Abstract
Because asymptomatic carriers of COVID‐19 produce respiratory droplets that can remain suspended in air for several hours, social distancing may not be a reliable physical barrier to transmission. During the COVID‐19 pandemic, however, some governments were reluctant to mandate public mask use out of concern this would worsen shortages of respirators for healthcare workers. Cloth masks with a filtering effectiveness of 70–90% can be made from widely available materials, and are a better option than respirators for the public. Countries could rapidly implement Effective Fiber Mask Programs (EFMPs) to use local resources to mass produce effective and affordable cloth masks, and to engage the public in their correct use. EFMPs could be a cost‐effective measure to ease isolation while limiting new infections during pandemics. EFMPs could also protect healthcare workers by increasing the supply of respirators for their use, reducing their risk of acquiring the illness from their communities, and by reducing the number of patients they must treat.
Keywords: Cloth masks, COVID‐19 transmission risk, pandemic preparedness
1. INTRODUCTION
This article was written during the COVID‐19 pandemic, but its analysis can apply to pandemics in general. Novel human viruses have been reported at the rate of one to two per year, a trend epidemiologists expect will continue (Woolhouse et al., 2008). Several interrelated issues emerged during the COVID‐19 epidemic. First, healthcare facilities experienced shortages of respirators because of a limited capacity to manufacture the electrospun filter materials used in their manufacture (Wu, Huang, Zhang, He, & Ming, 2020). Second, social distancing and isolation were used as physical barriers to reduce transmission, but social distancing is not always practical, and isolation brings significant economic and social costs. Authorities faced the challenging question of how to end lockdowns without triggering successive waves of infections (Lawton, 2020). Third, guidance from health authorities regarding the use of masks in public varied widely. Some countries mandated the public use of masks while others asked people not to wear masks out of concern that doing so would reduce the supply of masks for healthcare workers (Javid, Weekes, & Matheson, 2020). What if countries used local resources to produce effective masks for the public? How could these masks be made, and how effective would they need to be? Addressing these questions requires insights from epidemiology, virology, biochemistry, physics, mathematics, environmental science, material sciences, building engineering, psychology, and public policy. This article draws on current research in these fields to propose the development and public use of more effective cloth face masks during pandemics. The article does not provide medical advice but offers information to professionals who advise governments on public policy.
2. TRANSMISSION OF COVID‐19
2.1. Asymptomatic Transmission and Respiratory Droplets
COVID‐19 can be transmitted to susceptible individuals by asymptomatic individuals, who are less likely to sneeze or cough (Asadi, Bouvier, Wexler, & Ristenpart, 2020). He et al. (2020) estimated that “44% (95% confidence interval, 25–69%) of secondary cases were infected during the index cases’ presymptomatic stage.” Lauer et al. (2020) reported a median incubation period for COVID‐19 of five days.
Coughing and sneezing produce the largest respiratory droplets at 10 µm and up to 1,000 µm respectively. Breathing and speaking produce the smallest, in the ranges of 0.8–1 µm and 3.5–5.5 µm respectively (Asadi et al., 2019; Han, Weng, & Huang, 2013; Morawska et al., 2009). Leung, Lam, and Cheng (2020) reported “Viral RNA was identified from respiratory droplets and aerosols for all three viruses, including 30, 26, and 28% of respiratory droplets and 40, 35, and 56% of aerosols collected while not wearing a face mask, from coronavirus, influenza virus, and rhinovirus‐infected participants, respectively.“ Interestingly, Milton, Fabian, Cowling, Grantham, and McDevitt (2013) found the number of virus copies in the exhaled breath of influenza patients was 8.8 times higher in particles smaller than 5 µm than in larger particles. Stadnytskyi, Bax, Bax, and Anfinrud (2020) reported that “At an average viral load of 7 × 106 per milliliter, we estimate that one minute of loud speaking generates at least 1,000 virion‐containing droplet nuclei that remain airborne for more than eight minutes.”
2.2. Respiratory Droplets and Aerosols
The size of droplets and particles is a continuum, but there are important differences between aerosols and larger droplets or particles. In physics the upper limit of the size of an aerosol is 100 µm (Baron, Kulkarni, & Willeke, 2011; Hinds, 1999; Thomas, Charvet, Bardin‐Monnier, & Appert‐Collin, 2017). Aerosols behave differently from larger droplets and particles in significant ways:
Under normal conditions, the high surface‐to‐volume ratio of liquid aerosols causes them to evaporate rapidly.
Smaller aerosols are affected more strongly by air currents than by gravity (Baron et al., 2011).
While larger particles and larger aerosols tend to be deposited in the upper respiratory tract, smaller aerosols (<2.5 µm) can be deposited in the lungs (Roy & Milton, 2004).
Because respiratory droplets contain salts, proteins, and carbohydrates, their droplet nuclei are hygroscopic and do not become completely dehydrated (O'Shaughnessy et al., 2020; Vejerano & Marr, 2018). Droplets containing salt evaporate more slowly than pure water droplets, but at isotonic concentrations this difference is negligible (Qu, Escobar, Li, Rao, & Xu, 2020). Except under conditions of high relative humidity (RH), most of the water they contain evaporates to leave droplet nuclei that are 60–70% smaller than the original droplet. These residual droplet nuclei are also aerosols, having even greater mobility than the original droplets.
Some descriptions of small droplet behavior in the literature, however, are incomplete. For example, one source refers to particles larger than 10 µm as “large particles” that fall to the ground in a few seconds (Public Health Agency of Canada, 2017). Based on the relationships provided by Holterman (2003) and the characteristics of saliva described by Liu, Wei, Li, and Ooi (2016), however, it can be calculated that at 20 °C and 50% RH, 10 µm droplets of saliva evaporate to 3.5 µm residual droplet nuclei in under a second, which would then require more than an hour to settle in still air. Under real life conditions, these droplet nuclei are more likely to travel with air currents than to reach the ground directly. Although some medical scientists and physicists may discuss the upper size limit of aerosols differently, these differences are unimportant to the question of how respiratory droplets from asymptomatic individuals behave. These droplets are smaller than 10 µm, and their behavior is well understood.
To illustrate how the behavior of droplets and aerosols differs by size, the time for a droplet to settle through a vertical distance of 1.5 m to the ground in still air, and the time to form a droplet nucleus through evaporation were calculated for a temperature of 20 °C and RH of 50% and the results are shown in Table I. The calculations are based on the relationships provided by Holterman (2003) and the characteristics of saliva described by Liu et al. (2016). The calculations ignore the initial speed of droplets, and assume they are spherical and electrically neutral. The initial concentrations of salts and solids in the respiratory droplet were assumed to be 0.9% and 1.8% respectively, after Liu et al. (2016).
Table I.
The Fate of Respiratory Droplets by Size at 20°C and 50% Relative Humidity
| Original size | Estimated final size | Fate of droplets at 20 °C and 50% relative humidity |
|---|---|---|
| 1 mm | 350 µm | Sneezing produces droplets of this size. These droplets are not aerosols but are comparable in size to raindrops and would settle in 0.4 seconds. |
| 100 µm | 35 µm | A 100 µm droplet will settle in six seconds. |
| 10 µm | 3.5 µm | Coughing produces droplets of this size, which evaporate in 0.2 seconds to 3.5 µm droplet nuclei. The nuclei would theoretically require one hour to settle in still air, but in practice are entrained in air currents. |
| 5 µm | 1.8 µm | Speaking produces droplets of this size, which evaporate in 0.1 seconds to 1.8 µm droplet nuclei. The nuclei would theoretically require four hours to settle in still air, but in practice are entrained in air currents. |
| 0.8 µm | 0.3 µm | Breathing produces droplets of this size, which evaporate in a few milliseconds to 0.3 µm droplet nuclei (comparable in size to smoke particles). The nuclei would theoretically require 20 hours to settle in still air, but in practice are entrained in air currents. |
Under ambient conditions of 20 °C and RH of 50%, droplets initially smaller than approximately 80 µm form smaller droplet nuclei before they can fall 1.5 m to the floor or ground. Similar results have been found for droplets containing salt (Ferron & Soderholm, 1990; Yang & Marr, 2011) and for sputum droplets expelled by a cough.
The evaporation of respiratory droplets is one reason that loose‐fitting masks reduce the risk of infection for others more than for the wearer: the concentration and diameter of respiration droplets are at a maximum as they are expelled (Redrow, Mao, Celik, Posada, & Feng, 2011). It is therefore easier to reduce respiration droplets at their source than to filter out their smaller and more diffuse residual droplet nuclei later. This outcome matches experience with pollution control, where it is more efficient and cost‐effective to reduce contaminants at the source than to remove them from the environment later.
2.3. Limitations of Social Distancing
After respiratory droplets leave the body their temperature decreases and their concentration of salt ions increases through evaporation; both affect the viability of the virus (Lin & Marr, 2019). Van Doremalen et al. (2020) measured the viability of SARS‐CoV‐2 and SARS‐CoV‐1 in aerosols smaller than 5 µm produced in a laboratory and found the half‐life of each virus was approximately one hour. Their study measured viability by end point titration rather than detection of RNA. Other researchers found that SARS‐CoV‐1 was transmitted among individuals in aerosol form (Booth et al., 2005; Morawska & Cao, 2020; Yu, Wong, Chiu, Lee, & Li, 2005).
During the COVID‐19 pandemic, social distancing of 2 m was recommended. Here, distance is used as a proxy for time: the time for larger droplets to reach the ground or floor in the spaces among individuals, the time for smaller droplet nuclei to disperse in air currents, and the time for virus particles in droplet nuclei to become inactive. The challenge is that ambient conditions vary so widely that time cannot reliably be represented by a fixed distance.
Aerosol droplet nuclei produced by individuals disperse in air, so their concentration decreases with distance. In most buildings, however, ventilation systems remove air contaminants relatively slowly. For example, if a building ventilation system achieves six air changes per hour, then (assuming air in a room moves vertically, so the calculated velocity is independent of floor area) the resulting average air velocity would be 4 mm per second. In contrast, a light outdoor breeze of 6 km/h moves 400 times faster. Higher air change rates in buildings can remove contaminants more quickly, but can also move aerosols more rapidly toward others (Ghia et al., 2012; Liu et al., 2020). Issues of ventilation rates, indoor air quality, and sick building syndrome have been extensively researched (Fisk, Mirer, & Mendell, 2009; Jaakkola & Miettinen, 1995). Interestingly, a study of COVID‐19 outbreaks in China in January and February 2020 found only one of 318 outbreaks could be traced to an outdoor contact: the balance occurred indoors (Qian et al., 2020).
The shortcomings of social distancing have been researched by Anderson, Turnham, Griffin, and Clarke (2020); Asadi et al. (2020); Bahl et al. (2020); Drossinos and Stilianakis (2020); Feng, Marchal, Sperry, and Yi (2020); and Setti et al. (2020) among others. Guzman (2020) concluded “A SARS‐CoV‐2 carrier person talking, sneezing, or coughing at distance of 2 m can still provide a pathogenic bioaerosol load with submicron particles that remain viable in air for up to three hours for exposure of healthy persons near and far from the source in a stagnant environment.” Professionals who advise governments on public policy could therefore consider:
Medical science shows asymptomatic individuals produce respiratory droplets from speaking and breathing smaller than 5 µm that contain virus particles.
Physics shows droplets of this size rapidly evaporate to smaller droplet nuclei, that remain suspended in air for several hours. The original droplets and residual droplet nuclei are both aerosols.
Medical science shows the SARS‐CoV‐2 virus survives in aerosol droplet nuclei for several hours, which is significantly longer than the time required for droplet nuclei produced by an infected individual to reach a susceptible one.
Physics shows that indoors, air currents move droplet nuclei more slowly toward others, but also disperse them more slowly. Outdoors, air currents can disperse droplet nuclei more quickly, but can also move them more rapidly toward others.
Together, medical science and physics strongly suggest social distancing is not a reliable barrier to transmission of COVID‐19. The question of whether an illness can spread through aerosols is less important if effective masks are used as barriers to transmission, because they can reduce emissions of respiratory droplets to air and consequently contamination of surfaces and fomites, and can also reduce inhalation of aerosols.
3. MASKS AS BARRIERS
Estimating the risk of transmission between two individuals would be highly complex, because of the large number of variables involved. Such mechanistic modeling is unnecessary because the goal is not to determine if a given individual will become ill, but to estimate how the risk of transmission may be reduced in a population. Tian et al. (2020) developed a model to show how general mask use can reduce transmission of infection, and hence R 0. They developed a “semiquantitative model to show that mask‐wearing reduces βeff and hence by a factor , where is the efficacy of trapping viral particles inside the mask, and is the percentage of mask‐wearing population” (Tian et al., 2020). Based on Tian et al. (2020) the relationship between the rate of transmission between two individuals, the effectiveness of masks, and the percentage of people who wear them in public can be expressed as:
Equation 1. Mask Effectiveness and Mask Use
| (1) |
where:
β is the rate of transmission of infection from an infected person to a susceptible person in the case where neither person wears a mask,
β mask is the rate of transmission of infection from an infected person to a susceptible person in the case where a percentage of people wear masks,
P is the percentage of people who wear masks in public,
Ei is the % reduction in the risk of transmission from others to the wearer by masks
E 0 is the % reduction in the risk of transmission to others from the wearer by masks.
Equation 1 is based on the assumption that the mask of person “A” reduces transmission from “A” to the shared space between them, and the mask of person “B” reduces transmission from the shared space to “B.” Because the two masks act in series, their effect on transmission is compounded. Even if each mask is only 50% effective, the two masks together would reduce the risk of transmission by 75%. The reduction in transmission is simply:
Equation 2. Reduction in Transmission
| (2) |
Equations 1 and 2 can be combined to find the required “incoming effectiveness” of masks:
Equation 3. Mask Effectiveness as a Function of Mask Use
| (3) |
where:
P is the percentage of people who wear masks in public,
Ei is the % reduction in the risk of transmission from others to the wearer by masks
E 0 is the % reduction in the risk of transmission to others from the wearer by masks.
The required combinations of P, Ei, and E0 to reduce transmission by 50% are shown in Fig. 1.
Fig 1.
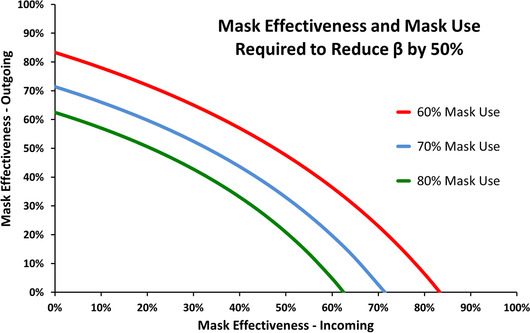
Impact of mask effectiveness and mask use on β
In this high‐level model it does not matter whether transmission is reduced by masks that reduce the risk of infection to the wearer (but not to others), or masks that reduce the risk of infection to others (but not to the wearer). The model also shows even imperfect masks can reduce the risk of transmission. Similarly, Kai, Goldstein, Morgunov, Nangalia, and Rotkirch (2020) concluded if 80% of people wear masks in public, and if masks have an effectiveness of 70%, then daily case growth rates could be significantly reduced.
In this model the relationship between the filtering effectiveness of masks and the risk of transmission is linear, which may be inaccurate. If a minimum infectious dose is required for an airborne pathogen to cause illness, then models could account for the potential of masks to reduce exposure to a level below this dose. Further, a higher initial dose may cause more severe illness (Paulo, Correia‐Neves, Domingos, Murta, & Pedrosa, 2010). Research has shown that the severity of illness caused by the influenza A virus depends on whether infection began in the nose or in the lower respiratory tract (Nikitin, Petrova, Trifonova, & Karpova, 2014; Tellier, 2006). If this proves true for COVID‐19, then research could determine if a second benefit of public mask could be less severe illnesses.
The model also shows a small improvement in mask use can strongly affect outcomes because the effect on β is proportional to the square of P. Further, R 0 is proportional to β, so reducing β will cause an exponential reduction in cases over time. This effect is discussed by Abaluck et al. (2020) who found “If masks reduce the transmission rate of the virus by only 10%, epidemiological models suggest that hundreds of thousands of deaths could be prevented globally, creating trillions of dollars in economic value. According to one commonly used epidemiological model, a 10% reduction in transmission probabilities would generate $3,000–6,000 in value per capita from reduced mortality risk in the United States alone.”
4. TYPES OF MASK
When reading studies of the performance of masks, it is helpful to note:
In some studies masks are sealed to a machine or mannequin for testing. The results show the filtering efficiency of the mask's materials, but not the effectiveness of the mask as worn (Davidson et al., 2013). Other studies use quantitative fit tests of masks worn by people, which better indicate their effectiveness.
Studies may report the effectiveness of masks as filter penetration, filtering efficiency, or fit factor. In this article, results are presented as filtering efficiency.
Results do not always include breathing rate (L·min‐1·cm‐2) and differential pressure. These are important aspects of performance because filtering efficiency and breathability change with air velocity.
Particles used for testing can be monodisperse or polydisperse and may not be charge‐neutralized. Further, particle counting equipment may not report results for different particle sizes. This is important since filtering efficiency varies with particle size.
4.1. N95 and FFP Respirators
N95 and FFP respirators are made with highly efficient electret filter materials, and with a means of forming a close fit and seal with the wearer's face. These masks were originally designed to protect industrial workers and are certified to filter more than 95% of particles 0.3 µm in diameter. N95 respirators are unsuitable for public use during pandemics because they are needed by healthcare and industrial workers, and because their effectiveness cannot be assured without individual fit testing and training (U.S. FDA, 2020).
4.2. Surgical Masks
Surgical masks (also called procedure masks or medical masks) are designed to resist penetration by fluids under pressure, and to reduce emissions of droplets from the wearer. A confusion regarding certified surgical masks is that while they are made with materials having a high filtration efficiency, their effectiveness in actual use is lower. The reason is that the filtration efficiency of surgical mask material is measured by devices that do not allow air to bypass the material, but in use surgical masks cannot provide a tight fit. The incoming filtering effectiveness of surgical masks has been measured as 53–74% (Mueller, Eden, Oakes, Bellini, & Fernandez, 2020), 20–80% (Bałazy et al., 2005), 16–80% (Bałazy et al., 2006), and 10–86% (Oberg & Brosseau, 2008). The U.S. FDA (2020) states: “While a surgical mask may be effective in blocking splashes and large‐particle droplets, a face mask, by design, does not filter or block very small particles in the air that may be transmitted by coughs, sneezes, or certain medical procedures. Surgical masks also do not provide complete protection from germs and other contaminants because of the loose fit between the surface of the face mask and your face.” 3M (2020), the Milton et al. (2013), U.S. CDC (2020), and Oberg and Brosseau (2008) give similar descriptions of surgical masks. Surgical masks are unsuitable for public use during pandemics because they are needed by healthcare workers for their intended purpose.
4.3. Cloth Masks
Cloth masks have been made with a wide variety of designs and materials. Some disadvantages of cloth masks include:
Their filtering effectiveness varies widely.
Education is required to ensure they are properly used.
Less area of a cloth mask is available for filtration than in a respirator.
Breathing resistance in some cloth masks is high.
They require more time and effort to make than disposable masks.
They are not normally certified to ensure they meet minimum requirements.
To date, highly effective cloth masks have not been widely available.
Some advantages of cloth masks include:
Cloth masks can be made with widely available materials and low‐tech methods (Konda et al., 2020; Mueller et al., 2020).
Resources needed to make cloth masks do not compete with those needed to make respirators.
Making cloth masks can employ people who might otherwise be unemployed during pandemics.
Making cloth masks indirectly increases availability of respirators for healthcare workers, by providing the public with an alternative.
Cloth masks can be designed to provide a better fit than pleated disposable masks (Mueller et al., 2020).
Cloth masks do not require fit testing and can be made for different shapes and sizes of faces, including the faces of children.
Cloth masks can be disinfected in an autoclave, or laundered since the lipid membrane of the SARS‐CoV‐2 virus is disrupted by surfactants (Welch et al., 2020).
New cloth masks have no expiry date and can be stored in preparation for future pandemics, and against air pollution from wildfires and fossil fuel combustion.
The cost per hour of use of cloth masks is lower than that of disposable masks. (These costs are discussed in Section 7.3.)
Cloth masks have strengths and weaknesses: how can they be improved?
5. IMPROVING CLOTH MASKS
Hand‐sewn cloth masks are often made with tightly woven fabrics, in the hope they will screen droplets and particles. However, this screening or sieving effect cannot block small droplets and particles. Air filters actually remove small particles in four ways, which are summarized in Fig. 2 (Kowalski, Bahnfleth, & Whittam, 1999; Lee & Liu, 1982).
Fig 2.
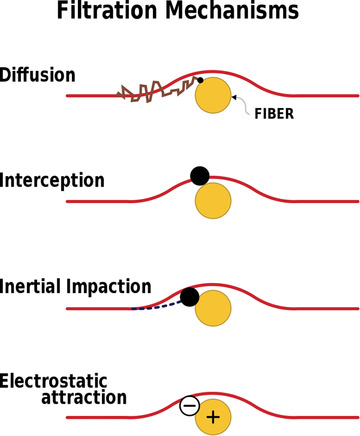
Filtration mechanisms
Filtration mechanisms is licensed by Andrew Jarvis under creative commons.
Diffusion. Collisions between particles and gas molecules cause Brownian motion, which randomly moves particles out of the path of the air stream and toward filter fibers.
Interception. Particles adhere to fibers when the path of the air stream is within approximately one radius of the fiber.
Inertial Impaction. Because of their inertia, particles are unable to follow the air stream around fibers, and instead adhere to them.
Electrostatic Attraction. Surface charges on the fiber cause electrostatic fields, which attract particles to the fiber.
Woven fabrics are not ideal air filters. As Fig. 3 shows, the spaces between threads are larger than the spaces between fibers in threads: most air flows through these spaces. Further, individual fibers in a thread lie parallel to each other, so less than 5% of their surface area is exposed to air moving through the fabric.
Fig 3.

Woven fabric
Photo credit: Edal Anton Lefterov / CC‐BY‐SA‐3.0
Because smaller particles are adsorbed onto the surfaces of fibers in a filter, increasing the surface area of fibers exposed to moving air improves filtering efficiency. In nonwoven fabrics the area of fibers exposed to moving air is larger than in woven fabrics, which is one reason they are used in N95 respirators (Lam et al., 2019). Cotton batting is a common three‐dimensional nonwoven material. Cotton fibers are typically 15 µm wide and 7 µm thick, and 95% of the surface area of fibers in cotton batting is exposed to air moving through the material. If a cloth mask with a filter area of 120 cm2 incorporates cotton batting with a basis weight of 200 g.m−2, the fibers would have a total surface area of approximately 6,000 cm2.
The surface of cotton fibers is physically irregular, as Fig. 4 shows, and is also chemically heterogeneous. During processing, cotton fibers twist arbitrarily in a left‐handed or right‐handed direction, causing approximately five convolutions per millimeter. Cotton fibers are 90% cellulose, and their surfaces include pectins, proteins, minerals, and waxes. Each beta glucose monomer in cellulose has several hydroxyl groups, which cause hydrogen bonding among cellulose molecules in cotton fibers. Surface hydroxyl groups in cotton may attract and hold small particles though dipole‐induced‐dipole forces. Cotton batting also gives particles more time to interact with and adhere to fibers. For example, a surgical mask is typically 0.4 mm thick (Leonas & Jones, 2003). For a given mask area and breathing rate, a particle would spend twelve times longer moving through 5 mm cotton batting than it would moving through a surgical mask. Konda et al. (2020) reported that a traditional cotton quilt (5 mm of blended cotton batting sandwiched between cotton fabric) had a filtering efficiency of 96% for particles smaller than 0.3 µm.
Fig 4.
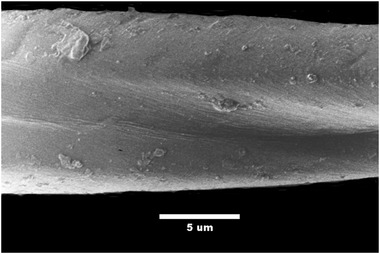
Cotton fiber
Photo Credit: CSIRO / CC‐BY‐SA‐3.0
5.1. Cotton Batting Masks
To evaluate the effectiveness of cotton batting as a filter, cloth masks were made from the following materials:
Outer and inner fabrics: 100% cotton, 120 threads per inch
Inner filter: 100% cotton batting (2.5 mm thick and 150 g.m−2 or 3.5 mm thick and 200 g.m−2)
Nose wire: 20 gauge stainless steel
Ties: hockey skate laces
The wholesale value of these materials was US$1.50. The area of fabric in each mask was 120 cm2, although the area through which air can flow was reduced by contact of the mask with the face. Fig. 5 shows the inner cotton batting layer of the mask, and Fig. 6 shows how the mask wraps around the face to give closer contact than a pleated mask design.
Fig 5.

Cotton batting mask interior
Fig 6.

Cotton batting face mask
5.2. Test Results
In May and June of 2020, 17 cotton batting masks underwent 35 tests. The tests were carried out by three independent people using commercial quantitative fit testing equipment and quantitative fit testing methods. The three tests reported filtering effectiveness of 90.2% (95% CI 88.4–92%), 77.3% (95% CI 75.1–79.4%), and 76.5% (95% CI 72.3–80.6%). Some limitations of the tests are that particle size was not known in all cases, particles were not charge‐neutralized, filtering effectiveness was not measured for a range of particle sizes, and pressure drop was not measured. The thickness of cotton batting used in the tested masks varied from 3.5 mm to 7 mm, but the results did not show a correlation between thickness and effectiveness. Although the tests showed the fit of the mask and variability among masks needs improvement, they also showed that cloth masks made by novices can reduce the amount of small particles inhaled by the wearer. Test methodologies and detailed results are described in the Supporting Information.
6. ARGUMENTS FOR AND AGAINST THE GENERAL USE OF MASKS
During the COVID‐19 pandemic, guidance from officials regarding public use of masks varied widely. Some of the arguments for and against cloth masks and general mask use are discussed here.
6.1. General Use of Masks Will Deprive Healthcare Workers of Personal Protective Equipment
This dilemma can be avoided if effective cloth masks are available to the public during pandemics.
6.2. Wearing a Mask Makes People Careless
Objective evidence to support this concern does not appear to be available (Cheng, Lam, & Leung, 2020). If the public must wear masks they must be educated about their correct use, and about the ongoing need for other measures.
6.3. There is No Scientific Evidence Masks Reduce the Risk of Transmission
Epidemiological studies to determine whether general mask use reduces transmission of diseases like COVID‐19 have examined areas where masks are commonly used. A weakness of these studies is that they do not report data regarding the filtering effectiveness of masks against outgoing respiratory droplets and incoming droplet nuclei, nor how effectively masks were used. Despite the limitations of epidemiological studies of general mask use, research by Abaluck et al. (2020); Cheng et al. (2020); Eikenberry et al. (2020); Esposito, Principi, Leung, and Migliori (2020); and Howard et al. (2020) have concluded that general mask use is helpful.
6.4. Cloth Masks Can Become Contaminated
All masks can become contaminated, and in pandemics, some people reuse disposable masks (Leung et al., 2020). During pandemics, people must be educated to use masks safely. The government of France, for example, has recommended that cloth masks be worn for no longer than four hours (AFNOR, 2020).
6.5. Cloth Masks Are Ineffective
The term “cloth mask” is ambiguous since it refers to materials rather than effectiveness. For example, one study of 1,607 hospital healthcare workers in Vietnam compared the rates of infection among two groups with a control group (MacIntyre et al., 2015). One group in the study wore cloth masks, and a second group was issued with two disposable medical masks per shift. The study found that “The rates of all infection outcomes were highest in the cloth mask arm.” The study also measured the filtering effectiveness of the cloth masks and medical masks that were used, and reported that “Penetration of cloth masks by particles was almost 97% and medical masks 44%.” In other words, the study showed that healthcare workers wearing cloth masks with a filtering efficiency of 3% became infected more often than those who wore masks with a filtering efficiency of 56%. The issue found by the study was not that “cloth masks” per se are ineffective, but that cloth masks with a filtering efficiency of 3% are ineffective.
7. AN EFFECTIVE FIBER MASK PROGRAM
If cloth masks are to be useful during pandemics, they must be produced as part of an integrated program. the proposed elements of an effective fiber mask program (EFMP) are shown in Fig. 7.
Fig 7.
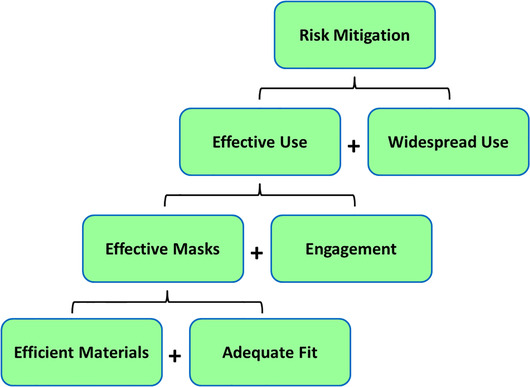
Elements of an effective fiber mask program
7.1. Effective Masks
Masks proven to reduce both incoming droplet nuclei and outgoing droplets could be called EFM. For example, the minimum requirements for an “EFM90” mask could include 90% filtering effectiveness against incoming and outgoing particles, breathability, and durability of at least 30 laundering cycles. Approved masks could be labeled. To avoid solving one problem while creating others, the EFM Program could mandate that manufacturers:
Use commonly available materials and local manufacturing resources to reduce dependence on long supply chains across borders, which are subject to logistical challenges and political forces during pandemics.
Ensure workers in their supply chain are treated ethically, by contractually requiring compliance with the Conventions of the International Labor Organization Declaration on Fundamental Principles and Rights at Work.
Minimize lifecycle environmental impacts of materials and production.
Require consumers to return masks that have reached their end of life for safe recycling.
7.2. Engagement
To ensure effective masks are used effectively, the public must be educated to understand how infections are transmitted, how masks help reduce transmission, how to use and care for masks, and why other measures such as hand hygiene are important.
7.3. Widespread Use
EFMs must be produced locally at reasonable cost. If EFMs cost US$6, at 30 uses of four hours each the cost per hour of use would be US$0.05. The economic benefits of general mask usage during the COVID‐19 pandemic were evaluated by Abaluck et al. (2020), who concluded: “…the benefits of each additional cloth mask worn by the public are conservatively in the $3,000–6,000 range due to their impact in slowing the spread of the virus.” This cost‐benefit ratio suggests governments should consider subsidizing the cost of masks for the public.
7.4. Continuous Improvement
Manufacturers can use quality assurance techniques to reduce the variability of cloth masks. Manufacturers can also use production methods not available to individuals and can undertake research and development work. For example:
A thin layer of medical‐grade silicone could be applied around a mask's edges to improve contact with the skin and reduce leakage.
A hybrid design could be based on the elastomeric half‐mask but designed to accept replaceable and reusable fiber filters.
Fiber masks could include a hemispherical polymer sieve to hold materials away from the face. This would increase the area of material involved in filtering, which improves breathability and filtering effectiveness.
Much harvested cotton is wasted because fibers are lower than desirable (Dashtbani & Afra, 2015). Could this material be used in cotton batting masks?
Natural fibers can be functionalized to increase their surface energy and fibrillated to increase roughness and surface area. Would doing so improve their efficiency as nonwoven filters?
Cotton used in air filters can be treated with antimicrobial agents to reduce the activity of microorganisms in bioaerosols (Ali, Pan, Tilly, Zia, & Wu, 2018; de Freitas Rosa, Aguiar, & Bernardo, 2017. Could treated fibers be safely used in face masks?
7.5. Cloth Mask Production in France
In early 2020 France used isolation to slow the transmission of COVID‐19. As part of its program for ending confinement, on May 11, 2020, France mandated mask use on public transport, in high schools, and in some other public spaces (République Français 2020a, 2020b; Santé Publique France, 2020). France gave the public information about transmission of COVID‐19, the intended purpose of masks, and instructions for properly using cloth masks. In March 2020 France announced new categories of nonmedical cloth masks: “Masque alternatif à Usage Non Sanitaire Catégorie 1” (UNS‐1) for people who work with the public, and UNS‐2 for people who work together, for example in an office. “AFNOR Spec S76‐001—Barrier Masks” defines performance requirements, and UNS‐1 and UNS‐2 masks must have filtering efficiencies for 3 µm particles over 90% and 70%, respectively (AFNOR, 2020). Manufacturers submitted masks for testing by the French Direction Générale de L'armement. By June 16 2020, over 800 masks had been tested, and the average reported filtering efficiencies for 3 µm particles were 96% for UNS‐1 masks and 82% for UNS‐2 masks (Government of France, 2020). In June 2020, UNS‐1 masks were available in France at US$3.
One unanticipated outcome of the French program was that the public continued to buy disposable masks, which resulted in unsold stocks of cloth masks (Willsher, 2020). This outcome can be avoided if EFMPs mandate that the public use only approved fiber masks.
8. CONCLUSIONS
Medical research and physics show social distancing is not a reliable barrier to aerosol respiratory droplets and their residual droplet nuclei. The question of whether COVID‐19 is transmitted by aerosols as well as by droplets is less important if masks are used by the public, because masks reduce transmission of droplets from infected individuals to air and to surfaces, and also reduce inhalation of droplet nuclei by susceptible individuals. During the COVID‐19 pandemic, however, some governments expressed concern that mandating general mask use would reduce the supply of respirators for healthcare workers. This is an unnecessary dilemma, as we have shown that effective fiber masks can be made by novices.
Individual countries or regions could implement EFMPs to encourage local manufacturers to use locally available resources to mass produce “EFMs” that meet high standards for filtering effectiveness, breathability, and durability. EFMPs could protect healthcare workers by safeguarding their supplies of respirators, reducing their risk of acquiring COVID‐19 from their communities, and by reducing the number of patients they must treat. In the interval between the onset of a pandemic and its resolution, an EFMP could help societies find a viable balance between supporting the economy, protecting vulnerable groups, and reducing illness. Public policy must often be made despite a degree of uncertainty, especially when societies face novel challenges (Greenhalgh, Schmid, Czypionka, Bassler, & Gruer, 2020). In the case of general mask use, available information suggests that EFMPs can improve the capacity of societies to face pandemics.
9. SUGGESTIONS FOR FURTHER WORK
-
(1)
Cloth masks could become “mobile air samplers.” Volunteers willing to share their location data could send their masks at the end of each day for testing to identify virus RNA. Dynamic maps to show the movement of a virus through communities could be created from the location data and RNA test data. The relationship between positive mask RNA tests and negative COVID‐19 tests of wearers could also indicate the protective effectiveness of masks in actual use.
-
(2)
If a minimum infectious dose is required for a pathogen to cause illness, research could evaluate the ability of effective masks to reduce exposure below this threshold. Further, if higher initial doses cause more severe illness, research could determine if another benefit of effective masks could be less severe illnesses.
-
(3)
Epidemiological studies have tried to determine if general mask use reduces transmission of droplet‐borne or airborne illnesses. Future studies should characterize the actual effectiveness of masks in use as a key variable.
-
(4)
Van Doremalen et al. (2020) found the SARS‐CoV‐2 virus lives longer on plastic than on cardboard. Research is needed to measure the viability of viruses on synthetic and natural fibers.
-
(5)
It will be helpful to research some psychological aspects of wearing masks in public. For example:
-
a
Are people more likely to voluntarily wear a mask if they believe it can not only reduce the risk that they will infect others, but also the risk that others will infect them?
-
b
What are the pros and cons of mandating mask use? Do mandates help by removing social judgements regarding mask use? Alternatively, could enough people be persuaded to voluntarily wear masks that mandates become unnecessary?
-
c
While masks interfere with nonverbal communication and can somewhat muffle speech, effective masks may allow people who would otherwise be isolated to interact with others. Could effective masks support mental health during pandemics?
-
d
The COVID‐19 pandemic left many people feeling helpless and depressed. Could wearing a mask give people a sense of empowerment in the face of pandemics?
-
a
DECLARATION OF COMPETING INTEREST
The authors report no conflicts of interest relevant to this article.
FUNDING
No funding was received to carry out the work described in this article.
Supporting information
Table 1. Fit Test Results: First Set
Table 2. Fit Test Results: Second Set
Table 3. Fit Test Results: Third Set
ACKNOWLEDGMENTS
The author gratefully acknowledges the help of Fernanda Oliveira, Tara Zala, and Mark Salter for their helpful comments; Odete Oliveira for making masks for testing; Ken Sasaki, Rachel Baaske, and Amy Mueller for testing cotton batting masks; and Leihan Tang for discussions regarding modeling of mask effectiveness.
REFERENCES
- 3M (2020). Respirators and surgical masks: A comparison. Retrieved from https://multimedia.3m.com/mws/media/957730O/respirators-and-surgical-masks-contrast-technical-bulletin.pdf
- Abaluck, J. , Chevalier, J. A. , Christakis, N. A. , Forman, H. P. , Kaplan, E. H. , Ko, A. , & Vermund, S. H. (2020). The case for universal cloth mask adoption and policies to increase supply of medical masks for health workers. SSRN Electronic Journal, 10.2139/ssrn.3567438 [DOI] [Google Scholar]
- Ali, A. , Pan, M. , Tilly, T. B. , Zia, M. , & Wu, C. Y. (2018). Performance of silver, zinc, and iron nanoparticles‐doped cotton filters against airborne E. coli to minimize bioaerosol exposure. Air Quality, Atmosphere, & Health, 11(10), 1233–1242. 10.1007/s11869-018-0622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, E. L. , Turnham, P. , Griffin, J. R. , & Clarke, C. C. (2020). Consideration of the aerosol transmission for COVID‐19 and public health. Risk Analysis, 40(5), 902–907. 10.1111/risa.13500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadi, S. , Bouvier, N. , Wexler, A. S. , & Ristenpart, W. D. (2020). The coronavirus pandemic and aerosols: Does COVID‐19 transmit via expiratory particles? Aerosol Science and Technology, 0(0), 1–4. 10.1080/02786826.2020.1749229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadi, S. , Wexler, A. S. , Cappa, C. D. , Barreda, S. , Bouvier, N. M. , & Ristenpart, W. D. (2019). Aerosol emission and superemission during human speech increase with voice loudness. Scientific Reports, 9(1), 2348–2348. 10.1038/s41598-019-38808-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association Française de Normalisation (AFNOR) . (2020). AFNOR SPEC S76‐001. Barrier masks – Guide to minimum requirements, methods of testing, making and use. March 27, 2020.
- Association Française de Normalisation (AFNOR) . (2020). FAQ ‘barrier masks’. Retrieved from https://www.afnor.org/en/faq-barrier-masks/
- Bahl, P. , Doolan, C. , de Silva, C. , Chughtai, A. A. , Bourouiba, L. , & MacIntyre, C. R. (2020). Airborne or droplet precautions for health workers treating COVID‐19? Journal of Infectious Diseases, jiaa189. 10.1093/infdis/jiaa189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bałazy, A. , Toivola, M. , Adhikari, A. , Sivasubramani, S. K. , Reponen, T. , & Grinshpun, S. A. (2006). Do N95 respirators provide 95% protection level against airborne viruses, and how adequate are surgical masks? American Journal of Infection Control, 34(2), 51–57. 10.1016/j.ajic.2005.08.018 [DOI] [PubMed] [Google Scholar]
- Bałazy, A. , Toivola, M. , Reponen, T. , Podgórski, A. , Zimmer, A. , & Grinshpun, S. A. (2005). Manikin‐based performance evaluation of N95 filtering‐facepiece respirators challenged with nanoparticles. Annals of Occupational Hygiene, 50(3). 10.1093/annhyg/mei058. [DOI] [PubMed] [Google Scholar]
- Baron, P. A. , Kulkarni, P. , & Willeke, K. (2011). Aerosol measurement: Principles, techniques, and applications. Hoboken, NJ: Wiley. [Google Scholar]
- Booth, T. F. , Kournikakis, B. , Bastien, N. , Ho, J. , Kobasa, D. , Stadnyk, L. , … Plummer, F. (2005). Detection of airborne severe acute respiratory syndrome (SARS) coronavirus and environmental contamination in SARS outbreak units. Journal of Infectious Diseases, 191(9), 1472–1477. 10.1086/429634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canada, Public Health Agency of Government of Canada . (2017). Routine practices and additional precautions for preventing the transmission of infection in healthcare settings. Retrieved from https://www.canada.ca/en/public-health/services/infectious-diseases/nosocomial-occupational-infections/routine-practices-additional-precautions-preventing-transmission-infection-healthcare-settings.html [PubMed]
- Cheng, K. K. , Lam, T. H. , & Leung, C. C. (2020). Wearing face masks in the community during the COVID‐19 pandemic: Altruism and solidarity. Lancet. 10.1016/S0140-6736(20)30918-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, V. C.‐C. , Wong, S.‐C. , Chuang, V. W.‐M. , So, S. Y.‐C. , Chen, J. H.‐K. , Sridhar, S. , … Yuen, K.‐Y. (2020). The role of community‐wide wearing of face mask for control of coronavirus disease 2019 (COVID‐19) epidemic due to SARS‐CoV‐2. Journal of Infection, 81(1), 107–114. 10.1016/j.jinf.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashtbani, R. , & Afra, E. (2015). Producing cellulose nanofiber from cotton wastes by electrospinning method. International Journal of Nano Dimension, 6(1), 1–9. 10.7508/ijnd.2015.06.001 [DOI] [Google Scholar]
- Davidson, C. S. , Green, C. F. , Gibbs, S. G. , Schmid, K. K. , Panlilio, A. L. , Jensen, P. A. , & Scarpino, P. V. (2013). Performance evaluation of selected N95 respirators and surgical masks when challenged with aerosolized endospores and inert particles. Journal of Occupational and Environmental Hygiene, 10(9), 461–467. 10.1080/15459624.2013.818243 [DOI] [PubMed] [Google Scholar]
- de Freitas Rosa, P. , Aguiar, M. L. , & Bernardo, A. (2017). Modification of cotton fabrics with silver nanoparticles for use in conditioner air to minimize the bioaerosol concentration in indoor environments. Water, Air, & Soil Pollution, 228(7). 10.1007/s11270-017-3429-y [DOI] [Google Scholar]
- Drossinos, Y. , & Stilianakis, N. I. (2020). What aerosol physics tells us about airborne pathogen transmission. Aerosol Science and Technology, 54(6), 639–643. 10.1080/02786826.2020.1751055 [DOI] [Google Scholar]
- Eikenberry, S. E. , Mancuso, M. , Iboi, E. , Phan, T. , Eikenberry, K. , Kuang, Y. , … Gumel, A. B. (2020). To mask or not to mask: Modeling the potential for face mask use by the general public to curtail the COVID‐19 pandemic. Infectious Disease Modelling, 5, 293–308. 10.1016/j.idm.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito, S. , Principi, N. , Leung, C. C. , & Migliori, G. B. (2020). Universal use of face masks for success against COVID‐19: Evidence and implications for prevention policies. European Respiratory Journal, 55(6), 2001260. 10.1183/13993003.01260-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, Y. , Marchal, T. , Sperry, T. , & Yi, H. (2020). Influence of wind and relative humidity on the social distancing effectiveness to prevent COVID‐19 airborne transmission: A numerical study. Journal of Aerosol Science, 147, 105585–105585. 10.1016/j.jaerosci.2020.105585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferron, G. A. , & Soderholm, S. C. (1990). Estimation of the times for evaporation of pure water droplets and for stabilization of salt solution particles. Journal of Aerosol Science, 21(3), 415–429. 10.1016/0021-8502(90)90070-e [DOI] [Google Scholar]
- Fisk, W. J. , Mirer, A. G. , & Mendell, M. J. (2009). Quantitative relationship of sick building syndrome symptoms with ventilation rates. Indoor Air, 19(2), 159–165. 10.1111/j.1600-0668.2008.00575.x [DOI] [PubMed] [Google Scholar]
- Ghia, U. , Gressel, M. , Konangi, S. , Mead, K. , Kishore, A. , & Earnest, G. (2012). Assessment of health‐care worker exposure to pandemic flu in hospital rooms. ASHRAE Transactions, 118(1), 442–449. [PMC free article] [PubMed] [Google Scholar]
- Government of France, Direction Générale des Entreprises . Covid 19: Les informations relatives aux masques grand public. Retrieved from https://www.entreprises.gouv.fr/covid-19/liste-des-tests-masques-de-protection. (2020).
- Greenhalgh, T. , Schmid, M. B. , Czypionka, T. , Bassler, D. , & Gruer, L. (2020). Face masks for the public during the covid‐19 crisis. British Medical Journal, m1435. 10.1136/bmj.m1435 [DOI] [PubMed] [Google Scholar]
- Guzman, M. I. (2020). Bioaerosol size effect in COVID‐19 transmission. Preprints. 10.20944/preprints202004.0093.v2 [DOI] [Google Scholar]
- Han, Z. Y. , Weng, W. G. , & Huang, Q. Y. (2013). Characterizations of particle size distribution of the droplets exhaled by sneeze. Journal of the Royal Society, Interface, 10(88), 20130560–20130560. 10.1098/rsif.2013.0560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, X. , Lau, E. H. Y. , Wu, P. , Deng, X. , Wang, J. , Hao, X. , … Leung, G. M. (2020). Temporal dynamics in viral shedding and transmissibility of COVID‐19. Nature Medicine, 26(5), 672–675. 10.1038/s41591-020-0869-5 [DOI] [PubMed] [Google Scholar]
- Hinds, W. C. (1999). Aerosol technology: Properties, behavior, and measurement of airborne particles. Hoboken, NJ: Wiley. [Google Scholar]
- Holterman, H. J. (2003). Kinetics and evaporation of water drops in air. Wageningen, Netherlands: IMAG. [Google Scholar]
- Howard, J. , Huang, A. , Li, Z. , Tufekci, Z. , Zdimal, V. , van der Westhuizen, H. , … Rimoin, A. W. (2020). Face masks against COVID‐19: An evidence review. Preprints. 10.20944/preprints202004.0203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaakkola, J. J. , & Miettinen, P. (1995). Ventilation rate in office buildings and sick building syndrome. Occupational and Environmental Medicine, 52(11), 709–714. 10.1136/oem.52.11.709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javid, B. , Weekes, M. P. , & Matheson, N. J. (2020). Covid‐19: Should the public wear face masks? British Medical Journal, m1442. 10.1136/bmj.m1442 [DOI] [PubMed] [Google Scholar]
- Kai, D. , Goldstein, G.‐P. , Morgunov, A. , Nangalia, V. , & Rotkirch, A. (2020). Universal masking is urgent in the COVID‐19 pandemic: SEIR and agent based models, empirical validation, policy recommendations. arXiv:2004.13553.
- Konda, A. , Prakash, A. , Moss, G. A. , Schmoldt, M. , Grant, G. D. , & Guha, S. (2020). Aerosol filtration efficiency of common fabrics used in respiratory cloth masks. ACS Nano, 14(5), 6339–6347. 10.1021/acsnano.0c03252 [DOI] [PubMed] [Google Scholar]
- Kowalski, W. J. , Bahnfleth, W. P. , & Whittam, T. S. (1999). Filtration of airborne microorganisms: Modeling and prediction. ASHRAE Transactions, 105, 7–10. [Google Scholar]
- Lam, T.‐N. , Wu, C.‐H. , Huang, S.‐H. , Ko, W.‐C. , Huang, Y.‐L. , Ma, C.‐Y. , … Huang, E.‐W. (2019). Multi‐Scale microstructure investigation for a PM2.5 air‐filter efficiency study of non‐woven polypropylene. Quantum Beam Science, 3(4), 20. 10.3390/qubs3040020 [DOI] [Google Scholar]
- Lauer, S. A. , Grantz, K. H. , Bi, Q. , Jones, F. K. , Zheng, Q. , Meredith, H. R. , … Lessler, J. (2020). The incubation period of Coronavirus disease 2019 (COVID‐19) from publicly reported confirmed cases: Estimation and application. Annals of Internal Medicine, 172(9), 577–582. 10.7326/M20-0504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton, G. (2020). How do we leave lockdown? New Scientist (1971), 246(3277), 10–12. 10.1016/S0262-4079(20)30706-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, K. W. , & Liu, B. Y. H. (1982). Theoretical study of aerosol filtration by fibrous filters. Aerosol Science and Technology, 1(2), 147–161. 10.1080/02786828208958584 [DOI] [Google Scholar]
- Leonas, K. , & Jones, C. R. (2003). The relationship of fabric properties and bacterial filtration efficiency for selected surgical face masks. Journal of Textile and Apparel, Technology and Management, 3(2), 1–8. [Google Scholar]
- Leung, C. C. , Lam, T. H. , & Cheng, K. K. (2020). Mass masking in the COVID‐19 epidemic: People need guidance. Lancet, 395(10228), 945–945. 10.1016/S0140-6736(20)30520-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung, N. H. L. , Chu, D. K. W. , Shiu, E. Y. C. , Chan, K‐H. , McDevitt, J. J. , Hau, B. J. P. , … Cowling, B. J. (2020). Respiratory virus shedding in exhaled breath and efficacy of face masks. Nature Medicine, 26(5), 676–680. 10.1038/s41591-020-0843-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, K. , & Marr, L. C. (2019). Humidity‐dependent decay of viruses, but not bacteria, in aerosols and droplets follows disinfection kinetics. Environmental Science & Technology, 54(2), 1024–1032. 10.1021/acs.est.9b04959 [DOI] [PubMed] [Google Scholar]
- Liu, L. , Wei, J. , Li, Y. , & Ooi, A. (2016). Evaporation and dispersion of respiratory droplets from coughing. Indoor Air, 27(1), 179–190. 10.1111/ina.12297 [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Ning, Z. , Chen, Y. , Guo, M. , Liu, Y. , Gali, N. K. , … Lan, K. (2020). Aerodynamic Characteristics and RNA Concentration of SARS‐CoV‐2 Aerosol in Wuhan Hospitals during COVID‐19 Outbreak. Nature, 582, 557–560. [DOI] [PubMed] [Google Scholar]
- MacIntyre, C. R. , Seale, H. , Dung, T. C. , Hien, N. T. , Nga, P. T. , Chughtai, A. A. , … Wang, Q. (2015). A cluster randomised trial of cloth masks compared with medical masks in healthcare workers. British Medical Journal Open, 5(4), e006577–e006577. 10.1136/bmjopen-2014-006577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton, D. K. , Fabian, M. P. , Cowling, B. J. , Grantham, M. L. , & McDevitt, J. J. (2013). Influenza virus aerosols in human exhaled breath: Particle size, culturability, and effect of surgical masks. PLoS Pathogens, 9(3), e1003205–e1003205. 10.1371/journal.ppat.1003205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska, L. , & Cao, J. (2020). Airborne transmission of SARS‐CoV‐2: The world should face the reality. Environment International, 139, 105730–105730. 10.1016/j.envint.2020.105730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska, L. , Johnson, G. R. , Ristovski, Z. D. , Hargreaves, M. , Mengersen, K. , Corbett, S. , … Katoshevski, D. (2009). Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. Journal of Aerosol Science, 40(3), 256–269. 10.1016/j.jaerosci.2008.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller, A. V. , Eden, M. J. , Oakes, J. J. , Bellini, C. , & Fernandez, L. A. (2020). Quantitative method for comparative assessment of particle filtration efficiency of fabric masks as alternatives to standard surgical masks for PPE. medRxiv. 10.1101/2020.04.17.20069567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikitin, N. , Petrova, E. , Trifonova, E. , & Karpova, O. (2014). Influenza virus aerosols in the air and their infectiousness. Advances in Virology, 2014, 859090–859090. 10.1155/2014/859090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shaughnessy, P. T. , LeBlanc, L. , Pratt, A. , Altmaier, R. , Rajaraman, P. K. , Walenga, R. , & Lin, C.‐L. (2020). Assessment and validation of a hygroscopic growth model with different water activity estimation methods. Aerosol Science and Technology, 54(10), 1169–1182. 10.1080/02786826.2020.1763247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberg, T. , & Brosseau, L. M. (2008). Surgical mask filter and fit performance. American Journal of Infection Control, 36(4), 276–282. 10.1016/j.ajic.2007.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulo, A. C. , Correia‐Neves, M. , Domingos, T. , Murta, A. G. , & Pedrosa, J. (2010). Influenza infectious dose may explain the high mortality of the second and third wave of 1918–1919 influenza pandemic. PloS One, 5(7), e11655–e11655. 10.1371/journal.pone.0011655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, H. , Miao, T. , Liu, L. , Zheng, X. , Luo, D. , & Li, Y. (2020). Indoor transmission of SARS‐CoV‐2. medRxiv, 10.1101/2020.04.04.20053058 [DOI] [PubMed] [Google Scholar]
- Qu, J. , Escobar, L. , Li, J. , Rao, Z. , & Xu, B. (2020). Experimental study of evaporation and crystallization of brine droplets under different temperatures and humidity levels. International Communications in Heat and Mass Transfer, 110, 104427. 10.1016/j.icheatmasstransfer.2019.104427 [DOI] [Google Scholar]
- Redrow, J. , Mao, S. , Celik, I. , Posada, J. A. , & Feng, Z. G. (2011). Modeling the evaporation and dispersion of airborne sputum droplets expelled from a human cough. Building and Environment, 46(10), 2042–2051. 10.1016/j.buildenv.2011.04.011 [DOI] [Google Scholar]
- République Français, Ministry of Labour . (2020a). Protocole National de Deconfinement Pour les Entreprises Pour Assurer La Santé et la Sécurité de Salariés [National de‐confinement protocol for companies to ensure the health and safety of employees]. Published May 9, 2020, Ministry of Labour, France.
- République Français . (2020b). Décret n° 2020–545 du 11 mai 2020 prescrivant les mesures générales nécessaires pour faire face à l'épidémie de covid‐19 dans le cadre de l'état d'urgence sanitaire. [Decree No. 2020–545 of 11 May 2020 prescribing the general measures necessary to deal with the epidemic of covid‐19 in the context of the state of health emergency] Retrieved from https://www.legifrance.gouv.fr/affichTexte.do;jsessionid=4EDAE9FD38F057E82136122A0F42514B.tplgfr37s_2?cidTexte=JORFTEXT000041858681&dateTexte=&oldAction=rechJO&categorieLien=id&idJO=JORFCONT000041858676.
- Roy, C. J. , & Milton, D. K. (2004). Airborne transmission of Communicable infection: The elusive pathway. New England Journal of Medicine, 350(17), 1710–1712. 10.1056/nejmp048051 [DOI] [PubMed] [Google Scholar]
- Santé Publique France (French National Public Health Agency) . COVID‐19: État des connaissances sur la généralisation de l'utilisation des masques dans l'espace public. Retrieved from https://www.santepubliquefrance.fr/les-actualites/2020/covid-19-etat-des-connaissances-sur-la-generalisation-de-l-utilisation-des-masques-dans-l-espace-public.
- Setti, L. , Passarini, F. , De Gennaro, G. , Barbieri, P. , Perrone, M. G. , Borelli, M. , … Miani, A. (2020). Airborne transmission route of covid‐19: Why 2 meters/6 feet of inter‐personal distance could not be enough. International Journal of Environmental Research and Public Health, 17(8), 2932. 10.3390/ijerph17082932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadnytskyi, V. , Bax, C. E. , Bax, A. , & Anfinrud, P. (2020). The airborne lifetime of small speech droplets and their potential importance in SARS‐CoV‐2 transmission. Proceedings of the National Academy of Sciences of the United States of America, 117(22), 11875–11877. 10.1073/pnas.2006874117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellier, R. (2006). Review of aerosol transmission of influenza A virus. Emerging Infectious Diseases, 12(11), 1657–1662. 10.3201/eid1211.060426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, D. , Charvet, A. , Bardin‐Monnier, N. , & Appert‐Collin, J. C. (2017). Aerosol filtration. London, UK: ISTE Press. [Google Scholar]
- Tian, L. , Li, X. , Qi, F. , Tang, Q. , Tang, V. , Liu, J. , … Tang, L. (2020). Calibrated intervention and containment of the COVID‐19 pandemic. arXiv:2003.07353 [DOI] [PMC free article] [PubMed]
- U.S. Centers for Disease Control and Prevention (CDC) . (2020). Respiratory protection during outbreaks: Respirators versus surgical masks. Retrieved from https://blogs.cdc.gov/niosh-science-blog/2020/04/09/masks-v-respirators/
- U.S. Food and Drug Administration (USFDA) . (2020). N95 respirators and surgical masks (face masks) Retrieved from https://www.fda.gov/medical-devices/personal-protective-equipment-infection-control/n95-respirators-and-surgical-masks-face-masks
- Van Doremalen, N. , Bushmaker, T. , Morris, D. H. , Holbrook, M. G. , Gamble, A. , Williamson, B. N. , … Munster, V. J. (2020). Aerosol and surface stability of SARS‐CoV‐2 as compared with SARS‐CoV‐1. New England Journal of Medicine, 382(16), 1564–1567. 10.1056/NEJMc2004973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vejerano, E. P. , & Marr, L. C. (2018). Physico‐chemical characteristics of evaporating respiratory fluid droplets. Journal of the Royal Society, Interface, 15(139), 20170939. 10.1098/rsif.2017.0939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch, S. , Davies, K. , Buczkowski, H. , Hettiarachchi, N. , Green, N. , Arnold, U. , … Killip, M. J. (2020). Inactivation analysis of SARS‐CoV‐2 by specimen transport media, nucleic acid extraction reagents, detergents and fixatives. Journal of Clinical Microbiology, 10.1128/JCM.01713-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willsher, K. (2020). Coronavirus cases fall in France despite easing of lockdown. The Guardian. (June, 11) Retrieved from https://www.theguardian.com/world/2020/jun/11/coronavirus-cases-fall-in-france-despite-easing-of-lockdown [Google Scholar]
- Woolhouse, M. E. J. , Howey, R. , Gaunt, E. , Reilly, L. , Chase‐Topping, M. , & Savill, N. (2008). Temporal trends in the discovery of human viruses. Proceedings of the Royal Society B Biological Sciences, 275(1647), 2111–2115. 10.1098/rspb.2008.0294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, H.‐L. , Huang, J. , Zhang, C. J. P. , He, Z. , & Ming, W.‐K. (2020). Facemask shortage and the novel coronavirus disease (COVID‐19) outbreak: Reflections on public health measures. EClinicalMedicine, 21, 100329–100329. 10.1016/j.eclinm.2020.100329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, W. , & Marr, L. C. (2011). Dynamics of airborne influenza A viruses indoors and dependence on humidity. PloS One, 6(6), e21481–e21481. 10.1371/journal.pone.0021481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, I. T. S. , Wong, T. W. , Chiu, Y. L. , Lee, N. , & Li, Y. (2005). Temporal‐spatial analysis of severe acute respiratory syndrome among hospital inpatients. Clinical Infectious Diseases, 40(9), 1237–1243. 10.1086/428735 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1. Fit Test Results: First Set
Table 2. Fit Test Results: Second Set
Table 3. Fit Test Results: Third Set


