Highlights
-
•
Epidemiology of COVID-19 and life cycle of SARS-CoV-2 in human cells was explored.
-
•
SARS-CoV-2-induced cell-mediated immunity plays a critical role in COVID-19.
-
•
SARS-CoV-2 infection induces immune cell-mediated cytokine storms that promote pulmonary fibro-inflammation and organ failure.
-
•
This study highlights the immunotherapeutic effects of IL-15 on innate immunity to promote the clearance of SARS-CoV-2 viral loads.
Keywords: COVID-19, SARS-CoV-2, Pulmonary inflammation, Cytokine storm, Immune cells, Interleukin-15
Abstract
Coronavirus disease 2019 (COVID-19) is a pulmonary inflammatory disease induced by a newly recognized coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). SARS-CoV-2 infection was detected for the first time in the city of Wuhan in China and spread all over the world at the beginning of 2020. Several millions of people have been infected with SARS-CoV-2, and almost 382,867 human deaths worldwide have been reported so far. Notably, there has been no specific, clinically approved vaccine or anti-viral treatment strategy for COVID-19. Herein, we review COVID-19, the viral replication, and its effect on promoting pulmonary fibro-inflammation via immune cell-mediated cytokine storms in humans. Several clinical trials are currently ongoing for anti-viral drugs, vaccines, and neutralizing antibodies against COVID-19. Viral clearance is the result of effective innate and adaptive immune responses. The pivotal role of interleukin (IL)-15 in viral clearance involves maintaining the balance of induced inflammatory cytokines and the homeostatic responses of natural killer and CD8+ T cells. This review presents supporting evidence of the impact of IL-15 immunotherapy on COVID-19.
1. Introduction
Previously known four different coronaviruses (CoV), namely HKU1, NL63, 229E, and OC43, can induce mild respiratory diseases. The first outbreak of coronavirus was reported to have originated in bats and crossed over to humans via the intermediary host palm civet cats in the province of Guangdong in China in 2002 [1]. In 2012, coronavirus was reported again in the Middle East that originated in bats in Saudi Arabia with dromedary camels as the intermediate host, and it was named Middle East respiratory syndrome coronavirus (MERS-CoV) [2]. This virus also caused acute respiratory disease. The third outbreak caused by the worst type of coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was reported to originate from the city of Wuhan in China in Jan 2020 and spread worldwide to cause the coronavirus disease (COVID-19). SARS-CoV-2 is a fast-spreading virus between humans by close contact, which has infected approximately 6.4 million people and caused almost 382,867 deaths as of June 04, 2020. The current means of stopping viral transmission is only by restricting social contact. SARS-CoV-2 is an enveloped positive-sense RNA virus of 60 nm–140 nm in diameter with spike-like projections identified by electron microscopy [3]. Herein, this review highlights the biology of SARS-CoV-2 and possible novel immunotherapy with interleukin (IL)-15 for COVID-19 infection in humans.
2. IL-15 is required for the maintenance of innate immunity and promotes viral clearance
IL-15 is a critical immunoregulatory cytokine with anti-viral properties [4]. IL-15 is expressed by myeloid cells to aid in T cell responses, activate natural killer (NK) cells, and modulate inflammation [5]. In lymphocytes, activated IL-15 binds to the IL-2/15Rβγ heterodimer and induces signal transduction via phosphorylation of Janus-associated kinases (JAK) and signal transducer and activator of transcription (STAT) proteins. JAK1 activation phosphorylates STAT3 via the β chain, whereas JAK3/STAT5 activation occurs via the γ chain. The STAT3/STAT5 form heterodimers upon phosphorylation [6,7] and translocate to the nucleus to activate Bcl-2, c-Myc, c-Fos, c-Jun and NF-κB [[8], [9], [10], [11]]. Akt is activated via a phosphatidylinositol 3-kinase (PI3K)-dependent pathway. Shc is an adaptor protein that binds to a phosphotyrosine residue on the IL-2/15Rβ heterodimer and activates Grb2, which then activates Akt, resulting in an increase in cell proliferation and/or survival [12,13]. IL-15 trans-presentation to IL-2/15Rβγ and Shc-mediated activation of Grb2 lead to the formation of Grb2-SOS complex that further activates the Ras-Raf pathway by facilitating the removal of GDP from a member of the Ras subfamily, activating the mitogen-activated protein kinase (MAPK) pathway for cellular proliferation [[14], [15], [16]].] IL-15 deficiency has been previously shown to promote airway resistance in mice, whereas IL-15 inhibits pro-inflammatory cytokines, reduces goblet cell hyperplasia, and regulates allergen-induced airway obstruction in mice by inducing Interferon (IFN)-γ and IL-10-producing regulatory CD4+CD25+Foxp3+ T cells [17]. In another study, rIL-15 treatment has been showed to further protect mice from chronic fibro-inflammation via the induction of IFN-γ-responsive invariant NK T cells [18]. Human IL-15 cytokine integration into the genome of the Wyeth strain of vaccinia induces powerful immunogenicity in mice [19]. IL-15 enhances the activity of NK cells, whereas blocking IL-15 delays NK cell entry into mouse lung airways infected with influenza, resulting in dysregulated control of viral replication. IL-15 regulates innate and adaptive responses to influenza infection by mobilizing NK cells to control early viral replication. Depletion of NK1.1+ cells is associated with a decrease in the migration of influenza-specific CD8+ T cells at the site of infection [20]. IL-15 may be a novel therapeutic molecule, and we have previously showed that IL-15-responsive RORγ + T regulatory cells are expressed in IL-15-overexpressing allergen-challenged mice to restrict pulmonary fibrosis [21]. A clinical trial is under way using intravenous infusions of Natural Killer Group 2D (NKG2D)-Angiotensin-converting enzyme 2 (ACE2) chimeric antigen receptor (CAR)-NK cells with an IL-15 superagonist and Granulocyte-macrophage colony-stimulating factor (GM-CSF) neutralizing single-chain variable fragments for the treatment of COVID-19. This therapy aims to target SARS-CoV-2 and Natural killer group 2D ligand (NKG2DL) with ACE2 and NKG2D on the surface of infected cells for effective removal of SARS-CoV-2 virus particles (https://clinicaltrials.gov/ct2/show/NCT04324996). An earlier report has shown that plasma IL-15 levels are increased in MERS-CoV infected patients, which demonstrates that IL-15 induced NK and CD8+ T cell responses are effective in eliminating virus-infected cells [22]. Further, Wyeth/IL-15/5Flu and Wyeth/mutIL-15/5Flu vaccines have been reported to promote defense against clade 2.2 H5N1 infection [23]. These data demonstrate that induced IL-15 improves both humoral and cellular responses against respective viral antigens and protects infected individuals. Most recent report indicates that IFN-α2b with or without arbidol reduces virus load in the upper respiratory tract [24]. Since, IL-15 is critical for the development, survival and function of several innate cells including NK cells that regulates IFN-α/β; we hope that the innate immune responses associated with IL-15 overexpression may also be critical in the treatment of SARS-CoV-2 infection. Therefore, a double-blind clinical trial with IL-15 immunotherapy is warranted to establish the critical therapeutic effects of IL-15-induced innate immune responses for patients infected with COVID-19.
3. Epidemiology of COVID-19
Coronavirus belongs to the family Coronaviridae and the order of Nidovirales, which is a type of enveloped positive-sense RNA viruses distributed in mammals. Earlier outbreaks of coronavirus diseases that led to severe threats to human health at the beginning of the 21st century were caused by the severe acute respiratory syndrome (SARS)-CoV and the MERS-CoV [25]. The natural reservoir for these viruses includes wild animals like bats, from which the virus may be transmitted to a secondary host and humans [26]. SARS-CoV-2 is 96 % similar to bat coronaviruses at the whole genome level [27]. Zhang et al. determined the probable pangolin origin of SARS-CoV-2, which was 91.02 % similar to SARS-CoV-2 at the genome level [28]. COVID-19, an inflammatory viral disease caused by the novel coronavirus SARS-CoV-2, is a deadly disease emerged in December 2019 in Wuhan city, Hubei province of China. A recent study identified the novel coronavirus by deep sequencing analysis of isolated human airway epithelial cells from patients with pneumonia, which was later named SARS-CoV-2 [29]. The initial events of virus spread was associated with animal-to-animal contact, and subsequent spread to humans was linked to the Huanan seafood market [30]. It has affected several countries across the globe with a wide community spread and high mortality. There are numerous clinical trials under way in developing potent vaccines and potential anti-viral and neutralizing therapies for COVID-19 [31]. The mortality of COVID-19 aggravates in patients with co-morbid conditions like hypertension, diabetes, obesity, cancer, chronic respiratory disease, chronic kidney disease, and liver diseases. Besides chronic obstructive pulmonary disease, a history of asthma worsens disease severity and increases mortality rate in COVID-19 patients [32,33]. The expression of ACE2 receptor is upregulated in the lung tissues of tobacco smokers, suggesting that smoking is a critical risk factor for viral infections [34]. Current data indicate that older adults and people of any age with comorbidities might be at an elevated risk of severe illness and mortality from COVID-19 (https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-at-higher-risk.html).
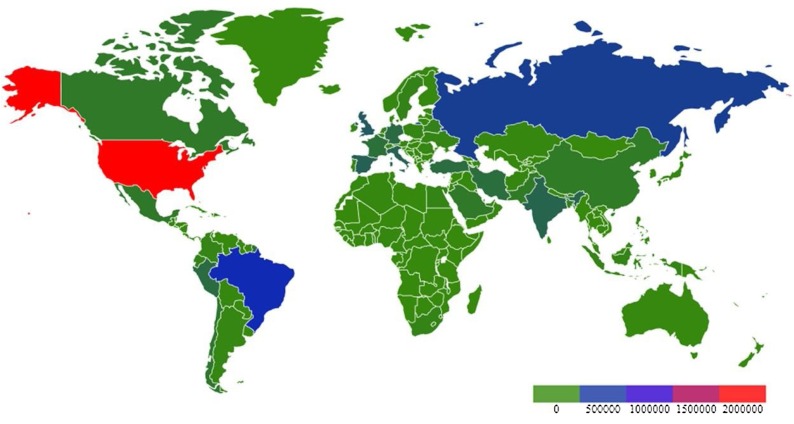
SARS-CoV-2 infected patient’s manifest dry cough, itchy throat, and increased body temperature at the onset. As the disease progresses, most patients exhibit pneumonia with dyspnea, pulmonary inflammation, myalgia, fatigue, and reduced leukocyte counts. SARS-CoV-2 infected patients can be identified by radiological evaluation of pneumonia and laboratory detection of viral infection. Recent report indicates that the infection of SARS-CoV-2 not only damage the lung, but also affect multiple organs in virus infected patients [35]. Males have been shown to exhibit higher rate of SARS-CoV-2 infection and mortality than females, which may be attributed to the female X chromosome that is associated with less viral loads, lower levels of IL-6 and inflammation, higher levels of CD4+ T cells, antibodies, and immune cells, along with the activation of Toll-like receptor 7 (TLR7) and IFN in females [36]. A study with 38 pregnant women with COVID-19 shows no evidence of intrauterine or transplacental transmission of SARS-CoV-2 from infected pregnant women to their fetuses [37], which still needs to be verified in future research. SARS-CoV-2 also infects children, who are less susceptible to the infection with milder disease course, better prognosis, and a lower mortality rate than those in adults [38,39]. There has been an increasing concern over pediatric multi-system inflammatory syndrome that requires intensive care to be potentially associated with COVID-19, and the commonly reported symptoms include fever, abdominal pain, vomiting, diarrhea, rashes and cardiac inflammation. The blood work is consistent with severe pediatric COVID-19 cases, with overlapping features of toxic shock syndrome and atypical Kawasaki disease [40]. Mutation hotspots have been identified by sequencing analysis of the spike protein of SARS-CoV-2 that is involved in viral virulence [41]. As of June 04, 2020, 6.4 million cases of COVID-19 have been reported globally with 382,867 deaths. The worldwide spread of COVID-19 is presented in the heat map modified from https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200517-covid-19-sitrep-118.pdf?sfvrsn=21c0dafe_8 (Fig. 1 ).
Fig. 1.
Global cases of COVID-19 as of 06/04/2020.
4. COVID-19 entry and replication
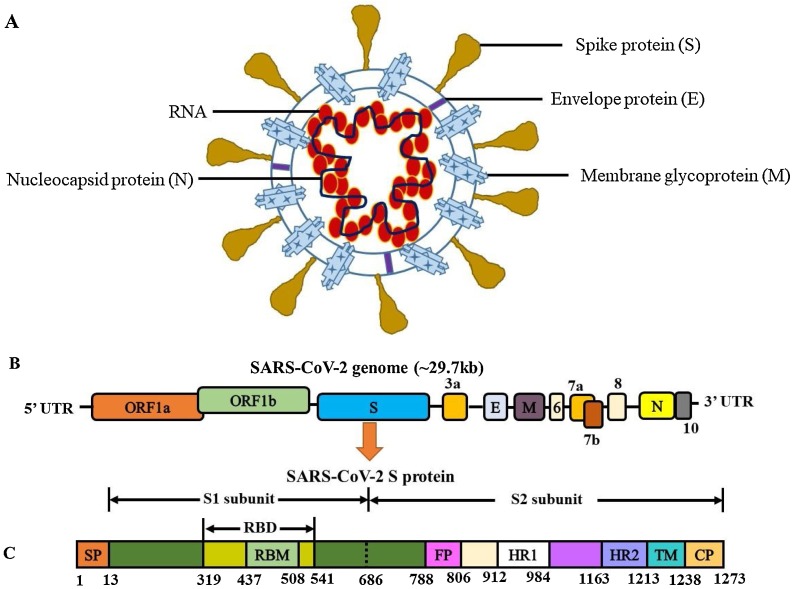
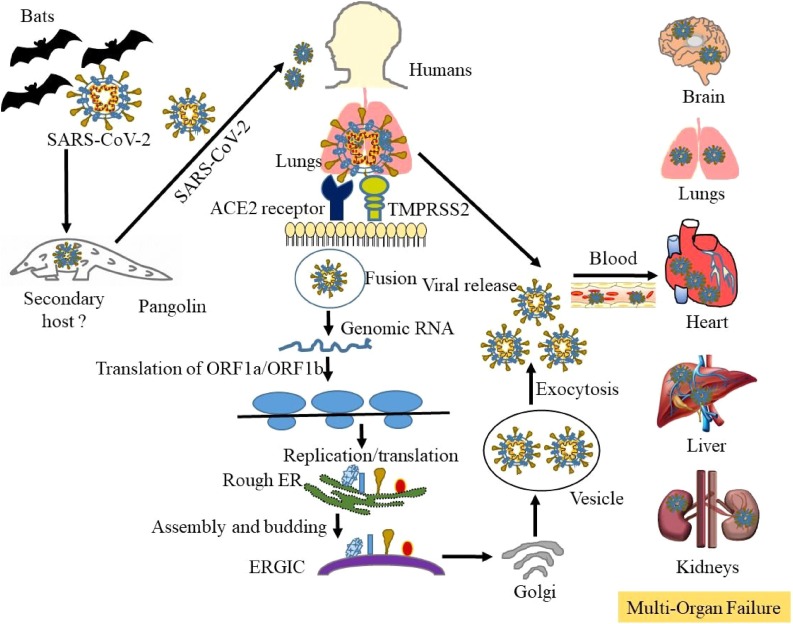
The human SARS-CoV-2 genome encompasses the 5′-untranslated region (5′-UTR), open reading frame (ORF) 1a/b encoding non-structural proteins that aid in replication, ORFs encoding structural proteins including spike (S), envelop (E), membrane (M), and nucleocapsid (N) proteins, ORFs 3, 6, 7a, 7b, 8 and 9b encoding accessory proteins, and the 3′-UTR. The M and E proteins help with viral assembly, and the N protein is critical for RNA synthesis as shown in Fig. 2 A–C. The S protein plays a vital role in viral entry into host cells by biding to the ACE2 receptor. Membrane fusion of SARS-CoV-2 is primed by proteases cathepsin L and Transmembrane Serine Protease 2 (TMPRSS2) via cleavage at the S1/S2 and the S2 sites [42]. The conformation change in the S protein facilitates the fusion of viral envelope with the cell membrane through the endosomal pathway, followed by RNA release from SARS-CoV-2 into the host cell. Genome RNA is translated into viral replicase polyproteins pp1a and pp1ab, which are cleaved by viral proteinases. Subgenomic mRNAs produced by polymerases are translated into relevant viral proteins by discontinuous transcription, assembled into virions in the ER and Golgi, transported via vesicles, and released out of the host cell [43]. The released SARS-CoV-2 infects not only the lungs, but key organs like the brain, heart, intestine, kidney, and liver through ACE2-mediated pathways. Its pathogenesis involves induced cytokine storms, immune cell infiltration, and the depletion of T cells. These pathological changes lead to acute respiratory distress syndrome (ARDS), hypoxia with myocardial, hepatic, renal, and central nervous system injuries, and may contribute to organ failure and increased mortality as depicted in Fig. 3 [44,45]. COVID-19 is also detected in asymptomatic carriers by CT imaging and RT-PCR tests, and the wide spread of asymptomatic transmission makes it challenging to prevent COVID-19 infections [46,47].
Fig. 2.
Schematic of the virion, the genome and the spike protein of SARS-CoV-2 that causes COVID-19, a human respiratory syndrome. A) The viral surface, spike, envelope, and membrane proteins are embedded in a lipid bilayer and the single-stranded positive-sense viral RNA is associated with the nucleocapsid protein. The SARS-CoV-2 genome encompasses the 5′-untranslated region (5′-UTR), open reading frame (ORF) 1a/b encoding non-structural proteins for replication, ORFs encoding structural proteins including spike, envelop, membrane, and nucleocapsid proteins, ORFs encoding accessory proteins such as ORF 3a, 6, 7a, 7b, 8 and 10, and the 3′-UTR. B) Genome organization of SARS-CoV-2. C) SARS-CoV-2 spike glycoprotein. The S1/S2 cleavage sites are indicated by dotted lines. In the S protein, the S1 subunit is comprised of signal peptide (SP), receptor (ACE2)-binding motif (RBM), and receptor-binding domain (RBD); the S2 subunit is comprised of fusion peptide (FP), heptad repeat (HR), transmembrane domain (TM), and cytoplasm domain (CP).
Fig. 3.
Transmission of SARS-CoV-2, replication in humans, and induction of pulmonary fibro-inflammation and organ failure. SARS-CoV-2 that causes COVID-19 may originate from the primary host bats or unknown secondary hosts and cross the species barrier to humans. The spike protein on SARS-CoV-2 binds to the cell surface receptor ACE2 and the enzyme TMPRSS2, which aid the virion entry. The virion releases its RNA, part of which is translated into proteins. Proteins and the RNA are assembled into a new virion in the Golgi and released. Exposure to SARS-CoV-2 induces immune cell infiltration that promotes inflammatory cytokine storms and multi-organ failure via the acute respiratory distress syndrome.
5. Immune cell infiltration and cytokine storms with SARS-CoV-2 infection
Immune cell infiltration and cytokine storms have been reported in patients with SARS-CoV-2 infection. The levels of inflammatory cells including eosinophils were low at disease onset but returned to normal before patients are discharged, indicating that COVID-19 patients may benefit from continued use of lopinavir, a strategy that needs to be verified in future studies [48]. The neutrophil to lymphocyte ratio (NLR) is a marker for the overall inflammatory status of patients; increased NLR is a risk factor in various diseases and is reported to be increased in COVID-19 patients, suggesting that it can be an independent risk factor for mortality in SARS-CoV-2 infected hospitalized patients [49]. Necropsy of two patients infected with SARS-CoV-2 showed pulmonary hemorrhage, epithelial injury, spherical hyaline degeneration bodies with macrophage infiltration and fibrosis, and desquamated alveolar cells in the lung. Immunohistology identification has also confirmed the expression of chemokines and inflammatory cytokines IL-6, IL-10, tumor necrosis factor (TNF)-α, programmed death-ligand (PDL)-1, and CD68+ macrophages. Incubation of purified and Fc-tagged spike proteins of SARS-CoV-2 that have receptor binding domains to enter white blood cells showed evidence of the S protein interacting with CD68-expressing monocytes or macrophages but not with T or B lymphocytes. The expression of ACE2 was also observed on macrophages. This study demonstrates the critical role of macrophages as the host cells for SARS-CoV-2 and the potential driver of cytokine storms [50].
6. T cell immunity in viral infections
T cell cytotoxic subsets and NK cells play an important role in viral clearance; exhaustion of such cells increases disease severity. NKG2A is an inhibitory receptor associated with NK cells to restrict viral replication [51]. A recent study showed a decrease in NK cells and CD8+ T cells with an increased expression of NKG2A, whereas recovering patients showed restored levels of NK and CD8+ T cells with a decreased expression of NKG2A. Interestingly, the levels of CD107a+ CD8+, IFN-γ+CD8+, IL-2+CD8+, and granzyme B+CD8+ T cells as well as CD107a+, IFN-γ+, IL-2+, TNF-α+, and granzyme B+ NK cells were also decreased in COVID-19 patients [52]. Regulatory T cells (Tregs) suppress activated CD4+ or CD8+ T lymphocytes, while IL-10 enhances Tregs and inhibits T cell activation. Decreased levels of Tregs have been observed in COVID-19 patients [53]. IL-10 is an anti-inflammatory cytokine produced in viral, fungal, bacterial, and parasitic infections. It suppresses macrophages and dendritic cells, while inhibiting cytokines and chemokines [54]. IL-10 adjunct therapy has been shown to be effective against viral encephalitis caused by a recombinant coronavirus (J2.2-V-1 [rJ2.2]) in mice [55]. However, elevated IL-10 levels have been observed in COVID-19 patients with severe infection [56], possibly due to a compensatory anti-inflammatory response of IL-10 for high disease severity. Taken together, the evidence strongly supports IL-15 immunotherapy as a useful strategy to control SARS-CoV-2 infection in patients. The rationale is based on our recent findings that IL-15 can induce INF and IL-10 by increasing the number of Treg subsets [17]. Further, mesenchymal stem cells (MSC) possess immunomodulatory effects, and ACE2− MSC transplantation has showed elevated levels of peripheral lymphocytes and IL-10, as well as decreased levels of C-reactive protein, TNF-α, CXCR3+CD4+ T cells, CXCR3+CD8+ T cells, and CXCR3+ NK cells that secrete cytokines. These MSC are ACE2- and TMPRSS2-, and are free from SARS-CoV-2 infection [57]. Therefore, it is helpful to assess cytokines and lymphocyte subsets in the initial screening and treatment of COVID-19.
7. SARS-CoV-2 mediated pulmonary fibrosis
Immune cell infiltration and inflammatory cytokine storms observed in SARS-CoV-2 infected patients can lead to acute pulmonary injury and edema via dysfunctional endothelial barriers and damaged alveolar walls in the lung [58]. The histological features of lung tissues in COVID-19 patients include pulmonary edema, interstitial fibrosis, mucin production, pulmonary hemorrhage, hyaline degeneration, vascular wall thickening, inflammatory cell infiltration, necrotizing bronchial and epithelial cells, and squamous cell metaplasia. Additionally, the pulmonary tissues showed positive staining of CD3, CD4, CD8, CD20, CD79a, CD5, CD38, CK7, and collagen IV [59]. SARS-CoV-2 induced cytokine storms may further lead to clotting, cell death, immune paralysis with inflammation, and organ failure.
8. Current and potential treatments for COVID-19
Humoral and cell-mediated responses are critical in fighting against SARS-CoV-2 infection. Various preclinical studies in mouse models showed protective responses against the S protein of SARS-CoV-2, and antibodies generated against the N protein of SARS-CoV-2 was reported in COVID-19 patients. Azithromycin and hydroxychloroquine combination are more efficient for viral load reductions against SARS-CoV-2 infection [60]. Kaletra anti-viral therapy, along with IFN and antibiotic treatment, has been shown to normalize T cells, NK cells, and NKG2A + Cytotoxic T lymphocytes (CTLs) in a small set of individuals [52]. Increased level of IL-6 is correlated with poor outcomes in COVID-19 patients and a study with IL-6 receptor-targeted antibodies, tocilizumab, has showed recovery of respiratory functions (ChiCTR2000029765) [61]. Cross-neutralizing human monoclonal antibody 47D11 that targets the conserved epitope in the SARS2-S-S1B domain and neutralizes both SARS-CoV and SARS-CoV-2 is identified in cell culture. This antibody alone or in combination with other anti-viral drugs may potentially prevent and/or treat COVID-19 [62]. Furthermore, convalescent plasma transfusion with SARS-CoV-2-IgG antibody and a neutralization titer has been shown to improve symptoms in a small study with 5 patients [63]. Convalescent plasma therapy is well tolerated with increased oxyhemoglobin saturation and lymphocyte counts, decreased C-reactive protein, and neutralized viremia in 10 COVID-19 patients [64]. However, these data are not independently reliable due to the absence of the control groups in these studies. The effectiveness of plasma therapy needs to be validated in reliable double-blind placebo-controlled clinical trials. Natural immunity and anti-oxidative capacity of the host are crucial in minimizing or preventing symptoms associated with viral attacks. Earlier reports have also indicated antiviral properties of micronutrients [65], and a recent study has demonstrated that vitamin D could improve clinical symptoms of COVID-19 [66]. Therefore, maximizing the body’s defense with antioxidant-rich diets supplemented with micronutrients might be beneficial in some individuals with healthy immune systems [67]. Several ongoing clinical trials across the globe for vaccines, anti-viral drugs, and neutralizing antibodies to restrict COVID-19 infection in humans are listed in Table 1 .
Table 1.
Pharmacology of selected COVID-19 treatments under investigation. Resources: () FDA, WHO, Clinical trials.gov.
| Drugs | Mechanism of action |
|---|---|
| Anti-inflammatory therapies for COVID-19 infection | |
| Actemra | IL-6 inhibitor |
| Lenzilumab | anti-GM-CSF |
| CD24Fc | IL-6 inhibitor |
| Colchicine | Tubulin disruption |
| Kevzara | IL-6 inhibitor |
| Leronlimab | CCR5 antagonist |
| Aviptadil | IL-6 inhibitor |
| SNG001 | IFN-β-1α |
| Gilenya | sphingosine 1-phosphate receptor modulator |
| Mesenchymal stem cells | Tissue regeneration |
| Gimsilumab | Anti-GM-CSF |
| Sylvant | IL-6 inhibitor |
| Anti-viral therapies for COVID-19 infection | |
| Remdesivir | Adenosine analog |
| Kaletra | HIV protease inhibitor |
| Arbidol | Broad-spectrum antiviral |
| Chloroquine/ Hydroxychloroquine | ACE-2 inhibitor |
| Avigan | RNA polymerase inhibitor |
| Pneumonia therapies for COVID-19 infection | |
| Ganovo-Ritonavir | Hepatitis C/HIV protease inhibitors |
| Prezcobix | HIV-1 protease inhibitor + CYP3A inhibitor + CYP3A inhibitor |
| Avastin | VEGF inhibitor |
| Airuika | PD-1 inhibitor |
| Plasma therapies for COVID-19 infection | |
| Plasmapheresis | Antibodies from recovered patients |
| Therapies for organ failure with COVID-19 infection | |
| Losartan | AT1R inhibitor |
| Vaccines under investigation for COVID-19 infection | |
| mRNA-1273 | S-protein mRNA vaccine |
| Ad5-nCoV | Non-replicating viral vector |
| ChAdOx1 nCoV-19 | Non-replicating viral vector |
| LV-SMENP-DC | Lentiviral |
| BCG Vaccine | Live attenuated Virus |
9. Conclusions
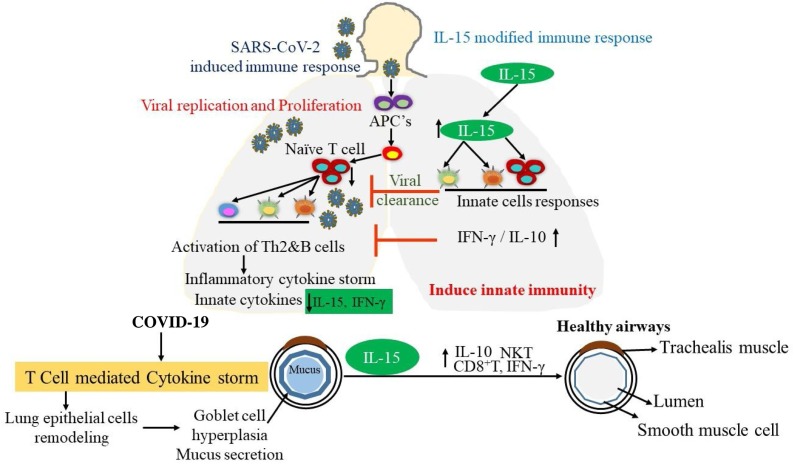
The COVID-19 pandemic is a worldwide public health concern and is worse than the influenza pandemic of 1918. Almost 382,867 deaths have been reported as of June 04, 2020. Several clinical trials are currently under way in search of therapies for COVID-19. SARS-CoV-2 infection induces cytokine storms via several immune cells. The IL-15 immunotherapy may be a viable strategy for COVID-19, as it promotes innate immune responses via the induction of NK cells, CD8+ T cells, and T regulatory cells to neutralize Th2 cytokine storms, resulting in decreased levels of IL-4, IL-5, and IL-13. These events mitigate SARS-CoV-2 induced inflammation and fibrosis through IFN-γ and IL-10, which inhibit viral replications and reduce viral loads. The current review highlights the importance of IL-15 immunotherapy in decreasing viral loads and neutralizing cytokine storms induced by SARS-CoV-2 in COVID-19 patients. A summarized mechanistic pathway for IL-15 immunotherapy is presented in Fig. 4 .
Fig. 4.
Significance of IL-15 immunotherapy in inducing innate immunity in COVID-19 patients. In response to SARS-CoV-2 infection, cytokine storms occur via the induction of several immune cells. IL-15 overexpression promotes innate immune responses via the induction of NK, CD8+ T and T regulatory cells that neutralize the induced Th2 cytokine storms, resulting in decreased levels of IL-4, IL-5, and IL-13. These events mitigate SARS-CoV-2 induced inflammation and fibrosis via IFN-γ and IL-10, which inhibit viral replications and reduce viral loads.
Funding
Dr. Mishra is the Endowed Schlieder Chair. All authors would like to thank Edward G. Schlieder Educational Foundation for the major support. The work is also partially supported by the NIH R01 AI080581 grant (AM).
Transparency document
Biographies

Dr. Hemanth Kumar Kandikattu, PhD is Postdoctoral Research Fellow in the Section of Pulmonary Diseases at Tulane School of Medicine, New Orleans, LA. Dr. Hemanth K Kandikattu is working on role of eosinophils in allergic diseases, and on pancreatitis and its progression to pancreatic cancer. Dr. Hemanth had an exceptional background, foundation with his renowned education at Sri Venkateswara University, Tirupati, India where he graduated with Bachelor of Science in Biotechnology followed by Master of Science in Biochemistry. Having excelled in science education, he then undertook PhD training at Defence Food Research Laboratory and University of Mysore, Mysore, India. Dr. Hemanth completed his Post-Doctoral training in Cardiovascular Medicine from University of Missouri, Columbia, Missouri, USA. He has published over 45 articles, book chapters, and reviews. He is a member of American Academy of Allergy, Asthma & Immunology (AAAAI), Milwaukee, WI, USA, European Academy of Allergy and Clinical Immunology, Zurich, Switzerland, American Gastroenterological Association, Bethesda, MD, USA, National Environmental Science Academy (NESA), Delhi, India.

Dr. Sathisha Upparahalli Venkateshaiah, PhD is Assistant Professor in the Section of Pulmonary Diseases at Tulane School of Medicine, New Orleans, LA. Dr. Sathisha UV had an exceptional background, foundation with his renowned education at University of Mysore, Mysore, India where he graduated with Bachelor of Science. Having excelled in science education, he then undertook PhD training at University of Mysore, Mysore, India. Dr. Sathisha UV skilled in Cancer Biology and Molecular Biology training from an esteemed Central food Technological Research Institute (CSIR), Mysore, India. Dr. Sathisha UV completed his postdoctoral training at Myeloma Institute for Research and Therapy, Winthrop P. Rockefeller Cancer Institute, UAMS, Little Rock, AR, USA, Tulane School of Medicine, New Orleans, LA. Dr. Sathisha UV most important contribution was role of bone marrow microenvironment in Myeloma tumorigenesis. Dr. Sathisha UV’s most important contribution was IL-15′s protective role in the pathogenesis of asthma, Journal of Allergy and Clinical Immunology (PMID: 28,606,589); Critical role for IL-18 in transformation and maturation of naive eosinophils to pathogenic eosinophils, J Allergy Clin Immunology (PMID: 29,499,224).

Dr. Sandeep Kumar, PhD is Postdoctoral Research Fellow in the Section of Pulmonary Diseases at Tulane School of Medicine, New Orleans, Louisiana. Dr. Sandeep Kumar is working on role of eosinophils in pancreatic cancer. Dr. Sandeep Kumar did PhD on Cytokine gene polymorphism on adult women with metabolic syndrome at King George’s Medical University, Lucknow, India. Dr. Sandeep completed National Post-Doctoral Fellowship training in Reactive arthritis from Sanjay Gandhi PGIMS, Lucknow, India. He has published over 25 articles and reviews.

Dr. Anil Mishra, PhD is Professor of Medicine and Endowed Chair, Edward G. Schlieder Educational Foundation in the Section of Pulmonary Diseases at Tulane School of Medicine, New Orleans, LA. He is also the Director of Eosinophilic Disorder Center at Tulane School of Medicine, New Orleans, LA. Earlier, Dr. Mishra served as a faculty member at Allergy and Immunology Division, Cincinnati Childrens Hospital Medical Center, Cincinnati, OH and Case Western Reserve University Medical College, OH before joining the Endowed Chair and Professor of Medicine at Tulane University School of Medicine, New Orleans, LA. Dr. Mishra’s made several important contribution in science. He establish that eosinophils are the resident cell that home prenatally in the gastrointestinal tract (Mishra et al. J. Clin. Invest. 1999, 103, 1719-27). Dr. Mishra also developed a first murine model of asthma associated eosinophilic esophagitis (Mishra et al. J. Clin. Invest. 2001, 107, 83–90). These findings implicated aeroallergens in the etiology of EoE and suggested that esophageal eosinophilic inflammation is mechanistically associated with pulmonary inflammation (Mishra et al. J. Immunology 2002, 168, 2464-69 and Gastroenterology, 2003, 125. 1419-27). Dr. Mishra’s is an elected fellow of the American Academy of Allergy Asthma Immunology (FAAAAI), and American Gastrointestinal Association (FAGA). Dr. Mishra was the active member of Cincinnati Center for Eosinophilic Disorder (CCED) from the start of the center. He has over a 100 articles on molecular mechanisms of the pulmonary gastrintestinal toxicity and allergic responses. Some of his publications are cited more than 800 till 2018. Dr. Mishra’s research has been supported by the 2-differen intitutes of National Institutes of Health (NIDDK and NIAID) to understand the mechanism that induce EoE. He is serving in a number of study sections as member in NIH. Further, his research implicated iNKT cells to the food and indoor insect allergens induce EoE and iNKT cell neutralization as a possible future therapy of EoE. He patents anti-IL-18 futhere therapy for eosinophils associated allergic diseases including EoE, based on his recent findings that Il-18 is critical in transforming IL-5 generted naivere eosinophils to CD101/Cd274 double positive pathogeneic eosinophils that promotes tissue pathogenesis.]If interested in his research activities then please visit Mishra’s Research Laboratory and Eosinophilic Disorder Center at Tulane
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cytogfr.2020.06.008.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Wang L.F., Eaton B.T. Bats, civets and the emergence of SARS. Curr. Top. Microbiol. Immunol. 2007;315:325–344. doi: 10.1007/978-3-540-70962-6_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Omrani A.S., Al-Tawfiq J.A., Memish Z.A. Middle East respiratory syndrome coronavirus (MERS-CoV): animal to human interaction. Pathog. Glob. Health. 2015;109(8):354–362. doi: 10.1080/20477724.2015.1122852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richman DD W.R., Hayden F.G. 4th ed. ASM Press; Washington: 2016. Clinical Virology. [Google Scholar]
- 4.Verbist K.C., Klonowski K.D. Functions of IL-15 in anti-viral immunity: multiplicity and variety. Cytokine. 2012;59(3):467–478. doi: 10.1016/j.cyto.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kandikattu H.K., Upparahalli Venkateshaiah S., Mishra A. Synergy of Interleukin (IL)-5 and IL-18 in eosinophil mediated pathogenesis of allergic diseases. Cytokine Growth Factor Rev. 2019;47:83–98. doi: 10.1016/j.cytogfr.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyazaki T., Kawahara A., Fujii H., Nakagawa Y., Minami Y., Liu Z.J., Oishi I., Silvennoinen O., Witthuhn B.A., Ihle J.N. Functional activation of Jak1 and Jak3 by selective association with IL-2 receptor subunits. Science. 1994;266(5187):1045–1047. doi: 10.1126/science.7973659. [DOI] [PubMed] [Google Scholar]
- 7.Lin J.X., Migone T.S., Tsang M., Friedmann M., Weatherbee J.A., Zhou L., Yamauchi A., Bloom E.T., Mietz J., John S. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity. 1995;2(4):331–339. doi: 10.1016/1074-7613(95)90141-8. [DOI] [PubMed] [Google Scholar]
- 8.Waldmann T.A., Tagaya Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu. Rev. Immunol. 1999;17:19–49. doi: 10.1146/annurev.immunol.17.1.19. [DOI] [PubMed] [Google Scholar]
- 9.Shibuya H., Yoneyama M., Ninomiya-Tsuji J., Matsumoto K., Taniguchi T. IL-2 and EGF receptors stimulate the hematopoietic cell cycle via different signaling pathways: demonstration of a novel role for c-myc. Cell. 1992;70(1):57–67. doi: 10.1016/0092-8674(92)90533-i. [DOI] [PubMed] [Google Scholar]
- 10.Miyazaki T., Liu Z.J., Kawahara A., Minami Y., Yamada K., Tsujimoto Y., Barsoumian E.L., Permutter R.M., Taniguchi T. Three distinct IL-2 signaling pathways mediated by bcl-2, c-myc, and lck cooperate in hematopoietic cell proliferation. Cell. 1995;81(2):223–231. doi: 10.1016/0092-8674(95)90332-1. [DOI] [PubMed] [Google Scholar]
- 11.Lord J.D., McIntosh B.C., Greenberg P.D., Nelson B.H. The IL-2 receptor promotes lymphocyte proliferation and induction of the c-myc, bcl-2, and bcl-x genes through the trans-activation domain of Stat5. J. Immunol. 2000;164(5):2533–2541. doi: 10.4049/jimmunol.164.5.2533. [DOI] [PubMed] [Google Scholar]
- 12.Gu H., Maeda H., Moon J.J., Lord J.D., Yoakim M., Nelson B.H., Neel B.G. New role for Shc in activation of the phosphatidylinositol 3-kinase/Akt pathway. Mol. Cell. Biol. 2000;20(19):7109–7120. doi: 10.1128/mcb.20.19.7109-7120.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellery J.M., Nicholls P.J. Alternate signalling pathways from the interleukin-2 receptor. Cytokine Growth Factor Rev. 2002;13(1):27–40. doi: 10.1016/s1359-6101(01)00023-5. [DOI] [PubMed] [Google Scholar]
- 14.Adunyah S.E., Wheeler B.J., Cooper R.S. Evidence for the involvement of LCK and MAP kinase (ERK-1) in the signal transduction mechanism of interleukin-15. Biochem. Biophys. Res. Commun. 1997;232(3):754–758. doi: 10.1006/bbrc.1997.6367. [DOI] [PubMed] [Google Scholar]
- 15.Steelman L.S., Pohnert S.C., Shelton J.G., Franklin R.A., Bertrand F.E., McCubrey J.A. JAK/STAT, Raf/MEK/ERK, PI3K/Akt and BCR-ABL in cell cycle progression and leukemogenesis. Leukemia. 2004;18(2):189–218. doi: 10.1038/sj.leu.2403241. [DOI] [PubMed] [Google Scholar]
- 16.Mishra A., Sullivan L., Caligiuri M.A. Molecular pathways: interleukin-15 signaling in health and in cancer. Clin. Cancer Res. 2014;20(8):2044–2050. doi: 10.1158/1078-0432.CCR-12-3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venkateshaiah S.U., Zhu X., Rajavelu P., Niranjan R., Manohar M., Verma A.K., Lasky J.A., Mishra A. Regulatory effects of IL-15 on allergen-induced airway obstruction. J. Allergy Clin. Immunol. 2018;141(3):906–917. doi: 10.1016/j.jaci.2017.05.025. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manohar M., Kandikattu H.K., Verma A.K., Mishra A. IL-15 regulates fibrosis and inflammation in a mouse model of chronic pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2018;315(6):G954–G965. doi: 10.1152/ajpgi.00139.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perera L.P., Waldmann T.A., Mosca J.D., Baldwin N., Berzofsky J.A., Oh S. Development of smallpox vaccine candidates with integrated interleukin-15 that demonstrate superior immunogenicity, efficacy, and safety in mice. J. Virol. 2007;81(16):8774–8783. doi: 10.1128/JVI.00538-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verbist K.C., Rose D.L., Cole C.J., Field M.B., Klonowski K.D. IL-15 participates in the respiratory innate immune response to influenza virus infection. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0037539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Upparahalli Venkateshaiah S., Niranjan R., Manohar M., Verma A.K., Kandikattu H.K., Lasky J.A., Mishra A. Attenuation of allergen-, IL-13-, and TGF-alpha-induced lung fibrosis after the treatment of rIL-15 in mice. Am. J. Respir. Cell Mol. Biol. 2019;61(1):97–109. doi: 10.1165/rcmb.2018-0254OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahallawi W.H., Khabour O.F., Zhang Q., Makhdoum H.M., Suliman B.A. MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine. 2018;104:8–13. doi: 10.1016/j.cyto.2018.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poon L.L., Leung Y.H., Nicholls J.M., Perera P.Y., Lichy J.H., Yamamoto M., Waldmann T.A., Peiris J.S., Perera L.P. Vaccinia virus-based multivalent H5N1 avian influenza vaccines adjuvanted with IL-15 confer sterile cross-clade protection in mice. J. Immunol. 2009;182(5):3063–3071. doi: 10.4049/jimmunol.0803467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Q., Chen V., Shannon C.P., Wei X.-S., Xiang X., Wang X., Wang Z.-H., Tebbutt S.J., Kollmann T.R., Fish E.N. Interferon-α2b treatment for COVID-19. Front. Immunol. 2020;11:1061. doi: 10.3389/fimmu.2020.01061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coronaviridae V. Study Group of the International Committee on Taxonomy of, The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17(3):181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang T., Wu Q., Zhang Z. Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Curr. Biol. 2020;30(8):1578. doi: 10.1016/j.cub.2020.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. I. China novel coronavirus, T. research, a novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mackenzie J.S., Smith D.W. COVID-19: a novel zoonotic disease caused by a coronavirus from China: what we know and what we don’t. Microbiol. Aust. 2020:MA20013. doi: 10.1071/MA20013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lythgoe M.P., Middleton P. Ongoing clinical trials for the management of the COVID-19 pandemic. Trends Pharmacol. Sci. 2020 doi: 10.1016/j.tips.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao Q., Meng M., Kumar R., Wu Y., Huang J., Lian N., Deng Y., Lin S. The impact of COPD and smoking history on the severity of Covid-19: a systemic review and meta-analysis. J. Med. Virol. 2020 doi: 10.1002/jmv.25889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X., Xu S., Yu M., Wang K., Tao Y., Zhou Y., Shi J., Zhou M., Wu B., Yang Z., Zhang C., Yue J., Zhang Z., Renz H., Liu X., Xie J., Xie M., Zhao J. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J. Allergy Clin. Immunol. 2020 doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai G., Bosse Y., Xiao F., Kheradmand F., Amos C.I. Tobacco smoking increases the lung gene expression of ACE2, the receptor of SARS-CoV-2. Am. J. Respir. Crit. Care Med. 2020 doi: 10.1164/rccm.202003-0693LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zaim S., Chong J.H., Sankaranarayanan V., Harky A. COVID-19 and multi-organ response. Curr. Probl. Cardiol. 2020 doi: 10.1016/j.cpcardiol.2020.100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conti P., Younes A. Coronavirus COV-19/SARS-CoV-2 affects women less than men: clinical response to viral infection. J. Biol. Regul. Homeost. Agents. 2020;34(2) doi: 10.23812/Editorial-Conti-3. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz D.A. An Analysis of 38 Pregnant Women with COVID-19, Their Newborn Infants, and Maternal-Fetal Transmission of SARS-CoV-2: Maternal Coronavirus Infections and Pregnancy Outcomes. Arch. Pathol. Lab. Med. 2020 doi: 10.5858/arpa.2020-0901-SA. [DOI] [PubMed] [Google Scholar]
- 38.Lee P.I., Hu Y.L., Chen P.Y., Huang Y.C., Hsueh P.R. Are children less susceptible to COVID-19? J. Microbiol. Immunol. Infect. 2020 doi: 10.1016/j.jmii.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ludvigsson J.F. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020 doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahase E. Covid-19: concerns grow over inflammatory syndrome emerging in children. BMJ. 2020;369:m1710. doi: 10.1136/bmj.m1710. [DOI] [PubMed] [Google Scholar]
- 41.Banerjee A.K.B., F, Ray U. Mutation hot spots in spike protein of COVID-19. Preprints. 2020 doi: 10.20944/preprints202004.0281.v1).). 2020040281. [DOI] [Google Scholar]
- 42.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181(2):281–292. doi: 10.1016/j.cell.2020.02.058. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shereen M.A., Khan S., Kazmi A., Bashir N., Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu Y., Xu X., Chen Z., Duan J., Hashimoto K., Yang L., Liu C., Yang C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020 doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geng Y.-J., Wei Z.-Y., Qian H.-Y., Huang J., Lodato R., Castriotta R.J. Pathophysiological characteristics and therapeutic approaches for pulmonary injury and cardiovascular complications of coronavirus disease 2019. Cardiovasc. Pathol. 2020 doi: 10.1016/j.carpath.2020.107228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bai Y., Yao L., Wei T., Tian F., Jin D.Y., Chen L., Wang M. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang J., Du Y., Bai L., Pu J., Jin C., Yang J., Guo Y. An asymptomatic patient infected with coronavirus disease 2019. Am. J. Respir. Crit. Care Med. 2020 doi: 10.1164/rccm.202002-0241IM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu F., Xu A., Zhang Y., Xuan W., Yan T., Pan K., Yu W., Zhang J. Patients of COVID-19 may benefit from sustained lopinavir-combined regimen and the increase of eosinophil may predict the outcome of COVID-19 progression. Int. J. Infect. Dis. 2020 doi: 10.1016/j.ijid.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Y., Du X., Chen J., Jin Y., Peng L., Wang H.H.X., Luo M., Chen L., Zhao Y. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J. Infect. 2020 doi: 10.1016/j.jinf.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang C., Cai J., Chen R., Shi Z., Bian X., Xie J., Zhao L., Fei X., Zhang H., Tan Y. 2020. Aveolar Macrophage Activation and Cytokine Storm in the Pathogenesis of Severe COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang C., Wang X.M., Li S.R., Twelkmeyer T., Wang W.H., Zhang S.Y., Wang S.F., Chen J.Z., Jin X., Wu Y.Z., Chen X.W., Wang S.D., Niu J.Q., Chen H.R., Tang H. NKG2A is a NK cell exhaustion checkpoint for HCV persistence. Nat. Commun. 2019;10(1):1507. doi: 10.1038/s41467-019-09212-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng M., Gao Y., Wang G., Song G., Liu S., Sun D., Xu Y., Tian Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020 doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian D.S. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mosser D.M., Zhang X. Interleukin-10: new perspectives on an old cytokine. Immunol. Rev. 2008;226:205–218. doi: 10.1111/j.1600-065X.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trandem K., Jin Q., Weiss K.A., James B.R., Zhao J., Perlman S. Virally expressed interleukin-10 ameliorates acute encephalomyelitis and chronic demyelination in coronavirus-infected mice. J. Virol. 2011;85(14):6822–6831. doi: 10.1128/JVI.00510-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pedersen S.F., Ho Y.C. SARS-CoV-2: a storm is raging. J. Clin. Invest. 2020 doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leng Z., Zhu R., Hou W., Feng Y., Yang Y., Han Q., Shan G., Meng F., Du D., Wang S., Fan J., Wang W., Deng L., Shi H., Li H., Hu Z., Zhang F., Gao J., Liu H., Li X., Zhao Y., Yin K., He X., Gao Z., Wang Y., Yang B., Jin R., Stambler I., Lim L.W., Su H., Moskalev A., Cano A., Chakrabarti S., Min K.J., Ellison-Hughes G., Caruso C., Jin K., Zhao R.C. Transplantation of ACE2(-) mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11(2):216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li L., Huang Q., Wang D.C., Ingbar D.H., Wang X. Acute lung injury in patients with COVID‐19 infection. Clin. Transl. Med. 2020 doi: 10.1002/ctm2.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luo W., Yu H., Gou J., Li X., Sun Y., Li J., Liu L. Clinical pathology of critical patient with novel coronavirus pneumonia (COVID-19) Pathology & Pathobiology. 2020 2020020407. [Google Scholar]
- 60.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T., Honore S., Colson P., Chabriere E., La Scola B., Rolain J.M., Brouqui P., Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Cao X. COVID-19: immunopathology and its implications for therapy. Nat. Rev. Immunol. 2020 doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang C., Li W., Drabek D., Okba N.M.A., van Haperen R., Osterhaus A., van Kuppeveld F.J.M., Haagmans B.L., Grosveld F., Bosch B.J. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat. Commun. 2020;11(1):2251. doi: 10.1038/s41467-020-16256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J., Wang F., Li D., Yang M., Xing L., Wei J., Xiao H., Yang Y., Qu J., Qing L., Chen L., Xu Z., Peng L., Li Y., Zheng H., Chen F., Huang K., Jiang Y., Liu D., Zhang Z., Liu Y., Liu L. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020 doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Duan K., Liu B., Li C., Zhang H., Yu T., Qu J., Zhou M., Chen L., Meng S., Hu Y., Peng C., Yuan M., Huang J., Wang Z., Yu J., Gao X., Wang D., Yu X., Li L., Zhang J., Wu X., Li B., Xu Y., Chen W., Peng Y., Hu Y., Lin L., Liu X., Huang S., Zhou Z., Zhang L., Wang Y., Zhang Z., Deng K., Xia Z., Gong Q., Zhang W., Zheng X., Liu Y., Yang H., Zhou D., Yu D., Hou J., Shi Z., Chen S., Chen Z., Zhang X., Yang X. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117(17):9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saul A.W. Nutritional treatment of coronavirus. Orthomolecular Medicine News Service. 2020;16(6):22. [Google Scholar]
- 66.Alipio M. 2020. Vitamin D Supplementation Could Possibly Improve Clinical Outcomes of Patients Infected With Coronavirus-2019 (COVID-2019) Available at SSRN 3571484. [Google Scholar]
- 67.Zhang L., Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J. Med. Virol. 2020;92(5):479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.