Abstract
While the number of coronavirus disease‐2019 (COVID‐19) cases is increasing day by day, there is limited information known about the hematological and laboratory findings of the disease. We aimed to investigate whether serum ferritin level predicts mortality is a marker for rapid progression for inpatients. Our study included 56 patients who were died due to COVID‐19 as the study group, and 245 patients who were hospitalized and recovered as the control group. The laboratory data of the patients were evaluated from the first blood tests (pre) taken from the first moment of admission to the hospital and the blood tests taken from before the patient's discharge or exitus (post) were evaluated retrospectively. The mean age of the nonsurvivor group was 62.0 ± 15.7 and the mean age of the control group was 54.34 ± 13.03. Age and length of stay are significantly higher in the nonsurvivor group. When comparing the pre‐ and postvalues of ferritin, according to the two groups separately, there was no significant difference in the control group and a high level of significance was observed in the nonsurvivor group (p < .01). COVID‐19 disease caused by severe acute respiratory syndrome coronavirus‐2 causes high mortality with widespread inflammation and cytokine storm. Ferritin is a cheap and widespread available marker, ferritin, which can be used for its predictivity of the mortality and hope it would be a useful marker for clinicians for the management of the disease.
Keywords: coronavirus, cytokine/chemokine, pandemics, immune system
1. INTRODUCTION
The new severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), defined for the first time in China at the end of 2019, rapidly affected the world and caused a pandemic. The disease identified as COVID‐19 can cause fatal acute respiratory distress syndrome (ARDS) with its severe course by severe pneumonia. 1 About 367,166 people died from the beginning of the pandemic by the end of May 2020 due to COVID‐19. 2
While the number of cases is increasing day by day, there is limited information known about the hematological and laboratory findings of the disease. 3 , 4 , 5 It is managed in light of clinical classification from simple pneumonia to severe respiratory failure recommended by World Health Organization and the scientific committee in our country. 6 COVID‐19 patients defined by the following criteria: (a) epidemiology history, (b) fever or other respiratory symptoms which are undefined by any condition, (c) typical CT image abnormities of viral pneumonia, (d) severe acute respiratory infections, and (e) positive result of reverse transcription polymerase chain reaction (RT‐PCR) for SARS‐CoV‐2 RNA.
While the disease may be asymptomatic, it can be encountered with severe ARDS, which is thought to be due to an inflammatory cytokine storm. Activation of the monocyte‐macrophage system is existing just before the disease produces pneumonia. In this period, an increase in ferritin levels is observed in COVID‐19 patients, along with an increase in many laboratory parameters such as d‐dimer and fibrinogen. 7 , 8 , 9
In this study, we aimed to investigate whether serum ferritin level predicts mortality, is a marker for rapid progression for inpatients and has got a cutoff level to forecast the negative affect to the treatment of the patient diagnosed with COVID‐19.
2. MATERIALS AND METHODS
Three hundred and one patients who were hospitalized for COVID‐19 according to who interim guidance to our education and research hospital, which was designated as Pandemic hospital between 11 March–20 April 2020, were retrospectively analyzed. Our study included 56 patients who were died due to COVID‐19 as the study group, and 245 patients who were hospitalized and recovered as the control group. This study was approved by the Clinical Research Ethics Committee of the Health Sciences University Istanbul Training and Research Hospital, the scientific board of our hospital and the Turkish Republic, the Ministry of Health General Directorate of Health Services.
Demographic data, medical history, laboratory findings, and thorax tomography of the patients were obtained from the hospital electronic information system retrospectively. The laboratory data of the patients were evaluated from the first blood tests (pre) taken from the first moment of admission to the hospital and the blood tests taken from before the patient's discharge or exitus (post) were evaluated. The hemogram data of the patients were studied in the biochemistry laboratory with the Beckman Coulter AU2700 device with the biochemical tests with the Sysmex XT4000i device. All patient data were double‐checked and analyzed by the research team.
PCR tests were performed in our hospital and Istanbul Cerrahpaşa Medical Faculty microbiology laboratory, which were both validated as Sars‐CoV‐2 laboratory. Swap samples were taken from the upper respiratory tract (naso‐oropharynx) when the patient was admitted to the hospital and transported via viral‐transport medium to the laboratory. All RNA extraction was obtained with the Genmark RNA Isolation Kit (Genome) previously described for the SARS‐CoV‐2 duration in 2 h. 10
2.1. Statistical methods
All statistical analyses were performed using the SPSS 21.0 software. Categorical variables were expressed as frequency rates and percentages (%), and continuous variables were expressed as median (interquartile range). Categorical variables between groups were compared using the χ 2 test or Fisher exact test. Kolmogorov–Smirnov test was used to check the normal distribution for continuous data. As the normal distribution hypothesis is not provided, the Mann–Whitney U test was conducted for the analysis of the differences between control and death groups. The pre‐ and postvalues of ferritin were compared with the Wilcoxon test. In addition, logistic regression was made after determining the dependent variable as being dead or alive due to COVID‐19 and independent variables as age, length of stay, and favipiravir. Furthermore, receiver operating characteristic (ROC) analysis was used to evaluate the cutoff value of ferritin. A p value of <.05 was considered statistically significant.
3. RESULTS
The demographic characteristics of our patients are summarized in Table 1, from the 301 patients, 56 (18.6%) of them died during the study. The mean age of the nonsurvivor group was 62.0 ± 15.7 and the mean age of the control group was 54.34 ± 13.03, and the nonsurvivor group was significantly older than the control group (p < .01). There was no significant difference in male/female ratio in the nonsurvivor group compared to the control group (p > .05).
Table 1.
Demographics and baseline characteristics of control and nonsurvivor groups
| Variables | No. (%) | |||
|---|---|---|---|---|
| Total (n = 301) | Control (n = 245) | Nonsurvivor (n = 56) | p | |
| Age, median (IQR) | 57 (18) | 55 (18) | 62 (23) | <.01 |
| Length of stay, median (IQR) | 8 (5) | 8 (4) | 11.5 (11) | <.01 |
| Gender | ||||
| Male | 206 (68.4) | 170 (69.4) | 36 (64.3) | .49 |
| Female | 95 (31.6) | 75 (30.6) | 20 (35.7) | |
| Comorbidities | ||||
| COPD | 38 (12.6) | 31 (12.7) | 7 (12.5) | .97 |
| DM | 9 (3.0) | 7 (2.9) | 2 (3.6) | .67 |
| HT | 43 (14.3) | 35 (14.3) | 8 (14.3) | 1.0 |
| CVD | 19 (6.3) | 11 (4.5) | 8 (14.3) | .01 |
| Lung cancer | 11 (3.7) | 7 (2.9) | 4 (7.1) | .12 |
| Favipiravir | 63 (20.9) | 19 (7.8) | 44 (78.6) | <.01 |
Abbreviations: COPD, chronic obstructive lung disease; CT, computed tomography; CVD, cardiovascular disease; DM, diabetes mellitus; HT, hypertension; IQR, interquartile range.
As can be seen in Table 1, age, length of stay, cardiovascular disease (CVD), and number of patients taken favipiravir treatment were statistically significant in the control and nonsurvivor group comparisons. Age and length of stay are significantly higher in the nonsurvivor group. In addition, when looking at categorical variables, the rates of CVD and number of patients taken favipiravir treatment were found significantly higher in the nonsurvivor group. While comorbidity was present in 39.8% of the patients, only CVD was found to be significantly higher as comorbidity in the exitus group.
When Table 2 is examined, the comparison of two groups of laboratory parameters, Ferritin (pre), ferritin (post), tropinin I (pre), tropinin I (post), d‐dimer (pre), d‐dimer (post), WBC (pre), WBC (post), HGB (post), HTC (post), LY (pre), LY (post), LY% (pre), LY%, procalcitonin (pre), procalcitonin (post), albumin (pre), albumin (post), LDH (pre), LDH (post), AST (pre), AST (post), ALT (post), CRP (pre), and CRP (post) were found statistically significant.
Table 2.
Comparison of laboratory parameters between control and nonsurvivor groups
| Median (IQR) | ||||
|---|---|---|---|---|
| Total (n = 301) | Control (n = 245) | Nonsurvivor (n = 56) | p | |
| Ferritin (pre) ng/ml | 262.5 (236.4) | 233.2 (186.6) | 451.25 (466.2) | <.01 |
| Ferritin (post) ng/ml | 249 (273.6) | 224.4 (165.3) | 1145.75 (1139.7) | <.01 |
| Fibribojen (pre) mg/dl | 481.3 (173.9) | 476.1 (162.5) | 509.4 (250.9) | .33 |
| Fibrinojen (post) mg/dl | 448.3 (180.8) | 448 (174.4) | 455.3 (255.1) | .72 |
| Tropinin I (pre) ng/L | 4.6 (5.55) | 3.8 (4.15) | 13.85 (39.8) | <.01 |
| Tropinin I (post) ng/L | 3.4 (8.85) | 2.9 (2.65) | 96.15 (291.15) | <.01 |
| d‐Dimer (pre) mg/L | 0.68 (0.73) | 0.615 (0.57) | 1.1 (1.31) | <.01 |
| d‐Dimer (post) mg/L | 0.79 (1.27) | 0.7 (0.6) | 4.6 (10.425) | <.01 |
| WBC (pre) ×10e3/μl | 7.03 (4.04) | 6.67 (3.01) | 14.43 (15.14) | <.01 |
| WBC (post) ×10e3/μl | 7.16 (4.89) | 7.07 (4.03) | 8.485 (8.75) | .04 |
| HGB (pre) g/dl | 13.6 (2.1) | 13.7 (2) | 13.25 (2.5) | .09 |
| HGB (post) g/dl | 12.6 (2.6) | 12.8 (2.1) | 10.75 (3.1) | <.01 |
| HCT (pre) % | 39.7 (5.5) | 40.2 (5.2) | 38.35 (7.2) | .07 |
| HTC (post) % | 37.8 (6.9) | 38.2 (6.3) | 33.9 (7.85) | <.01 |
| LY (pre) ×10e3/μl | 1.34 (0.88) | 1.39 (0.87) | 1.065 (0.64) | <.01 |
| LY (post) ×10e3/μl | 1.47 (0.81) | 1.61 (0.79) | 1.02 (0.98) | <.01 |
| LY% (pre) % | 19.1 (14.4) | 21.1 (14.3) | 14.35 (12.95) | <.01 |
| LY% (post) % | 23.2 (14) | 24.7 (12.1) | 7.1 (7.2) | <.01 |
| Procalcitonin (pre) ng/ml | 0.06 (0.105) | 0.05 (0.06) | 0.21 (0.95) | <.01 |
| Procalcitonin (post) ng/ml | 0.04 (0.08) | 0.04 (0.03) | 2.135 (7.44) | <.01 |
| Albumin (pre) g/L | 38.6 (7.1) | 39.4 (6.4) | 35.05 (6.0) | <.01 |
| Albumin (post) g/L | 35.4 (7.2) | 36.5 (5.2) | 24.5 (6.95) | <.01 |
| LDH (pre) U/L | 342.5 (199.0) | 318.0 (184) | 417.0 (209.0) | <.01 |
| LDH (post) U/L | 341.0 (247.0) | 314.0 (162.0) | 707.5 (800.0) | <.01 |
| AST (pre) U/L | 37.0 (22.0) | 36.5 (20.0) | 44.0 (57.5) | <.01 |
| AST (post) U/L | 40.0 (33.0) | 35.0 (22.0) | 92.5 (258.5) | <.01 |
| ALT (pre) U/L | 28.0 (23.0) | 28.0 (24.0) | 28.0 (22.0) | .78 |
| ALT (post) U/L | 42.0 (44.0) | 40.0 (43.0) | 56.5 (134.0) | <.01 |
| CRP (pre) mg/L | 66.9 (89.6) | 57.7 (82.9) | 126.4 (127.6) | <.01 |
| CRP (post) mg/L | 18.4 (56.7) | 13.4 (29.2) | 146.5 (148.1) | <.01 |
Abbreviations: ALT, alanine aminotransferase, U/L; AST, aspartate aminotransferase, U/L; CRP, C‐reactive protein, mg/L; IQR, interquartile range; HCT, hematocrit, %; HGB, hemoglobin, g/dL; LDH, lactate dehydrogenase; LY, lymphocyte count, ×10e3/μl; LY%, lymphocyte count %; WBC, white blood cell count, ×10e3/μl.
When these parameters are considered, the measurement values of ferritin (pre), ferritin (post), tropinin I (pre), tropinin I (post), d‐dimer (pre), d‐dimer (post), WBC (pre), WBC (post), procalcitonin (pre), procalcitonin (post), LDH (pre), LDH (post), AST (pre), AST (post), ALT (post), CRP (pre), CRP (post)are higher in the death group. HTC (post), LY (pre), LY (post), LY% (pre), LY% (post) values, albumin (pre), and albumin(post) are higher in the control group.
In Table 3, when comparing the pre‐ and postvalues of ferritin, according to the two groups separately, there was no significant difference in the control group and a high level of significance was observed in the nonsurvivor group. In the nonsurvivor group, post‐ferritin values are quite high compared to the pre‐ferritin.
Table 3.
Comparison pre‐ and post‐ferritin values among control and nonsurvivor groups
| Variables | Median (interquartile range) | |||
|---|---|---|---|---|
| Control (n = 245) | p | Nonsurvivor (n = 56) | p | |
| Ferritin (pre) ng/ml | 233.2 (186.6) | .67 | 451.25 (466.2) | <.01 |
| Ferritin (post) ng/ml | 224.4 (165.3) | 1145.75 (1139.7) | ||
A statistically significant difference was found in the comparison of the ferritin values of favipiravir users among the groups. Ferritin values are higher in the nonsurvivor group despite treatment (p < .01).
In Table 4, according to the results of logistic regression analysis, age, length of stay, CVD, lung cancer, and favipiravir are effective factors on the risk of death. However, when ORs are examined, this effect is low at the age and length of stay, and others are more effective in the risk of death.
Table 4.
Factors associated with COVID‐19 death using stepwise logistic regression analysis
| Independent variables | OR | 95% CI | p |
|---|---|---|---|
| Age | 1,085 | 1.041–1.130 | <.01 |
| Length of stay | 1,087 | 1.042–1.135 | <.01 |
| CVD | 15,331 | 3.394–69.272 | .01 |
| Lung cancer | 15,022 | 2.067–89.148 | .02 |
| Favipiravir | 43,613 | 19.713–96.251 | <.01 |
Abbreviations: CI, confidence interval; CVD, cardiovascular disease; OR, odds ratio.
According to ROC curve analysis results for ferritin, the cutoff value was 304.30 and the area under the curve was 0.762 (95% confidence interval: 0.690–0.835). Sensitivity and specificity values are 71.4% and 70.6%, respectively.
4. DISCUSSION
COVID‐19, caused by the new coronavirus SARS‐Cov‐2, is a new disease for humanity and also contains many unknowns. During the course of the disease, besides hematological abnormalities, variabilities are observed in many biochemical parameters. With the excessive activation of the immune system and monocyte‐macrophage system activation, damage occurs in many organs, especially the lungs. While variety in immunological response cannot be explained only by viral load, epidemiological and molecular differences play a role in the manifestation of the clinical presentation, and there are a limited number of studies to explain this in the literature. 11 , 12 In our study, we have shown that ferritin which is an indicator of systemic inflammation can be a predictor of disease severity and mortality.
In the current literature, it has been stated that COVID‐19 disease progresses more seriously, especially in elderly and male patients with comorbidity. While patients often have dyspnea, cough, fever, malaise, and myalgia, in severe cases clinical course can go up to ARDS and patients have to be intubated in intensive care. 4 In our study, the frequency of ARDS development and hematological abnormality was found to be increased in patients with a longer hospital stay, elderly and patients whose radiological involvement in CT is extensive so that treated with favipiravir. Our duration of hospital stay was determined in accordance with a multicenter study, and patients with an increased hospital stay had higher ARDS and increased ferritin levels. 13
While the correlation of lymphopenia from hematological abnormalities with disease severity is supported by the literature, it is emphasized that it is the first parameter that can be used due to being inexpensive, easy, and economical. 1 , 14 In our study, we think that lymphopenia may also be used with high ferritin levels in patients who have widespread inflammation leading to ARDS. In a meta‐analysis in which a limited number of literature are examined, hematological findings detected in COVID‐19 patients are considered as an indication of the increase in values such as d‐dimer, troponin, IL‐6, and the diffuse cytokine release in patients that led to multiple organ damage. 15 In our study, we have shown that the increase in the biochemical parameters such as ferritin, fibrinogen, d‐dimer, troponin, measured at the first time of hospital admission, as well as the detection of lymphopenia are associated with mortality.
Favipiravir, which is effective on viral RNA is activated with the host's enzyme system. 16 Favipiravir, whose efficacy was determined at the level of observation without clinical study, was used primarily in patients in the intensive care unit and in desaturated patients in clinics in accordance with the recommendation of the scientific committee of our country. Since the course of the disease was poor in our patients using favipiravir, the change in ferritin value was clinically significant in these patients.
As a result, COVID‐19 disease caused by SARS‐CoV‐2 causes high mortality with widespread inflammation and cytokine storm. A serious fight against the disease is active all around the world because of high mortality of this pandemic. In this pandemic period, we wanted to contribute to the literature with our study done with a cheap and widespread available marker, ferritin, which can be used for its predictivity of the mortality and hope it would be a useful marker for clinicians for the management of the disease (Figure 1).
Figure 1.
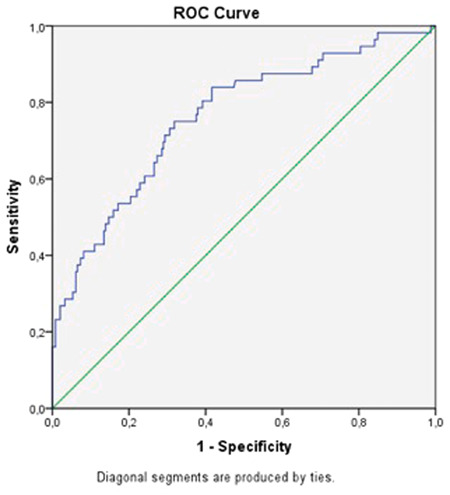
Receiver operating characteristic curve for ferritin
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Sedat Tural Önür, Sedat Altın, and Mehmet Toptaş. The first draft of the manuscript was written by Seda Tural Önür and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Tural Onur S, Altın S, Sokucu SN, et al. Could ferritin level be an indicator of COVID‐19 disease mortality? J Med Virol. 2021;93:1672‐1677. 10.1002/jmv.26543
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . Report of the Coronavirus Disease 2019 (COVID19) . Situation report 132. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200531-covid-19-sitrep-132.pdf?sfvrsn=d9c2eaef_2. Accessed July 10, 2020.
- 3. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang CL, Wang YM, Li XW, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen NS, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization . Clinical Management of Severe Acute Respiratory Infection When Novel Coronavirus (nCoV) Infection is Suspected: Interim Guidance . 2020. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-whennovel-coronavirus-(ncov)-infection-is-suspected. Accessed July 10, 2020.
- 7. Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID‐19) infections: a meta‐analysis. Clin Chim Acta. 2020;506:145‐148. 10.1016/j.cca.2020.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID‐19): a meta‐analysis. Clin Chim Acta. 2020;505:190‐191. 10.1016/j.cca.2020.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rosário C, Zandman‐Goddard G, Meyron‐Holtz EG, D'Cruz DP, Shoenfeld Y. The hyperferritinemic syndrome: macrophage activation syndrome, Still's disease, septic shock and catastrophic antiphospholipid syndrome. BMC Med. 2013;11:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. W‐J G, Liang W‐H, Zhao Y, et al. Comorbidity and its impact on 1,590 patients with COVID‐19 in China: a Nationwide analysis. medRxiv. 2020;55(5):2000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID‐19) infection: a systematic review and metaanalysis. Int J Infect Dis. 2020;94:91‐95. 10.1016/j.ijid.2020.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Imai Y, Kuba K, Rao S, et al. Angiotensin‐converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu J, Liu J, Zhao X, et al. Clinical characteristics of imported cases of COVID‐19 in Jiangsu Province: a multicenter descriptive study. Clin Infect Dis. 2020;71(15):706–712. 10.1093/cid/ciaa199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tan L, Wang Q, Zhang D, et al. Lymphopenia predicts disease severity of COVID‐19: a descriptive and predictive study. Version 2. Signal Transduct Target Ther. 2020;5(1):33. 10.1038/s41392-020-0148-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID‐19): a meta‐analysis. Clin Chem Lab Med. 2020;58(7):1021‐1028. 10.1515/cclm-2020-0369 [DOI] [PubMed] [Google Scholar]
- 16. Goldhill DH, te Velthuis AJW, Fletcher RA, et al. The mechanism of resistance to favipiravir in influenza. Proc Natl Acad Sci USA. 2018;115(45):11613–11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


