Case fatality rates (CFR) of the novel coronavirus disease 2019 (COVID‐19) have been reported ranging from 0% to 40% among patients with Parkinson's disease (PD). However, because of small sample sizes and the lack of large matched comparison groups, 1 , 2 , 3 , 4 previous studies have not clarified whether PD is an independent risk factor for death. The goal of our study was to determine whether patients with PD had a higher COVID‐19 CFR.
We compared COVID‐19 CFR in patients with PD with a large, demographically matched population via the TriNetX COVID‐19 research network, a health research database with deidentified medical records of >50 million patients mostly from the United States. As of July 15, 2020, this database listed 79,049 adult patients with COVID‐19, 694 of whom had PD. On September 9, 2020, we extracted mortality data for this cohort. We included an 8‐week delay to allow identified cases to resolve. Among 78,355 patients with COVID‐19 without PD, 4290 died compared with 148 of the 694 patients with PD (5.5% non‐PD vs. 21.3% PD; P < 0.001, χ2 test).
The non‐PD and PD groups had different age distributions (median age 50 vs. 78), sex balances (female 55.3% vs. 39.8%), and racial compositions (Black 19.7% vs. 9.7%). In addition, CFRs from COVID‐19 have been reported as higher in males versus females, 5 Blacks versus Whites, 6 and elderly versus younger patients. 5
We accounted for these differences using logistic regression with age, sex, and race as covariates. This analysis revealed that the risk of dying from COVID‐19 was significantly elevated in the PD group (odds ratio, 1.27; 95% confidence interval, 1.04–1.53; P = 0.016; Fig. 1A,B).
FIG. 1.
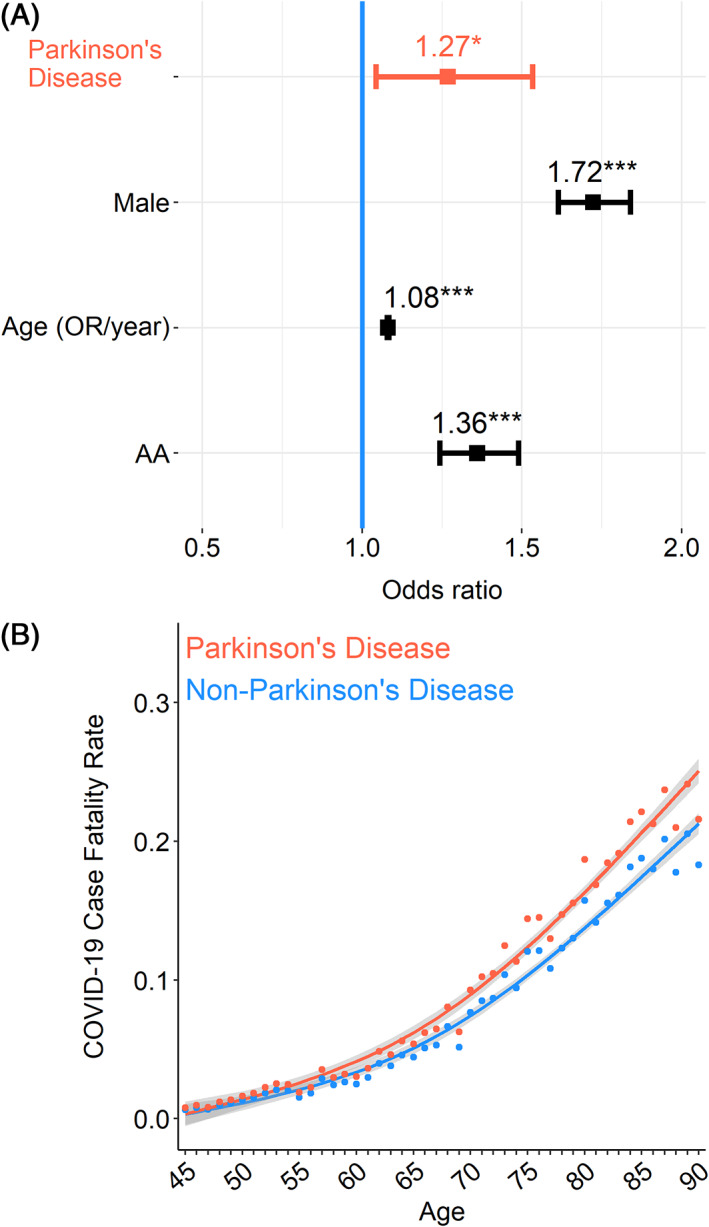
Patients with coronavirus disease 2019 (COVID‐19) with Parkinson's disease (PD) have an increased case fatality rate. (A) Patients with COVID‐19 with PD (n = 694) have an increased case fatality rate compared with those without PD (n = 78,355; odds ratio [OR], 1.27; 95% confidence interval, 1.04–1.53; P = 0.016). Logistic regression was performed with age, sex, and race included as covariates. Data from 79,049 patients with COVID‐19 in the TriNetX COVID‐19 research network. Each dot represents the model fit of each year for patients with PD and those without PD. (B) The COVID‐19 case fatality rate was higher across age groups >50 years.
To assess residual confounders, we matched 5 patients with COVID‐19 without PD to each patient with PD with the exact age, sex, and race. We then performed a conditional logistic regression and found that patients with PD had a significantly higher risk of dying from COVID‐19 compared with patients without PD (odds ratio, 1.30; 95% confidence interval, 1.13–1.49; P < 0.001). We further replicated the analysis with 1000 random matchings and found similar results with the effect being statistically significant in all but 2 replications.
In summary, we leveraged the TriNetX database and found that COVID‐19‐related CFR was increased in patients with PD, independent of age, sex, and race. These results are not without limitations. First, the TriNetX COVID‐19 research network includes >40 healthcare organizations primarily in the United States. We were unable to account for confounding regional factors that could increase mortality. In addition, this database lacks information on key comorbidities. Second, CFRs from COVID‐19 have decreased with increased access to testing. It will be important to conduct follow‐up studies. Third, this study only reports an association between COVID‐19‐related mortality and a diagnosis of PD; however, in this context, designs better suited to causal inference are challenging. Finally, the TriNetX database does not include information on recovery. Despite these limitations, our results indicate that it will be critical to develop effective strategies whereby healthcare providers can prevent the transmission of COVID‐19 while providing neurological care to patients with PD.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Manuscript: A. Writing of the First Draft, B. Review and Critique.
Q.Z.: 1A, 1C, 2A
J.L.S.: 1C, 2A
G.M.A.: 2A
J.E.S.: 1C, 2A
N.S.N.: 1A, 2A
Acknowledgments
The authors are grateful to Dr. George B. Richerson for his critical reading of the manuscript. Dr. Patrick Ten Eyck (Biostatistics, Epidemiology, and Research Design Core, Institute for Clinical and Translational Science at the University of Iowa) has provided insightful recommendations to the statistical analysis. Q.Z. is supported by NIH/NINDS R25 NS079173, the NIH/NINDS NeuroNext Fellowship, and the physician scientist training program at University of Iowa. Q.Z. is a trainee of the University of Iowa Clinical Neuroscientist Training Program (CNS‐TP). N.N. is supported by NIH R01 NS100849‐A1. G.M.A. is supported by NIH K08 NS109287 and the Iowa Neuroscience Institute. J.L.S. is supported by NIH KL2TR002536.
Relevant conflicts of interests/financial disclosures: Nothing to report.
References
- 1. Antonini A, Leta V, Teo J, Chaudhuri KR. Outcome of Parkinson's disease patients affected by COVID‐19. Mov Disord 2020;35:905–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cilia R, Bonvegna S, Straccia G, et al. Effects of COVID‐19 on Parkinson's disease clinical features: a community‐based case‐control study. Mov Disord 2020;35:1287–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fasano A, Cereda E, Barichella M, et al. COVID‐19 in Parkinson's disease patients living in Lombardy, Italy. Mov Disord 2020;35:1089–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fasano A, Elia AE, Dallocchio C, et al. Predictors of COVID‐19 outcome in Parkinson's disease. Parkinsonism Relat Disord 2020;78:134–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention . The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID‐19) in China. Zhonghua Liu, Xing Bing, Xue Za Zhi 2020;41:145–151. [DOI] [PubMed] [Google Scholar]
- 6. Ferdinand KC, Nasser SA. African‐American COVID‐19 mortality: a sentinel event. J Am Coll Cardiol 2020;75:2746–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]


