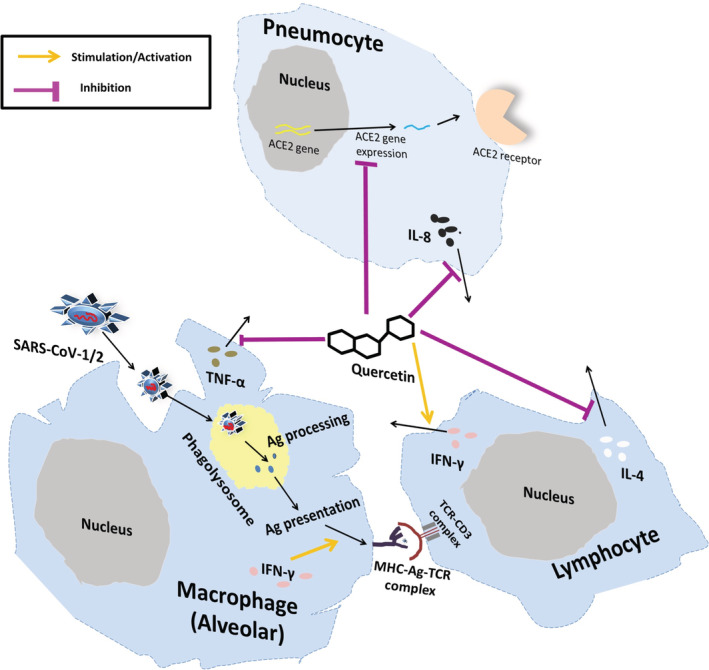
Schematic representation of possible antiinflammatory effects of quercetin against SARS‐CoV‐1/2. Quercetin has been demonstrated to inhibit SARS‐CoV‐1/2 entry inside cell by interfering with the expression of ACE2. Further, quercetin initiates IFN‐γ production by T lymphocytes, which enhances anti‐viral activity and antigen presentation by phagocytocytosis, and expression of MHC‐I and MHC‐II molecules. Decrement of inflammation and blood clotting in lungs is achieved by inhibition of IL‐8 production by quercetin. Importantly, quercetin also inhibits the production of IL‐4 and TNF‐α, which helps in ameliorating inflammation. ACE2, Angiotensin‐converting enzyme 2; IL, Interleukin; IFN‐γ, Interferon‐gamma; TNF‐α, Tumor necrosis factor‐alpha; SARS‐CoV, Severe acute respiratory syndrome coronavirus [Colour figure can be viewed at wileyonlinelibrary.com]
FIGURE 2.
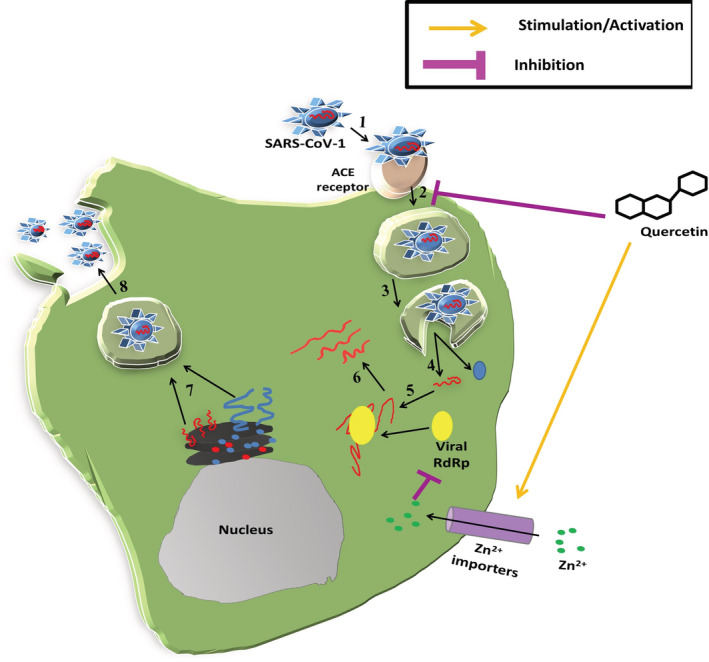
Schematic representation of different stages of SARS‐CoV‐1 replication cycle inhibited by quercetin and zinc. Viral attachment (1), viral entry (2), preparation for viral uncoating (3), viral uncoating, and release of viral genome and viral protein (4), viral transcription (5), viral protein translation (6), viral replication (5), virus assembly and maturation (7) and, finally mature viral particles release (8). Quercetin has been demonstrated to inhibit SARS‐CoV‐1 entry (2) step, whereas, zinc inhibits SARS‐CoV‐1 genome transcription by inhibiting viral RdRp in a dose dependent manner(Step 5). ACE2, Angiotensin‐converting enzyme 2; RdRp, RNA‐dependent RNA polymerase; SARS‐CoV, Severe acute respiratory syndrome coronavirus; Zn, zinc [Colour figure can be viewed at wileyonlinelibrary.com]
TABLE 1.
Interventional clinical trials with quercetin ± other drugs/dietary supplements for Covid‐19
| S.No. | Clinical trial study title | ClinicalTrial.Org identifier | Intervention (Drug/s) | Quercetin dose and duration | Types of intervention/Allocation | No. of subjects/age/sex |
|---|---|---|---|---|---|---|
| 1 | Effect of Quercetin on Prophylaxis and Treatment of COVID‐19 | NCT04377789 |
Dietary Supplement: Quercetin Prophylaxis Dietary Supplement: Quercetin Treatment |
#Intervention group 1; Dietary Supplement: Quercetin Prophylaxis a daily dose of quercetin (500 mg) will be taken by non‐COVID‐19 #Intervention group 2; Dietary Supplement: Quercetin Treatment a daily dose of quercetin (1,000 mg) will be taken by proven COVID‐19 cases |
Prevention/Nonrandomized‐Open label | 50/18 Years and older/All sex |
| 2 | The Study of Quadruple Therapy Zinc, Quercetin, Bromelain and Vitamin C on the Clinical Outcomes of Patients Infected With COVID‐19 | NCT04468139 |
Drug: Quercetin Dietary Supplement: bromelain Drug: Zinc Drug: Vitamin C |
Daily dose of quercetin (500 mg) will be taken orally by proven COVID‐19 cases | Treatment /Open Label | 60/18 Years and older/All sex |
Because of structural resemblance, it is possible that the molecular mechanisms of therapeutic actions of both quercetin and dexamethasone may have some resemblance. Whether quercetin can be used as an alternate therapy in mild and moderate patients and in dexamethasone nonresponsive severely ill COVID‐19 patients will be an interesting approach, which requires scientific validation by prospective RCTs. Besides, quercetin's use as adjunct therapy along with dexamethasone in severely ill COVID‐19 subjects, it will be also interesting to evaluate whether quercetin can augment the therapeutic effect of dexamethasone in dexamethasone nonresponsive COVID‐19 patients.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
ACKNOWLEDGEMENTS
The authors duly acknowledge the NCBI, NIH and WHO for providing free online clinical trials/research articles/information related to Covid‐19.
REFERENCES
- Abian, O. , Ortega‐Alarcon, D. , Jimenez‐Alesanco, A. , Ceballos‐Laita, L. , Vega, S. , Reyburn, H. T. , … Velazquez‐Campoy, A. (2020). Structural stability of SARS‐CoV‐2 3CLpro and identification of quercetin as an inhibitor by experimental screening. International Journal of Biological Macromolecules, 164, 1693–1703. 10.1016/j.ijbiomac.2020.07.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabi, Y. M. , Mandourah, Y. , Al‐Hameed, F. , Sindi, A. A. , Almekhlafi, G. A. , Hussein, M. A. , … Saudi Critical Care Trial Group . (2018). Corticosteroid therapy for critically ill patients with middle east respiratory syndrome. American Journal of Respiratory and Critical Care Medicine, 197(6), 757–767. 10.1164/rccm.201706-1172OC [DOI] [PubMed] [Google Scholar]
- Batiha, G. E. , Beshbishy, A. M. , Ikram, M. , Mulla, Z. S. , El‐Hack, M. , Taha, A. E. , … Elewa, Y. (2020). The pharmacological activity, biochemical properties, and pharmacokinetics of the major natural polyphenolic flavonoid: Quercetin. Foods (Basel, Switzerland), 9(3), 374. 10.3390/foods9030374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutto, Z. A. , He, F. , Zloh, M. , Yang, J. , Huang, J. , Guo, T. , & Wang, L. (2018). Use of quercetin in animal feed: Effects on the P‐gp expression and pharmacokinetics of orally administrated enrofloxacin in chicken. Scientific Reports, 8(1), 4400. 10.1038/s41598-018-22354-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushnie, T. P. , & Lamb, A. J. (2005). Antimicrobial activity of flavonoids. International Journal of Antimicrobial Agents, 26(5), 343–356. 10.1016/j.ijantimicag.2005.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, X. , Mei, Q. , Yang, T. , Li, L. , Wang, Y. , Tong, F. , … Pan, A. (2020). Low‐dose corticosteroid therapy does not delay viral clearance in patients with COVID‐19. The Journal of Infection, 81(1), 147–178. 10.1016/j.jinf.2020.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraets, L. , Moonen, H. J. , Brauers, K. , Wouters, E. F. , Bast, A. , & Hageman, G. J. (2007). Dietary flavones and flavonoles are inhibitors of poly(ADP‐ribose)polymerase‐1 in pulmonary epithelial cells. The Journal of Nutrition, 137(10), 2190–2195. 10.1093/jn/137.10.2190 [DOI] [PubMed] [Google Scholar]
- Glinsky, G. V. (2020). Tripartite combination of candidate pandemic mitigation agents: Vitamin D, quercetin, and estradiol manifest properties of medicinal agents for targeted mitigation of the COVID‐19 pandemic defined by genomics‐guided tracing of SARS‐CoV‐2 targets in human cells. Biomedicine, 8(5), 129. 10.3390/biomedicines8050129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, C. , Wang, Y. , Li, X. , Ren, L. , Zhao, J. , Hu, Y. , … Cao, B. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England), 395(10223), 497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal, M. , Ho, H. L. , Petropoulos, S. , Moisiadis, V. G. , Gibb, W. , & Matthews, S. G. (2012). Pro‐inflammatory cytokine regulation of P‐glycoprotein in the developing blood‐brain barrier. PLoS One, 7(8), e43022. 10.1371/journal.pone.0043022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, N. , Allen Chan, K. C. , Hui, D. S. , Ng, E. K. , Wu, A. , Chiu, R. W. , … Lo, Y. M. (2004). Effects of early corticosteroid treatment on plasma SARS‐associated Coronavirus RNA concentrations in adult patients. Journal of Clinical Virology: The Official Publication of the Pan American Society for Clinical Virology, 31(4), 304–309. 10.1016/j.jcv.2004.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjeet K, R., & Ghosh, B. (1999). Quercetin inhibits LPS‐induced nitric oxide and tumor necrosis factor‐alpha production in murine macrophages. International journal of immunopharmacology, 21(7), 435–443. 10.1016/s0192-0561(99)00024-7 [DOI] [PubMed] [Google Scholar]
- Mehta, P. , McAuley, D. F. , Brown, M. , Sanchez, E. , Tattersall, R. S. , Manson, J. J. , & HLH Across Speciality Collaboration, UK . (2020). COVID‐19: Consider cytokine storm syndromes and immunosuppression. Lancet (London, England), 395(10229), 1033–1034. 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabirotchkin, S. , Peluffo, A. , Bouaziz, J. , & Cohen, D. (2020). Focusing on the unfolded protein response and autophagy‐related pathways to reposition commonly approved drugs against COVID‐19. Preprints 2020030302. 10.20944/preprints202003.0302.v1 (preprints, not peer‐reviewed) [DOI] [Google Scholar]
- Nair, M. P. , Kandaswami, C. , Mahajan, S. , Chadha, K. C. , Chawda, R. , Nair, H. , … Schwartz, S. A. (2002). The flavonoid, quercetin, differentially regulates Th‐1 (IFNgamma) and Th‐2 (IL4) cytokine gene expression by normal peripheral blood mononuclear cells. Biochimica et Biophysica Acta, 1593(1), 29–36. 10.1016/s0167-4889(02)00328-2 [DOI] [PubMed] [Google Scholar]
- Read, S. A. , Obeid, S. , Ahlenstiel, C. , & Ahlenstiel, G. (2019). The role of zinc in antiviral immunity. Advances in nutrition (Bethesda, Md.), 10(4), 696–710. 10.1093/advances/nmz013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo, M. , Moccia, S. , Spagnuolo, C. , Tedesco, I. , & Russo, G. L. (2020). Roles of flavonoids against coronavirus infection. Chemico‐Biological Interactions, 328, 109211. 10.1016/j.cbi.2020.109211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj, V. , Dapaah‐Afriyie, K. , Finn, A. , & Flanigan, T. P. (2020). Short‐term dexamethasone in sars‐CoV‐2 patients. Rhode Island Medical Journal (2013), 103(6), 39–43. [PubMed] [Google Scholar]
- So, L. K. , Lau, A. C. , Yam, L. Y. , Cheung, T. M. , Poon, E. , Yung, R. W. , & Yuen, K. Y. (2003). Development of a standard treatment protocol for severe acute respiratory syndrome. Lancet (London, England), 361(9369), 1615–1617. 10.1016/s0140-6736(03)13265-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida, R. G. , Fittler, A. , Somogyi‐Végh, A. , & Poór, M. (2019). Dietary quercetin supplements: Assessment of online product informations and quantitation of quercetin in the products by high‐performance liquid chromatography. Phytotherapy Research, 33, 1912–1920. 10.1002/ptr.6382 [DOI] [PubMed] [Google Scholar]
- Villar, J. , Ferrando, C. , Martínez, D. , Ambrós, A. , Muñoz, T. , Soler, J. A. , … dexamethasone in ARDS network . (2020). Dexamethasone treatment for the acute respiratory distress syndrome: A multicentre, randomised controlled trial. The Lancet. Respiratory medicine, 8(3), 267–276. 10.1016/S2213-2600(19)30417-5 [DOI] [PubMed] [Google Scholar]
- von der Thüsen, J. , & van der Eerden, M. (2020). Histopathology and genetic susceptibility in COVID‐19 pneumonia. European Journal of Clinical Investigation, e13259. 10.1111/eci.13259. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Z. , Shi, L. , Wang, Y. , Zhang, J. , Huang, L. , Zhang, C. , … Wang, F. S. (2020). Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. The Lancet. Respiratory Medicine, 8(4), 420–422. 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi, L. , Li, Z. , Yuan, K. , Qu, X. , Chen, J. , Wang, G. , … Xu, X. (2004). Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells. Journal of Virology, 78(20), 11334–11339. 10.1128/JVI.78.20.11334-11339.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]


