Abstract
Background
COVID‐19 cutaneous manifestations have been recently described and classified in five different clinical patterns, including acral erythema‐edema (pseudo‐chilblain), maculopapular exanthemas, vesicular eruptions, urticarial lesions, and livedo or necrosis.
Objectives
The objective of this study was to examine the skin of hospitalized patients with a confirmed diagnosis of COVID‐19 disease and describe the real prevalence of skin manifestations.
Methods
A cross‐sectional study, which included hospitalized patients in Cruces University Hospital from April 14–30, 2020, with a laboratory‐confirmed diagnosis of COVID‐19 (with polymerase chain reaction and/or serology tests), was conducted. Entire body surface examination was performed by experienced dermatologists to search for cutaneous manifestations related to COVID‐19 disease.
Results
From a sample of 75 patients, 14 (18.7%) developed cutaneous manifestations possibly related to COVID‐19. We found six patients with acral erythema‐edema (pseudo‐chilblain) (42.8%), four patients with maculopapular exanthemas (28.6%), two patients with urticarial lesions (14.3%), one patient with livedo reticularis‐like lesions (7.15%), and one patient with vesicular eruption (7.15%).
Conclusions
Our study provides a more plausible relationship between the main cutaneous patterns and COVID‐19 in hospitalized patients as all of them had a confirmatory laboratory test. Skin manifestations are frequent but mild with spontaneous resolution. These findings are nonspecific and can be similar to other viral infections and adverse drug reactions in hospitalized patients.
Introduction
On January 7, 2020, a novel coronavirus, SARS‐CoV‐2, was isolated in patients with pneumonia in Wuhan, China. 1 This virus spreads rapidly, and in March 2020, a pandemic was declared. The first clinical findings associated with this virus were respiratory symptoms and fever. Later, diarrhea, anosmia, and ageusia were included. 2 , 3 It was not until March 26 that Recalcati's article 4 analyzed skin manifestations in COVID‐19 patients for the first time. Since this date, a series of cases began to emerge in Spanish hospitals, and similar case series were reported. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 For this reason, a nationwide study was conducted in Spain to describe the cutaneous manifestation patterns of COVID‐19 disease. 17 Most of these articles include confirmed and suspected COVID‐19 patients seen in an outpatient clinic and are restricted to patients with cutaneous manifestations.
We decided to perform this study to describe the real prevalence and directly examine the skin findings in hospitalized patients in our institution with a confirmed diagnosis of COVID‐19 disease.
Materials and methods
We performed a cross‐sectional study, which included COVID‐19 patients in Cruces University Hospital, Bizkaia, Spain, from April 14–30, 2020.
Inclusion criteria were patients admitted to the hospital with a laboratory‐confirmed diagnosis of COVID‐19 (with polymerase chain reaction and/or serology tests). All the patients gave their oral informed consent to participate in the study. Patients admitted to the Intensive Care Units were excluded due to the difficulties to obtain their informed consent. The final size of the sample was 75 patients.
Examination of the entire body surface was performed by experienced dermatologists to search for cutaneous manifestations possibly related to COVID‐19 disease. We recorded age, gender, treatment during admission, the presence of cutaneous manifestations and whether they appeared during prodromal (one week prior to the first clinical symptoms), illness or decline periods, their type and location, and the occurrence of familial cases.
Based on recent reports 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 and on the Spanish group classification in clinical patterns, 17 we included acral erythema‐edema (pseudo‐chilblain), maculopapular exanthemas, vesicular eruptions, urticarial lesions, and livedo or necrosis areas.
To examine the patients, we entered their rooms using personal protective equipment (PPE) and transparent ziplock bags to protect photographic devices along with dispensable biopsy instruments, as performed in other hospitals. 9
Results
We recruited a total of 75 patients admitted to the hospital because of clinical manifestations of COVID‐19 disease. The mean age of the sample was 67.5 years old (CI 95 64.5–70.5), and 64% (CI 95 53.1–74.9%) were men (Table 1).
Table 1.
Demographic characteristics and skin manifestations of patients with confirmed COVID‐19 disease in hospital setting
| Patient demographics | |||
| Age | Mean (CI 95%) | ||
| Years | 67.5 (64.5–70.5) | ||
| Sex | % (CI 95%) | ||
| Male | 64 (53.1–74.9) | ||
| Treatment | No (%) | Plus antibiotherapy | Without antibiotherapy |
| Hydroxychloroquine | 7 (9.3) | 6 | 1 |
| Hydroxychloroquine + lopinavir/ritonavir | 19 (25.3) | 10 | 9 |
| Hydroxychloroquine + lopinavir/ritonavir + methylprednisolone | 32 (42.7) | 23 | 9 |
| Hydroxychloroquine + methylprednisolone | 4 (5.3) | 3 | 1 |
| Methylprednisolone | 3 (4.0) | 0 | 3 |
| Antibiotherapy | 5 (6.7) | ||
| Supportive treatment | 5 (6.7) | ||
| Previous ICU stay, no (%) | 15 (20) | ||
| Results | |||
| Skin lesions | No (%) | ||
| No | 61 (81.3) | ||
| Yes | 14 (18.7) | ||
| Confirmed | 12 (16) | ||
| Self‐reported | 2 (2.7) | ||
| Lesion type | No | % Total lesions (n = 14) | % Total patients (n = 75) |
| Acral erythema‐edema | 6 | 42.9 | 8 |
| Rash | 4 | 28.6 | 5.3 |
| Urticarial | 2 | 14.3 | 2.7 |
| Vesicular | 1 | 7.1 | 1.3 |
| Livedoid | 1 | 7.1 | 1.3 |
| Median time from onset after COVID‐19 symptoms | Days | ||
| Any lesion | 6 | ||
| Acral erythema‐edema | ‐ | ||
| Rash | 20 | ||
| Urticarial | 1.5 | ||
| Vesicular | 4 | ||
| Livedoid | 2 | ||
We collected data of prescribed treatments during their hospital stay. The most frequently used therapy was a combination of hydroxychloroquine, lopinavir/ritonavir, and methylprednisolone pulses (42.7%) followed by double therapy of hydroxychloroquine and lopinavir/ritonavir (25.3%).
From the 75 patients, 14 (18.7%) developed cutaneous manifestations that could be attributed to COVID‐19. Twelve of the lesions were directly observed by the dermatologist, and two patients referred past lesions, not present during clinical evaluation. Pictures of all the cutaneous lesions were taken.
According to the recent classification in clinical patterns of cutaneous manifestations of COVID‐19 disease, 17 we found six patients with areas of acral erythema‐edema (pseudo‐chilblain), which represents 42.8% of the cases (Fig. 1); four patients with maculopapular exanthemas (28.6% of the cases) (Fig. 2a,b); two patients with urticarial lesions; one patient with livedo reticularis‐like lesions; and one patient with vesicular eruption.
Figure 1.
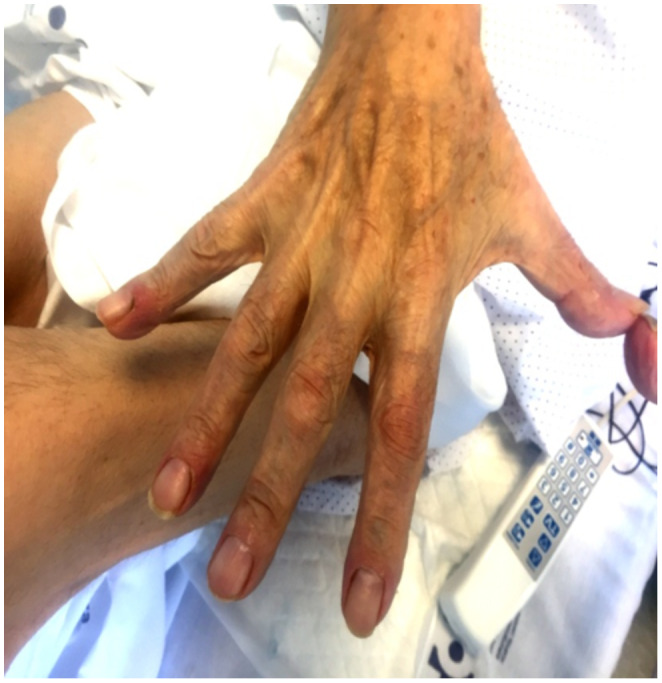
Clinical features of COVID‐19 positive patient with skin vascular symptoms. An 82‐year‐old male with pseudo‐chilblain pattern on distal fingers of the hand beginning 9 days before respiratory symptoms and lasting a duration of 2 weeks
Figure 2.
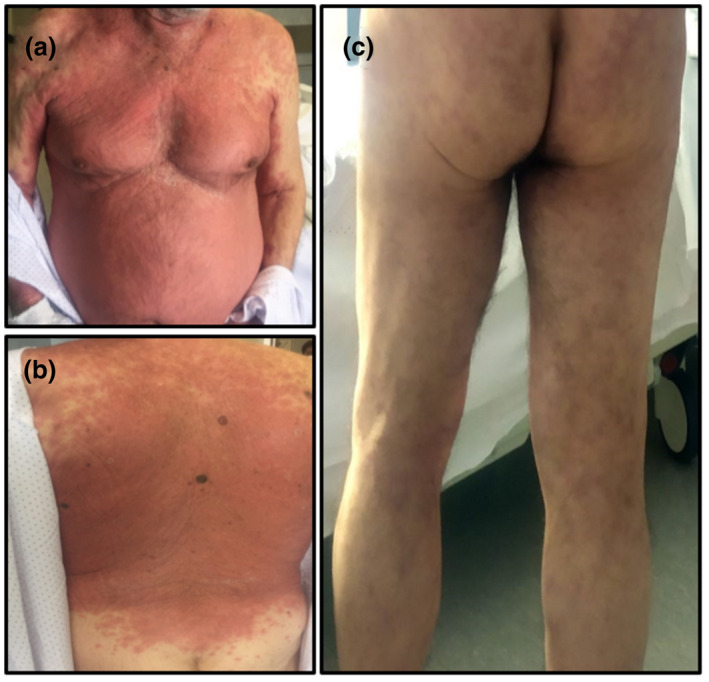
Clinical images (a) and (b) of widespread erythematous exanthema with predilection for the trunk in an 84‐year‐old man. The exanthema began 2 weeks after the onset of respiratory symptoms with spontaneous resolution after 15 days. (c) A 71‐year‐old man with livedo reticularis‐like lesions on both legs and buttocks
Acral erythema‐edema lesions were mainly observed in feet. These lesions were very subtle and asymptomatic, so they were unnoticed by patients. For this reason, the median time of onset since the beginning of respiratory symptoms is unknown. The maculopapular exanthemas showed predilection for trunk and proximal extremities (80%), and there was also one case of flexural involvement (20%). Some patients referred mild pruritus. The median time of onset of the rash was 17 days (range 15–28 days) from the first symptoms of COVID‐19. The patient with the vesicular pattern had lesions over the trunk (Fig. 3). A sample from one vesicle liquid was taken for PCR of COVID‐19, herpes simplex, and varicella zoster, which were all negative. The vesicles appeared 4 days after the onset of respiratory symptoms. One of the patients with urticarial pattern had a lumbar raised rash one week prior to COVID‐19 clinical findings, and the other had urticarial lesions on the arms, legs, and face 10 days after the symptoms. Finally, there was one patient with livedoid pattern on the trunk and extremities (Fig. 2c). Additionally, we asked all patients about the existence of family members with cutaneous lesions with the above‐mentioned patterns, and this was negative for all of them.
Figure 3.
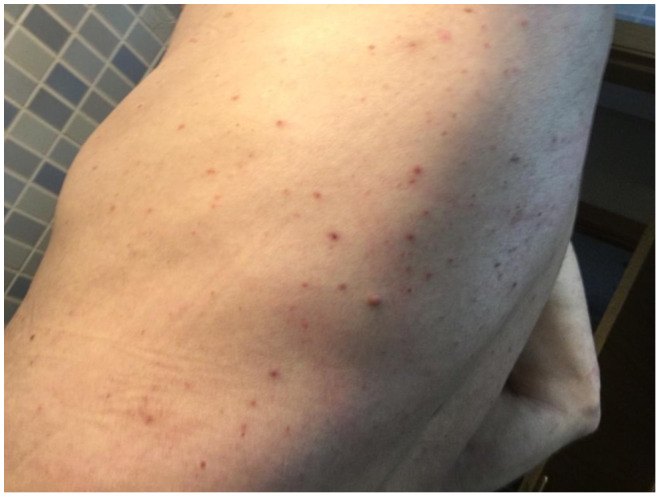
Vesicular pattern on the trunk of an 86‐year‐old male. The vesicles appeared 4 days after the onset of respiratory disease. A sample of one of the vesicle's fluid was taken for PCR of COVID‐19, herpes simplex, and varicella zoster virus with a negative result
Discussion
Analyzing the collected demographic data, the mean age in our sample is 67.5 years old, and 64% are men. Compared with previous studies, 9 , 17 our study includes older patients and a higher proportion of males. This can be explained because only hospitalized patients with laboratory confirmed SARS‐CoV‐2 were considered. As described in the existing literature, 18 , 19 elderly men suffering from COVID‐19 are more frequently hospitalized compared to women and young people. In this way, we aimed to achieve a representative sample of the profile of COVID‐19 hospitalized patients.
To our knowledge, this is the first series in which data collection has been directly performed by experienced dermatologists and with a systematic cutaneous exploration of all patients. All skin findings can be included in the recently described clinical patterns of cutaneous manifestations of COVID‐19 disease. 17 Results show a similar percentage of skin manifestations to other case series. 4 In our sample, the most frequently observed cutaneous pattern was acral erythema‐edema (pseudo‐chilblain), and the second in frequency was the maculopapular exanthema.
One of the latest studies 17 reported the pseudo‐chilblain pattern appeared in young patients, later in the course of the disease, was associated with less severe infection, and included patients who did not require hospitalization. As mentioned above, we carried out an exhaustive dermatological exploration on all admitted patients. For this reason, we may have included some lesions as pseudo‐chilblain patterns that could be explained by other causes or which were not exactly manifestations but rather a variety of normal skin conditions. Therefore, we may have overestimated the number of patients with acral erythema‐edema.
We would like to highlight that all the skin manifestations observed, regardless of the clinical pattern, were mild, did not affect mucous membranes, and did not cause epidermal detachment. In addition, these lesions did not require any specific treatment nor did they change the course, management, prognosis, or hospitalization days of the patients as they resolved spontaneously.
As described in other studies, 17 it would appear that each clinical dermatological pattern has a time of onset in the disease: vesicular lesions precede other symptoms, urticarial and maculopapular lesions concur with respiratory symptoms, and pseudo‐chilblain pattern appears later in the course of the disease. Given our low number of patients with skin findings, we are not able to make any conclusion on this area.
Galván et al. 17 referred there might be a correlation between the clinical patterns and the severity of the disease. In our study, we did not correlate the severity of the COVID‐19 disease with the different skin patterns because all of the patients we included had very similar clinical characteristics of COVID‐19 (moderate–severe infection that required hospitalization).
We are aware that our study has its limitations. Firstly, the limitations are inherent to a cross‐sectional study; it does not allow a monitoring over time; and it cannot determine cause and effect relationship. Secondly, we could not analyze other possible or alternative causes of skin manifestations such as medications. Finally, we are not able to correlate skin patterns with the time of onset of the disease and severity of the infection. Well‐designed prospective studies are needed to explore the cause–effect relationship of these skin manifestations.
Conclusions
This study provides a more plausible relationship between COVID‐19 disease and its main cutaneous patterns in hospitalized patients, since all of them had a confirmatory laboratory test. It seems important to emphasize that skin manifestations in this subgroup of patients are frequent but mild in nature and in all cases with spontaneous resolution. Furthermore, they are nonspecific because they are similar to other viral infections and drug reactions observed in patients admitted to the hospital.
Acknowledgments
The patients in this manuscript have given oral informed consent to publication of their case details. The authors did not receive any funding sources.
Conflict of interest: None.
Funding source: None.
The work is in accordance to ethical standards.
The work ensures patient privacy; there are no data or pictures that could identify patients. All patients gave their oral informed consent for skin examination and a posterior analysis of results.
References
- 1. Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA 2020; 323: 709–710. [DOI] [PubMed] [Google Scholar]
- 2. European Centre for Disease Prevention and Control . Case definition and European surveillance for COVID‐19, as of 2 March 2020. 2020. https://www.ecdc.europa.eu/en/case‐definition‐and‐european‐surveillance‐humaninfection‐novel‐coronavirus‐2019‐ncov. Last accessed 9th May 2020.
- 3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective. J Eur Acad Dermatol Venereol 2020; 34: e212–e213. [DOI] [PubMed] [Google Scholar]
- 5. Landa N, Mendieta‐Eckert M, Fonda‐Pascual P, et al. Chilblain‐like lesions on feet and hands during the COVID‐19 Pandemic. Int J Dermatol 2020; 59: 739–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ahouach B, Harant S, Ullmer A, et al. Cutaneous lesions in a patient with COVID‐19: are they related? Br J Dermatol 2020; 183: e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hedou M, Carsuzaa F, Chary E, et al. Comment on "Cutaneous manifestations in COVID‐19: a first perspective " by Recalcati S. J Eur Acad Dermatol Venereol 2020; 34: e299–e300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Estébanez A, Pérez‐Santiago L, Silva E, et al. Cutaneous manifestations in COVID‐19: a new contribution. J Eur Acad Dermatol Venereol 2020; 34: e250–e251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fernandez‐Nieto D, Ortega‐Quijano D, Segurado‐Miravalles G, et al. Comment on: cutaneous manifestations in COVID‐19: a first perspective. Safety concerns of clinical images and skin biopsies. J Eur Acad Dermatol Venereol 2020; 34: e252–e254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Henry D, Ackerman M, Sancelme E, et al. Urticarial eruption in COVID‐19 infection. J Eur Acad Dermatol Venereol 2020; 34: e244–e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Recalcati S, Barbagallo T, Frasin LA, et al. Acral cutaneous lesions in the time of COVID‐19. J Eur Acad Dermatol Venereol 2020; 34: e346–e347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Van Damme C, Berlingin E, Saussez S, et al. Acute urticaria with pyrexia as the first manifestations of a COVID‐19 infection. J Eur Acad Dermatol Venereol 2020; 34: e300–e301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fernandez‐Nieto D, Jimenez‐Cauhe J, Suarez‐Valle A, et al. Characterization of acute acro‐ischemic lesions in non‐hospitalized patients: a case series of 132 patients during the COVID‐19 outbreak. J Am Acad Dermatol 2020; 83: e61–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alramthan A, Aldaraji W. A case of COVID‐19 presenting in clinical picture resembling chilblains disease. First report from the Middle East. Clin Exp Dermatol 2020; 45: 746–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Su CJ, Lee CH. Viral exanthem in COVID‐19, a clinical enigma with biological significance. J Eur Acad Dermatol Venereol 2020; 34: e251–e252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bouaziz JD, Duong T, Jachiet M, et al. Vascular skin symptoms in COVID‐19: a French observational study. J Eur Acad Dermatol Venereol 2020; 34: e451–e452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol 2020; 183: 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li LQ, Huang T, Wang YQ, et al. COVID‐19 patients' clinical characteristics, discharge rate, and fatality rate of meta‐analysis. J Med Virol 2020; 92: 577–583. [DOI] [PMC free article] [PubMed] [Google Scholar]


