Abstract
It has been observed that the degree of pulmonary involvement shown in chest computed tomography (CT) scans tended to decrease as the prevalence of coronavirus disease 2019 (COVID‐19) infection decreased in the Turkish population. The purpose of this study was to investigate the relationship between the disease severity based on chest CT scans and the temporal evolution of the epidemic. This study recruited 179 patients with confirmed COVID‐19 disease who had received a chest CT scan between March 14 and April 28, 2020. The participants were divided into three successive temporal groups based on their date of CT examination. The early (March 14–29), mid (March 30–April 13), and late (April 14–28) groups were compared regarding the presence and extent of pulmonary involvement and CT characteristics of lesions. COVID‐19 pneumonia was less extensive in participants under 45 years of age and patients presenting late in the course of epidemic (i.e., the late group) compared those presenting earlier. When each group was subcategorized on the basis of age, older patients in the late group had less extensive lung involvement than older patients in the early group. However, there was no significant difference in the extent of lung involvement in younger patients between the late and early groups. The severity of COVID‐19 pneumonia appears to be variable at different temporal windows of the epidemic curve and decreases in patients presenting in the later weeks compared to the earlier weeks, particularly in older patients.
Keywords: coronavirus infections, COVID‐19, pandemic curve, pneumonia
1. INTRODUCTION
In December 2019, a novel coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) was first identified in Wuhan, China. The disease caused by SARS‐CoV‐2, called coronavirus disease 2019 (COVID‐19), has rapidly spread to many countries around the world and was declared a pandemic on March 11, 2020 by the World Health Organization. 1 , 2 In our country after the first officially reported case on March 11, the daily confirmed new cases reached a peak number of 5138 on April 11, 2020, and gradually declined until the end of May 2020. 3 In line with this, the number of patients who were admitted to Koç University Hospital′s radiology department with symptoms of possible COVID‐19 infection also decreased. A parallel trend towards a decreasing extent of pulmonary involvement as shown on chest computed tomography (CT) scans was observed in patients with confirmed COVID‐19 disease.
Determining the temporal changes in the severity of COVID‐19 pneumonia has several clinical implications. From a diagnostic point of view, limited lung involvement may theoretically result in normal or nearly normal CT findings, thereby hindering diagnosis, or may appear as less typical findings, complicating differentiation from other diseases. From an epidemiologic point of view, demonstrating a decrease in COVID‐19 pneumonia may show the efficacy of public health measures such as social distancing, mask use, and personal hygiene, which may reduce the viral load in patients. The purpose of this study was to confirm this anecdotal observation in a relatively large group of patients and show whether the disease severity on chest CT scans is stable or fluctuates with the temporal evolution of the epidemic curve.
2. METHODS
2.1. Data collection and patients
This single‐center retrospective study was conducted at Koç University Hospital. The study was approved by the Ethics Committee of Koç University (approval number: 2020.270.IRB1.093), which waived the requirement for informed consent. All adult patients over 18 years of age who had received a chest CT scan for COVID‐19 pneumonia between March 14 and April 28, 2020 and were confirmed to have COVID‐19 disease by reverse transcriptase‐polymerase chain reaction were selected from the radiology and hospital information systems. Three patients who were asymptomatic and four whose CT images included motion artifacts hindering evaluation of the CT scan were excluded, yielding a study group of 179 patients.
Patients’ demographic characteristics, including age, gender, and comorbidities (hypertension, Type 2 diabetes, cardiovascular diseases, chronic obstructive pulmonary disease, malignancy, and obesity), as well as the time interval between symptom onset and CT scan, were recorded. To analyze the severity of lung involvement in relation to the temporal development of the epidemic, patients were grouped by the date of chest CT examinations into three equal periods of 15 days. The early group included patients scanned in the first 15 days of the epidemic (March 14–29), the mid group was scanned between March 30 and April 13, and the late group between April 14 and 28. To homogenize the age distribution of the groups and remove any potential confounding effect of age on disease severity, each of the above groups was further divided into older (≥45 years of age) and younger (<45 years of age) subgroups (Figure 1). 4
Figure 1.
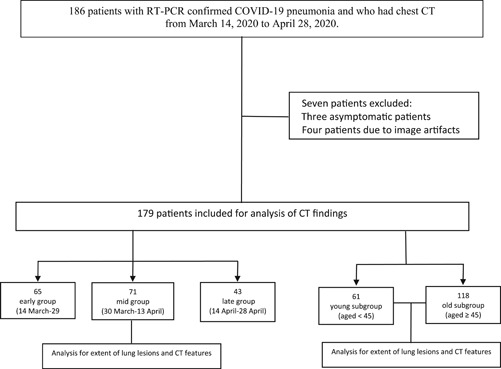
Flowchart of the study
2.2. CT protocol
Chest CT examinations were performed using a 64‐slice detector scanner (Somatom Definition AS, Siemens Healthineers) with patients in the supine position. The entire chest starting from the lung apices down to posterior costophrenic sulci was scanned with 0.625‐mm collimation, 80–120 kVp, and 20–150 mAs. Images were reconstructed with 1‐mm section thickness. No intravenous contrast medium was administered.
2.3. CT image analysis
All CT images were independently reviewed by two experienced radiologists with 13 years of experience each in chest imaging. Image review was blinded to the date of study and clinical data. All discrepancies were resolved by consensus.
A modified version of a previously described protocol was used to score the extent of pulmonary involvement, 5 , 6 whereby four lung lobes (the right middle and upper lobes were taken as one lobe) were scored semiquantitatively from 0 to 4 according to the percentage of lung involvement. Visually assessing the proportion of diseased areas to the whole area of the lobe, a score of 0 corresponded to 0% (no disease), 1 to 1%–25%, 2 to 26%–50%, 3 to 51%–75%, and 4 to 76%–100% of disease involvement. The total extent score was the sum of individual lobe scores and ranged from 0 to 16.
In patients who had pulmonary lesions, the greatest diameter of the largest lesions (up to three in number) were measured on lung window settings, and the average of these measurements was recorded as the mean lesion size. The total number of pulmonary lesions were categorized as solitary (1 lesion), few (2–4 lesions), moderate (5–9 lesions), and numerous (≥10 lesions). The following characteristics of CT findings were also noted: (a) unilateral or bilateral involvement; (b) focal or multifocal involvement; (c) craniocaudal predominance of abnormalities in relation to a horizontal line dividing the vertical height of lungs equally into an upper and lower zone: upper lung predominant, lower lung predominant, or no craniocaudal predilection 7 ; (d) lesion characteristics: ground‐glass opacity (GGO), consolidation, vascular enlargement, crazy‐paving pattern, traction bronchiectasis, subpleural curvilinear opacity, architectural distortion, and lobar volume loss; and (e) extrapulmonary findings: pleural effusion and lymph node enlargement (short‐axis diameter ≥10 mm).
GGO was defined as a hazy area of increased opacity not obscuring the underlying vessels. Consolidation was defined as a homogeneous opacification obscuring the underlying vessels. A crazy‐paving pattern was defined as GGO with superimposed interlobular septal thickening and/or intralobular lines. Subpleural curvilinear opacity referred to a thin curvilinear‐shaped opacity of 1–3 mm thick close to the pleural surface. Architectural distortion manifested as a loss of smooth course of the fissures, crowding of dilated bronchioles, or vessels with an angulated course. 8 Vascular enlargement was defined as an increased caliber of pulmonary vessels within the lesions, especially in areas of GGO, compared to surrounding disease‐free parenchyma. Focal was defined as a single focus of abnormality and multifocal as more than one focus. 9 , 10 , 11
2.4. Comparison among groups
The early, mid, and late groups, as well as their respective age subgroups, were compared regarding the score of the extent of pulmonary involvement, mean lesion size, lesion number, and frequency of CT features. The same comparisons were made between the older and younger age groups.
2.5. Statistical analyses
Statistical analyses were performed using IBM SPSS Statistics for Windows, version 24 (IBM Corp). Measurement data were expressed as means ± SDs, and categorical variables were reported as counts and percentages.
Quantitative data were tested first with the Shapiro–Wilk test for normality. The comparisons of paired quantitative data were evaluated using the Mann–Whitney U test for two groups and the Kruskal–Wallis test for multiple groups. A p value of less than .05 was considered statistically significant.
3. RESULTS
3.1. Demographic features
Table 1 shows the demographic features and the average time interval between symptom onset and CT scan for the entire patient groups as well as for the early, mid, and late groups. The mean patient age of the whole study group was 51.17 ± 17.61 years. The study group was divided almost equally by gender. Of the whole cohort, 38% had at least one comorbidity: 35.4% in the early group, 45.0% in the mid group, and 32.6% in the late group. There was no significant difference between the early, mid, and late groups regarding gender, frequency of comorbid disease, and time span between symptom onset and CT scan. The mean age of the early group was higher than the other groups. Pairwise comparison showed that the early group was significantly older than the late group (p = .002). However, there were no significant differences in age between the early and mid groups or between the mid and late groups. When the younger and older subgroups of the early, mid, and late groups were compared in terms of the factors that can influence the severity of COVID‐19 pneumonia, there was no significant difference regarding gender, age, frequency of comorbid disease, or the time span between symptom onset and CT date.
Table 1.
Demographic and clinical features in the whole cohort and the early, mid, and late groups
| Feature | All patients (n = 179) | Early group (n = 65) | Mid group (n = 71) | Late group (n = 43) | p |
|---|---|---|---|---|---|
| Female | 89 | 29 | 34 | 26 | .254 |
| Male | 90 | 36 | 37 | 17 | .254 |
| Age (year) | 51.17 ± 17.61 | 55.65 ± 15.18 | 51.17 ± 18.09 | 44.42 ± 18.46 | .003 |
| Comorbidity | 69 | 23 | 32 | 14 | .285 |
| Time from symptom onset to CT scan date (days) | 4,22 ± 3.11 | 4.25 ± 3.17 | 4.73 ± 3.66 | 3.34 ± 2.68 | .149 |
Note: The data are presented as count ±SDs. Bold values denote statistical significance at the p < .05 level.
Abbreviation: CT, computed tomography.
3.2. CT findings
Table 2 shows the extent of lung involvement in terms of the overall score, lesion number, and lesion size in the early, mid, and late groups (for all ages). Patients presenting later in the course of the epidemic had less lung involvement than patients presenting earlier. Mean lung involvement score was 4.6 in the early, 3.6 in the mid, and 2.2 in the late groups (p = .002; Figure 2). Lung lesions were more numerous in the early group compared to the late group (p = .001). The mean diameter of the largest lesion was 3.9 cm in the early group, 3.1 cm in the mid group, and 1.8 cm in the late group (p = .006; Figure 3). A pairwise comparison test showed that lung involvement score, lesion number, and lesion size were all significantly higher in the early group compared to the late group (p < .016). However, these were not significantly different between the early and mid groups or between the mid and late groups.
Table 2.
Extent of pulmonary involvement in the whole cohort and the early, mid, and late groups (all ages)
| Parameter | All patients (n = 179) | Early group (n = 65) | Mid group (n = 71) | Late group (n = 43) | p |
|---|---|---|---|---|---|
| Lung involvement score (0–16) | 3.6 ± .3.5 | 4.6 ± 3.8 | 3.6 ± 3.5 | 2.2 ± 2.4 | .002 |
| Number of lesions | 2.5 ± 1.7 | 2.9 ± 1.5 | 2.5 ± 1.7 | 1.7 ± 1.5 | .001 |
| Mean diameter of greatest three lesions (cm) | 3.1 ± 3.2 | 3.9 ± 3.6 | 3.1 ± 3.2 | 1.8 ± 2.4 | .006 |
Note: Data are means ± SDs. Bold values denote statistical significance at the p < .05 level.
Figure 2.
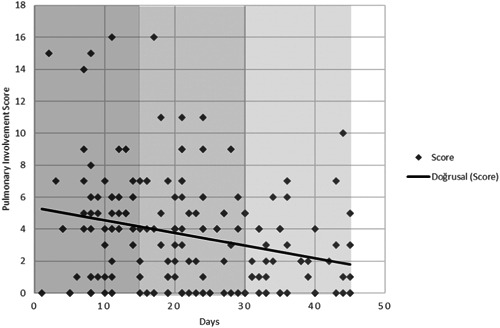
Scatter diagram showing the gradual decrease in the lung involvement score on patients' CT scans throughout the 45 days of study. CT, computed tomography
Figure 3.
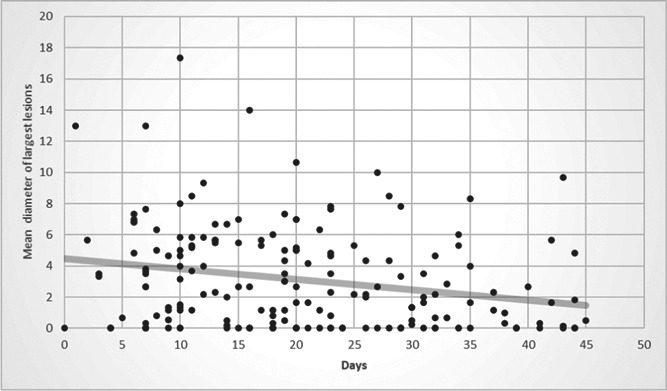
Scatter diagram showing the decrease in the mean diameter of the largest lesions throughout the 45 days of study
Similarly, older patients (≥45 years of age) who presented late had lesser lung involvement score, a lower number of lesions, and smaller lesions than patients from this age group who presented early in the course of the epidemic (Table 3). A pairwise comparison test showed that lung involvement score, lesion number, and lesion size were all significantly higher in the late group compared to the early group (p < .016; Figure 4). However, lung involvement score, lesion number, and lesion size were not statistically different between the early, mid, and late groups for younger patients (<45 years of age).
Table 3.
Extent of pulmonary involvement in the whole cohort and the early, mid, and late groups (patients ≥45 years old)
| Parameter | All patients (n = 118) | Early group (n = 52) | Mid group (n = 47) | Late group (n = 19) | p |
|---|---|---|---|---|---|
| Lung involvement score (0–16) | 4.7 ± 3.5 | 5.4 ± 3.7 | 4.72 ± 3.4 | 2.8 ± 2.4 | .026 |
| Number of lesions | 3.0 ± 1.5 | 3.3 ± 1.3 | 3.0 ± 1.5 | 1.6 ± 1.5 | .013 |
| Mean diameter of greatest three lesions (cm) | 4.1 ± 3.3 | 4.7 ± 3.5 | 4.1 ± 3.2 | 2.5 ± 2.5 | .047 |
Note: Data are means ± SDs. Bold values denote statistical significance at the p < .05 level.
Figure 4.
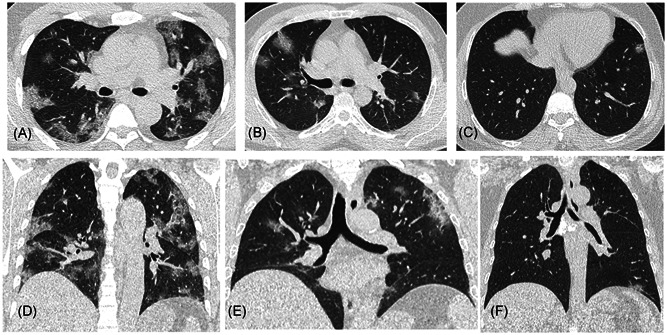
Representative axial (A–C) and coronal (D–F) images of three patients from the old subgroup show a decreasing extent of lung involvement in the later weeks of the study period. Figures (A, D) belong to a patient in the early group with a lung involvement score of 14, (B, E) to a patient in the mid group with a score of 9, and (C, F) to a patient from the late group with a score of 2
Of the whole study group, 45 patients (25.1%) had no CT findings suggesting pneumonia. Although 16.9% of patients (11/65) in the early group were CT negative compared to 29.6% of patients (21/71) in the mid and 30.2% (13/43) in the late groups, the differences were not statistically significant.
Table 4 shows the frequency and distribution of CT findings in the whole cohort. Bilateral disease was found in 62.6% of patients, and this was significantly less frequent in the late group compared to the other two groups. Multifocal lesions and lower zone predominance were found in 65.9% and 41.9% of patients, respectively; these were not significantly different between the three groups.
Table 4.
Frequency of CT features in the whole cohort and early, mid, and late groups (all ages)
| Feature | All patients (n = 179) | Early group (n = 65) | Mid group (n = 71) | Late group (n = 43) | p |
|---|---|---|---|---|---|
| Ground‐glass opacity | 130 (72.6%) | 52 (80.0%) | 49 (69.0%) | 29 (67.4%) | .245 |
| Crazy‐paving pattern | 72 (40.2%) | 31 (47.7%) | 29 (40.8%) | 12 (27.9%) | .122 |
| Consolidation | 69 (38.5%) | 29 (44.6%) | 26 (36.6%) | 14 (32.6%) | .414 |
| Vessel enlargement | 112 (62.6%) | 47 (72.3%) | 45 (63.4%) | 20 (46.5%) | .025 |
| Bronchial dilatation | 64 (35.6%) | 30 (46.2%) | 25 (35.2%) | 9 (20.9%) | .028 |
| Subpleural curvilinear opacity | 48 (26.8%) | 24 (36.9%) | 17 (23.9%) | 7 (16.3%) | .048 |
| Architectural distortion | 56 (31.3%) | 23 (35.4%) | 24 (33.8%) | 9 (20.9%) | .164 |
| Intrathoracic lymphadenopathy | 21 (11.7%) | 11 (16.9%) | 6 (8.5%) | 4 (9.3%) | .264 |
| Pleural effusion | 12 (6.7%) | 3 (4.6%) | 7 (9.9%) | 2 (4.7%) | .394 |
| Bilateral involvement | 112 (62.6%) | 47 (72.3%) | 45 (63.4%) | 20 (46.5%) | .025 |
| Focal involvement | 19 (10.6%) | 7 (10.8%) | 5 (7.0%) | 7 (16.3%) | .094 |
| Multifocal involvement | 118 (65.9%) | 47 (72.3%) | 45 (63.4%) | 23 (53.5%) | .178 |
| Lower lung predominance | 75 (41.9%) | 33 (50.8%) | 25 (35.2%) | 17 (39.5%) | .136 |
| No craniocaudal distribution | 51 (28.5%) | 19 (29.2%) | 23 (32.4%) | 9 (20.9%) | .418 |
Note: The counting data were presented as count (percentage of total). Bold values denote statistical significance at the p < .05 level.
Abbreviation: CT, computed tomography.
The most frequent CT findings were GGO (72.6% of patients), vessel enlargement within the lesion (62.6%), crazy‐paving pattern (40.2%), consolidation (38.5%), and bronchial dilatation (35.6%). Among these, vessel enlargement and bronchial dilatation were significantly more common in the early group than in the late group. Pleural effusion and lymph node enlargement were present in 6.7% and 11.7% of patients, respectively. The frequency of pleural effusion and lymph node enlargement did not differ significantly between the three groups (Table 5). The frequency of various lesion characteristics, bilateral disease, and multifocal involvement was not significantly different between the early, mid, and late groups or between the two age groups (Figure 5).
Table 5.
Frequency of CT features in the young (<45 years of age) and old (≥45 years of age) groups with pneumonia
| Feature | Young group (n = 61) | Old group (n = 118) | p |
|---|---|---|---|
| Ground‐glass opacity | 33 (54.1%) | 97 (82.2%) | <.001 |
| Crazy‐paving pattern | 10 (16.4%) | 62 (52.5%) | <.001 |
| Consolidation | 12 (19.7%) | 57 (48.3%) | <.001 |
| Vessel enlargement | 24 (39.3%) | 88 (74.6%) | <.001 |
| Bronchiectasis | 10 (16.4%) | 54 (45.8%) | <.001 |
| Subpleural curvilinear opacity | 8 (13.1%) | 40 (33.9%) | <.001 |
| Architectural distortion | 5 (8.2%) | 51 (43.2%) | <.001 |
| Intrathoracic lymphadenopathy | 1 (1.6%) | 17 (14.4%) | .150 |
| Pleural effusion | 0 (0%) | 12 (10.2%) | .012 |
| Bilateral involvement | 22 (36.1%) | 89 (75.4%) | <.001 |
| Focal involvement | 11 (18.0%) | 8 (6.8%) | .015 |
| Multifocal involvement | 22 (36.1%) | 96 (81.4%) | <.001 |
| Lower lung predominance | 19 (31.1%) | 56 (47.5%) | .271 |
| No craniocaudal distribution | 11 (18.0%) | 40 (33.9%) | .406 |
Note: The counting data were presented as count (percentage of total). Bold values denote statistical significance at the p < .05 level.
Abbreviation: CT, computed tomography.
Figure 5.
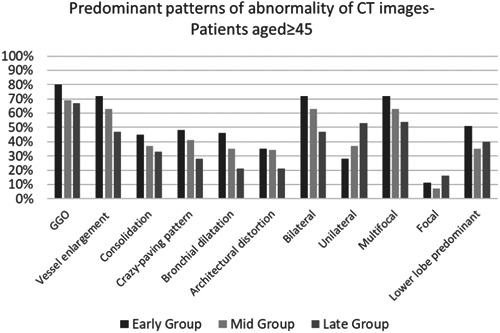
The frequency of pulmonary abnormalities in terms of distribution and frequency of high‐resolution computed tomography characteristics of lesions in older patients (≥45)
Table 5 shows the age‐related differences in the CT findings. Lung involvement was significantly more extensive in the older group, which had a higher mean involvement score (4.7 vs. 1.57), lesion size (4.07 vs. 1.22 cm), and lesion number (3.02 vs. 1.36) than the younger group.
A significantly higher proportion of the younger group (44.3%) had no CT findings related to pneumonia compared to 15.2% of the older group. Also, unilateral and focal involvement was more common in the younger group. GGO, consolidation, vascular enlargement, bronchiectasis, crazy paving, subpleural curvilinear opacity, architectural distortion, and pleural effusion were significantly more common in the older group. There were no statistically significant differences in terms of intrathoracic lymphadenopathy or upper/lower lung predominance of lesions between the two age groups.
4. DISCUSSION
The most important outcomes of this study were that the disease affected the lungs of older patients to a greater extent, and the severity of pneumonia varied in relation to the local outset of the epidemic. Specifically, patients presenting relatively late during the first wave of the epidemic were significantly less likely to have pneumonia as reflected by objective measurements of the extent of lung involvement, the number of lesions, and lesion size.
The SARS‐CoV‐2 virus causes a wide spectrum of disease presentations ranging from asymptomatic or mild cases to severe pneumonia that requires hospitalization and even intensive care. 12 The disease has been associated with high mortality and case‐fatality rates. 13 , 14 The degree of pulmonary involvement has been shown to be correlated with prognosis and mortality. 15 , 16 It has been previously demonstrated that patients 45 years of age or older had more extensive pulmonary involvement than younger patients. 4 , 17 This study confirmed that patients 45 years of age or older had a more severe disease as characterized by a higher semiquantitative score, larger lesion size, and higher abundance of lesions on a CT scan. In addition, several CT features—including vascular enlargement, bronchiectasis, crazy‐paving pattern, subpleural curvilinear opacity, and architectural distortion—were more common in older patients, which may also be correlated with a more severe disease course.
The underlying reason for the variation in the severity of COVID‐19 pneumonia on chest CT scans in relation to the temporal evolution of the epidemic is unclear. The disease is known to affect the elderly more severely than younger patients. Theoretically, the curfew on the older population (above 65 years of age) that was put into effect shortly after the disease appeared in Turkey could have resulted in less severe cases in the later weeks. However, in the subgroup analysis, which allowed for removal of the effects of age, decreased disease severity was still found in the older subgroup of the late group compared to the older subgroup of early group.
Although there is no evidence‐based explanation as to why the severity of pneumonia changed with time, a few speculations can be made. One possible cause may be reduced virulence of the offending agent, SARS‐CoV‐2. It is well known that the genetic material of viruses can undergo mutations, making them more or less virulent. 18 , 19 However, although this may be a matter of further research in viral genetics, it is quite unusual for a virus to undergo mutation in a very short time (i.e., within weeks to months).
Another explanation may be linked to the effect of community‐based preventive measures like social distancing, mask use, and personal hygiene, which have been promoted progressively in Turkey since the outset of infection. Indeed, it was recently demonstrated that transmission of viruses, including SARS‐CoV‐2, is decreased by health measures such as physical distancing and the use of facial masks. 20 It has also been suggested that patients with severe pneumonia tend to have a higher viral load of SARS‐CoV‐2, which may be associated with disease severity and prognosis. 21 It is quite possible that the aforementioned measures have resulted in a decreased rate of disease transmission as well as diminished clinical severity by decreasing the viral load.
Although a significantly different disease severity was observed in the early, mid, and late groups in older patients, this was not demonstrated in younger patients. This may have resulted from a lower number of patients and the limited extent of disease in the younger group owing to their stronger resistance to infection and well‐controlled immune response. 22 Patients in the early, mid, and late groups had similar frequencies of CT findings with the exception of vascular enlargement, bronchial dilatation, subpleural curvilinear opacity, and unilateral distribution, which were more common in the early group than in the late group. These features lost their significance when patient age was taken into account. Therefore, although the severity of COVID‐19 pneumonia decreased in the later periods of the epidemic with less extensive involvement and smaller and fewer lesions, all of the reported CT features (including signs of vascular enlargement) were retained, which can allow for differentiation from other causes of pneumonia. 23
The limitations of our study include its retrospective design and a relatively small number of cases. Studies on a larger number of patients over a longer course of the epidemic may offer further insight on the relationship between the severity of pneumonia and when patients present relative to the age of the epidemic.
In conclusion, this study was the first to demonstrate that the radiological severity of COVID‐19 pneumonia depends on when patients present relative to the curve of the epidemic. Older patients presenting later tended to have milder pneumonia than those presented earlier in the epidemic.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Terman Gumus, Duygu Cengiz, Zeynep Atceken, and Kayhan Cetin Atasoy had the idea for and designed the study and drafted the original manuscript. Terman Gumus, Süda Tekin, and Furkan Kartal collected the data. Zeynep Atceken, FK, and Kayhan Cetin Atasoy contributed to the literature search and checked the data. Terman Gumus and Duygu Cengiz contributed to the statistical analysis. Süda Tekin, Furkan Kartal, and Kayhan Cetin Atasoy contributed to the critical revision of the manuscript. All authors read and approved the final manuscript.
Gumus T, Cengiz D, Kartal F, Atceken Z, Tekin S, Atasoy KC. Changes in computed tomography findings of COVID‐19 pneumonia: Less extensive lung involvement with decreasing disease prevalence. J Med Virol. 2021;93:2056–2064. 10.1002/jmv.26573
Any of the authors have any funding sources.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061‐1069. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . WHO Director‐General's remarks at the media briefing on 2019‐nCoV on 11 February 2020. Published Feb 11, 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020. Accessed March 1, 2020.
- 3. Recent coronavirus status in Turkey , Ministry of Health Turkey. Published March 20, 2020. https://covid19.saglik.gov.tr. Accessed May 15, 2020.
- 4. Chen Z, Fan H, Cai J, et al. High‐resolution computed tomography manifestations of COVID‐19 infections in patients of different ages. Eur J Radiol. 2020;126:108972. 10.1016/j.ejrad.2020.108972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li K, Fang Y, Li W, et al. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID‐19). Eur Radiol. 2020;1:10‐4416. 10.1007/s00330-020-06817-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bernheim A, Mei X, Huang M, et al. Chest CT findings in coronavirus disease‐19 (COVID‐19): relationship to duration of infection. Radiology. 2020;295(3):200463. 10.1148/radiol.2020200463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ajlan AM, Ahyad RA, Jamjoom LG, Alharthy A, Maddani TA. Middle East respiratory syndrome coronavirus (MERS‐CoV) infection: chest CT findings. AJR Am J Roentgenol. 2014;203(4):782‐787. [DOI] [PubMed] [Google Scholar]
- 8. Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246:697‐722. [DOI] [PubMed] [Google Scholar]
- 9. Li Y, Xia L. Coronavirus disease 2019 (COVID‐19): role of chest CT in diagnosis and management. AJR Am J Roentgenol. 2020;214(6):1280‐1286. 10.2214/AJR.20.22954 [DOI] [PubMed] [Google Scholar]
- 10. Ojha V, Mani A, Pandey NN, Sharma S, Kumar SCT. CT in coronavirus disease 2019 (COVID‐19): a systematic review of chest CT findings in 4410 adult patients. Eur Radiol. 2020:1‐10. 10.1007/s00330-020-06975-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID‐19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol. 2020;215(1):87‐93. 10.2214/AJR.20.23034) [DOI] [PubMed] [Google Scholar]
- 12. Zhao W, Zhong Z, Xie X, Yu Q, Liu J. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID‐19) pneumonia: a multicenter study. AJR Am J Roentgenol. 2020;214(5):1072‐1077. 10.2214/AJR.20.22976 [DOI] [PubMed] [Google Scholar]
- 13. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Giangreco G. Case fatality rate analysis of Italian COVID‐19 outbreak. J Med Virol. 2020;92(7):919‐923. 10.1002/jmv.25894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li K, Wu J, Wu F, et al. The clinical and chest CT features associated with severe and critical COVID‐19 pneumonia. Invest Radiol. 2020;55(6):327‐331. 10.1097/RLI.0000000000000672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yuan M, Yin W, Tao Z, Tan W, Hu Y. Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan, China. PLoS One. 2020;15(3):e0230548. 10.1371/journal.pone.0230548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhu T, Wang Y, Zhou S, Zhang N, Xia L. A comparative study of chest computed tomography features in young and older adults with corona virus disease (COVID‐19). J Thorac Imaging. 2020;35:W97‐W101. 10.1097/RTI.0000000000000513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. CGeoghegan JL, Holmes EC. The phylogenomics of evolving virus virulence. Nat Rev Genet. 2018;19(12):756‐769. 10.1038/s41576-018-0055-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Berngruber TW, Froissart R, Choisy M, Gandon S. Evolution of virulence in emerging epidemics. PLoS Pathog. 2013;9(3):e1003209. 10.1371/journal.ppat.1003209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chu DK, Akl EA, Duda S, et al. Physical distancing, face masks, and eye protection to prevent person‐to‐person transmission of SARS‐CoV‐2 and COVID‐19: a systematic review and meta‐analysis. Lancet. 2020;S0140‐6736(20):31142‐31149. 10.1016/S0140-6736(20)31142-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu Y, Yan LM, Wan L, et al. Viral dynamics in mild and severe cases of COVID‐19. Lancet Infect Dis. 2020;20(6):656‐657. 10.1016/S1473-3099(20)30232-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mueller AL, McNamara MS, Sinclair DA. Why does COVID‐19 disproportionately affect older people? Aging. 2020;12(10):9959‐9981. 10.18632/aging.103344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bai HX, Hsieh B, Xiong Z, et al. Performance of radiologists in differentiating COVID‐19 from viral pneumonia on chest CT. Radiology. 2020;296:200823‐E54. 10.1148/radiol.2020200823 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


