Abstract
Background
The role of convalescent plasma therapy for patients with coronavirus disease 2019 (COVID‐19) is unclear.
Methods
We retrospectively compared outcomes in a cohort of critical COVID‐19 patients who received standard care (SC Group) and those who, in addition, received convalescent plasma (CP Group).
Results
In total, 40 patients were included in each group. The median patient age was 53.5 years (interquartile range [IQR] 42–60.5), and the majority of patients required invasive ventilation (69, 86.2%). Plasma was harvested from donors after a median of 37 days (IQR 31–46) from the first positive severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) polymerase chain reaction (PCR) result and 26 days (IQR 21–32) after documented viral clearance; it was administered after a median of 10 days (IQR 9–10) from the onset of symptoms and 2.5 days (IQR 2–4) from admission to intensive care unit. The primary endpoint of improvement in respiratory support status within 28 days was achieved in 26 patients (65%) in the SC Group and 31 patients (77.5%) in the CP Group (p = .32). The 28‐day all‐cause mortality (12.5% vs. 2.5%; p = .22) and viral clearance (65% vs. 55%; p = .49) were not significantly different between the two groups. Convalescent plasma was not significantly associated with the primary endpoint (adjusted hazard ratio 0.87; 95% confidence interval 0.51–1.49; p = .62). Adverse events were balanced between the two study groups.
Conclusion
In severe COVID‐19, convalescent plasma therapy was not associated with clinical benefits. Randomized trials are required to confirm our findings.
Keywords: convalescent plasma, coronavirus, COVID‐19, passive immunotherapy, SARS‐CoV‐2
Highlights
In patients with severe influenza, convalescent plasma was associated with improved viral clearance and reduced mortality.
In this retrospective observational study of patients with severe COVID‐19, convalescent plasma was not associated with improvement in respiratory support status within 28 days, all‐cause mortality, or viral clearance.
Randomized trials are urgently required to confirm these findings.
1. INTRODUCTION
The total global number of individuals diagnosed with coronavirus disease 2019 (COVID‐19), caused by the novel betacoronavirus severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), has surpassed 10 million, with more than 500,000 associated deaths. 1 Up to 10% of COVID‐19 patients may develop organ failure and may require admission to an intensive care unit (ICU). 2 Several investigational antiviral agents are currently being evaluated for the treatment of patients with severe and critical COVID‐19. 2 Thus far, hydroxychloroquine, with or without azithromycin, lopinavir–ritonavir, and remdesivir have not been shown to be associated with a clear clinical benefit in patients with severe COVID‐19. 3 , 4 , 5
In patients with severe influenza, convalescent plasma was associated with improved viral clearance and reduced mortality. 6 Similarly, convalescent plasma therapy for patients with severe acute respiratory syndrome (SARS) shortened hospital stays and lowered overall mortality. 7 Convalescent plasma may be useful in the treatment of severe COVID‐19. However, peer‐reviewed clinical data are limited. 8 Using a cohort of patients with severe COVID‐19, we aimed to assess the clinical benefits of convalescent plasma over and above standard care.
2. METHODS
2.1. Study design and patients
Hamad Medical Corporation (HMC) provides medical care for all COVID‐19 patients in Qatar. SARS‐CoV‐2 infection was confirmed on respiratory samples using reverse transcription polymerase chain reaction (RT‐PCR) assays TaqPath COVID‐19 Combo Kit (Thermo Fisher Scientific) or Cobas SARS‐CoV‐2 Test (Roche Diagnostics).
COVID‐19 is classified as severe if any one or more of the following is present: respiratory rate >30/min, oxygen saturation ≤90% while in ambient room air, partial pressure of oxygen–oxygen concentration (PaO2/FiO2) ≤ 300 mmHg, hypotension, or any organ failure. 2 Standard care for patients with severe COVID‐19 in HMC involved supportive care and antiviral therapy with hydroxychloroquine, azithromycin, and/or lopinavir–ritonavir. Tocilizumab could be added for those with evidence of significant systemic inflammation and methylprednisolone for acute respiratory distress syndrome (ARDS). Individual regimens were selected by the attending clinical teams based on patients' characteristics and needs.
From April 13, 2020, convalescent plasma derived from recovered COVID‐19 patients was available for clinical use for patients with severe COVID‐19. Plasma donors are adults whose COVID‐19 symptoms have resolved more than 2 weeks ago, with documented negative upper airway SARS‐CoV‐2 RT‐PCR and negative serological tests for syphilis and blood‐borne viruses.
We retrospectively included patients from HMC COVID‐19 database. The convalescent plasma group (CP Group) included patients who received SARS‐CoV‐2 convalescent plasma therapy within the first 7 days of admission to ICU, required mechanical ventilation, and completed 28 days of follow‐up by June 1, 2020. Criteria for inclusion in the comparator group, the standard care (SC Group), included laboratory‐confirmed SARS‐CoV‐2 infection, need for mechanical ventilation in ICU, and completion of 28 days of follow‐up by June 1, 2020. Starting from the earliest case of SARS‐CoV‐2 in Qatar, consecutive patients eligible for inclusion in the SC Group were selected until one control was identified for each individual in the CP Group.
2.2. Procedures
Donor apheresis was performed using Trima Accel Automated Blood Collection System (Terumo BCT) and inactivated using Mirasol PRT System (Terumo BCT). Each recipient received a total of 400 ml of ABO‐compatible convalescent plasma. Donation and receipt of plasma were subject to HMC standard clinical policies. SARS‐CoV‐2 antibody titers in the donated plasma were not available. The date on which a patient was admitted to ICU was designated as study day 1. All outcomes were recorded on the basis of the patients' disposition up to study day 28. Data including outcomes and adverse events were retrieved retrospectively from the electronic healthcare records. Radiological findings were reviewed and reported by a single radiologist who was blinded to the group allocation.
The results are reported according to the recommendations of STROBE initiative. 9 The study was approved by HMC's Institutional Review Board (MRC0120191), with a waiver of informed consent.
2.3. Outcomes
The primary outcome was improvement in the respiratory status, defined as two‐category improvement on a six‐level ordinal scale. 4 The scale in a descending order was death, invasive mechanical ventilation, noninvasive ventilation (NIV) or high‐flow nasal oxygen (HFNO) therapy, oxygen therapy other than NIV or HFNO, hospitalization without need for oxygen therapy, and discharge home or a community isolation facility. Secondary outcomes were as follows: discharged alive from ICU by study day 28 and viral clearance, defined as two consecutive negative RT‐PCR tests on airway samples taken within a gap of more than 24 hours.
2.4. Statistical analysis
In the absence of data on treatment effect, the sample size for the study was arbitrarily set at 40 patients in each group. This sample size provides approximately 80% power to detect an increase in the primary endpoint from 30% in the SC Group to 65% in the CP Group, at a two‐sided significance level of .05.
Categorical data were summarized as numbers (percentages) and compared using Fisher's exact test, whereas continuous data were presented as medians and interquartile ranges (IQR), and compared using Wilcoxon rank‐sum test. Cox proportional hazard was used to identify covariables associated with time to improvement in respiratory support status. To avoid overfitting the model, given the sample size and the number of events in the study, the number of variables included in the multivariate model was limited to four. In addition to receipt of convalescent plasma therapy and need for invasive mechanical ventilation, Acute Physiology and Chronic Health Evaluation (APACHE II) score was included due to its established role as a prognostic tool in critically ill patients. 10 The fourth variable in the multivariable Cox model was receipt of systemic corticosteroid therapy, which was recently reported to be associated with survival benefit in patients with severe COVID‐19. 11 Kaplan–Meier survival curve with log‐rank p value was used to compare time to improvement in respiratory status between the two study groups.
All p values were two‐sided with a threshold of <.05 for statistical significance. Statistical analyses were performed using Stata Statistical Software Release 15.1 (StataCorp LLC).
3. RESULTS
Eighty individuals were included, with 40 in each group. The majority were males (69, 86.3%) and the median age was 53.5 years (IQR 42–60.5). Diabetes mellitus (40, 50%) and hypertension (32, 40%) were common, and median body mass index (BMI) was 27.4 kg/m2 (IQR 25–32.4). The median duration between the onset of symptoms and admission to ICU was 7 days (IQR 6–9). Cough (73, 91.2%) and fever (68, 85.0%) were the most frequent presenting symptoms. The median APACHE II score was 12 (IQR 9–12), and all patients had pulmonary infiltrates on their chest radiographic images (Table 1).
Table 1.
Baseline characteristics of study groups
| Variable | Total (n = 80) | Standard care group (n = 40) | Convalescent plasma group (n = 40) | p value |
|---|---|---|---|---|
| Demographics and comorbidities | ||||
| Male sex | 69 (86.3%) | 35 (87.5%) | 34 (85%) | >.99 |
| Arab ethnicity | 22 (27.5%) | 15 (37.5%) | 7 (17.5%) | .078 |
| Age (years) | 53.5 (42–60.5) | 55.5 (46.5–60.5) | 47.5 (39–60.5) | .073 |
| Body mass index (kg/m2) | 27.4 (25–32.4) | 28.5 (24.7–32.4) | 27.2 (25.6–29.3) | .68 |
| Diabetes mellitus | 40 (50%) | 20 (50%) | 20 (50%) | >.99 |
| Hypertension | 32 (40%) | 19 (47.5%) | 13 (32.5%) | .25 |
| Chronic kidney disease | 6 (7.5%) | 4 (10%) | 2 (5%) | .68 |
| Chronic lung disease | 5 (6.2%) | 2 (5%) | 3 (7.5%) | >.99 |
| Current or past smokera | 12 (15%) | 9 (22.5%) | 3 (7.5%) | .12 |
| Presenting symptoms | ||||
| Symptoms at ICU admission (days) | 7 (6–9) | 7 (5.5–9) | 7 (6–8.5) | .85 |
| Fever | 68 (85%) | 36 (90%) | 32 (80%) | .35 |
| Cough | 73 (91.2%) | 35 (87.5%) | 38 (95%) | .43 |
| Sore throat | 20 (25%) | 9 (22.5%) | 11 (27.5%) | .80 |
| Dyspnea | 58 (72.5%) | 26 (65%) | 32 (80%) | .21 |
| Oxygen saturation at admission to ICU | 91% (85–95%) | 94% (85–96%) | 89% (86–94%) | .059 |
| APACHE II Score | 12.0 (9.0–14.0) | 12.0 (9.0–15.0) | 11.5 (9.0–13.0) | .23 |
| Laboratory results at admission to ICU | ||||
| Peripheral white cell count (×109/L) | 8.0 (5.3–10.4) | 8.0 (6.3–11.5) | 7.2 (5.0–9.6) | .28 |
| Lymphocyte count (×109/L) | 0.9 (0.6–1.2) | 0.90 (0.7–1.3) | 0.9 (0.6–1.1) | .43 |
| Platelet count (×109/L) | 234 (190–300) | 248.5 (200–300) | 219.5 (185–281.5) | .34 |
| Serum creatinine (µmol/L) | 86 (70.5–106) | 90 (80–119) | 81.0 (63.5–91.5) | .009 |
| CRP (mg/L) | 180 (103–259) | 180 (110.0–300.0) | 172.5 (93–231.4) | .16 |
| Serum ferritin (µg/L)b | 940 (671–1484) | 1200 (595.5–1571.5) | 920.5 (697.5–1288) | .61 |
| ALT (IU/L) | 38.5 (24–60) | 40 (24–69) | 37 (21.5–55) | .48 |
| Management | ||||
| Invasive mechanical ventilation | 69 (86.2%) | 36 (90%) | 33 (82.5%) | .52 |
| Vasopressor support | 42 (52.5%) | 22 (55%) | 20 (50%) | .82 |
| Hemodialysis | 7 (8.7%) | 5 (12.5%) | 2 (5.0%) | .43 |
| Methylprednisolone | 66 (82.5%) | 31 (78%) | 35 (88%) | .38 |
| Tocilizumab | 73 (91.2%) | 34 (85%) | 39 (98%) | .11 |
| Adverse events | ||||
| Acute kidney injury | 29 (36.2%) | 16 (40%) | 13 (32.5%) | .64 |
| Anemia | 48 (60.0%) | 20 (50%) | 28 (70%) | .11 |
| ALT rise | 66 (82.5%) | 35 (87.5%) | 31 (77.5%) | .38 |
| Bilirubin rise | 28 (35.0%) | 13 (32.5%) | 15 (37.5%) | .81 |
| Hypernatremia | 31 (38.7%) | 13 (32.5%) | 18 (45.0%) | .36 |
| Hypokalemia | 16 (20%) | 7 (17.5%) | 9 (22.5%) | .78 |
| QTc prolongation | 13 (16.2%) | 5 (12.5%) | 8 (20%) | .55 |
| Outcomes | ||||
| Improvement in respiratory support status | 57 (71.3%) | 26 (65%) | 31 (77.5%) | .32 |
| Discharged alive from ICU within 28 days | 52 (65%) | 26 (65%) | 26 (65%) | >.99 |
| The 28‐day all‐cause mortality | 6 (7.5%) | 5 (12.5%) | 1 (2.5%) | .22 |
| Viral clearance | 48 (60%) | 26 (65%) | 22 (55%) | .49 |
| Respiratory support at outcome | .42 | |||
| Ambient room air | 9 (11.3%) | 3 (7.5%) | 6 (15%) | |
| Supplemental oxygen | 50 (62.5%) | 25 (62.5%) | 25 (62.5%) | |
| Noninvasive ventilation | 1 (1.3%) | 0 | 1 (2.5%) | |
| Invasive ventilation | 20 (25%) | 12 (30%) | 8 (20%) |
Note: Data are presented as numbers (percentages) or median (interquartile range). p values are based on Fisher's exact or Wilcoxon rank‐sum tests.
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; CRP, C‐reactive protein; ICU, intensive care unit; IQR, interquartile range; LDH, lactate dehydrogenase.
Data missing for three patients.
Data missing for eight patients.
During their ICU stay, the majority of patients required invasive mechanical ventilation (69, 86.2%), and/or vasopressor support (42, 52.5%). All patients received hydroxychloroquine, azithromycin, and lopinavir–ritonavir, whereas tocilizumab was given to 73 patients (91.2%) and intravenous methylprednisolone to 66 patients (82.5%). Compared with the CP Group, the SC Group had higher median serum creatinine at admission to ICU (90 vs. 81 µmol/L, p = .009). There were no other significant baseline differences between the two groups (Table 1).
The median donor age was 36.5 years (IQR 26.3–41). All donors had infiltrates in their chest X‐rays. Seventeen donors (42.5%) required oxygen via nasal canulae, nine (22.5%) required noninvasive ventilation, and three (7.5%) required invasive mechanical ventilation. Plasma was harvested after a median of 37 days (IQR 31–46, range 26–56) from the first positive SARS‐CoV‐2 PCR result and 26 days (IQR 21–32, range 14–45) after documented viral clearance; it was administered after a median of 10 days (IQR 9–10) from the onset of symptoms and 2.5 days (IQR 2–4, range 1–7) from admission to ICU.
Adverse events occurred in 77 patients (96.3%), including grade 4 in eight, seven of which were from the SC Group. The frequency of adverse events was comparable between the two groups (Table 1).
Overall, the primary endpoint of improvement in respiratory support status was achieved in 57 patients (71.3%): 26 (65%) in the SC Group and 31 (77.5%) in the CP Group (p = .32). There were no significant differences between the SC Group and CP Group in the proportions of patients who were discharged alive from ICU within 28 days (65% for both groups; p > .99), viral clearance (65% vs. 55%; p = .49), or the 28‐day all‐cause mortality (12.5% vs. 2.5%; p = .22; Table 1).
Compared with patients in whom improvement in respiratory status was documented within 28 days, patients in whom it was not achieved were significantly older (median age 60 vs. 48 years; p < .001), had higher APACHE II scores (13 vs. 11; p = .017), and were more likely to have required hemodialysis (21.7% vs. 2 (3.5%; p = .019). Convalescent plasma recipients constituted 54.4% of those patients in whom the primary endpoint was achieved, compared with 39.1% of those in whom it was not achieved (p = .32; Table 2).
Table 2.
Baseline characteristics with respiratory status improvement at day 28 of admission to ICU
| Variable | No improvement (n = 23) | Improvement (n = 57) | p value |
|---|---|---|---|
| Demographics and comorbidities | |||
| Male sex | 20 (87%) | 49 (86%) | >.99 |
| Arab ethnicity | 6 (26.1%) | 16 (28.1%) | >.99 |
| Age (years) | 60 (55–66) | 48 (41–59) | <.001 |
| Body mass index (kg/m2) | 27 (25–32) | 27.8 (24.91–32.87) | .84 |
| Diabetes mellitus | 14 (60.9%) | 26 (45.6%) | .32 |
| Hypertension | 12 (52.2%) | 20 (35.1%) | .21 |
| Chronic kidney disease | 1 (4.3%) | 5 (8.8%) | .67 |
| Chronic lung disease | 1 (4.3%) | 4 (7.0%) | >.99 |
| Current of past smokera | 4 (17.4%) | 8 (14.0%) | .73 |
| Onset of symptoms at ICU admission (days) | 7 (4–8) | 7 (6–9) | .085 |
| Oxygen saturation at admission to ICU | 90% (85%–95%) | 90% (86–95%) | .77 |
| APACHE II Score | 13 (10–20) | 11 (8–13) | .017 |
| Laboratory results on the day of admission to ICU | |||
| Peripheral white cell count (×109/L) | 8.8 (6.4–13.8) | 7.2 (5.10–9.6) | .079 |
| Lymphocyte count (×109/L) | 1.0 (0.6–1.5) | 0.9 (0.6–1.0) | .39 |
| Platelet count (×109/L) | 238 (190–300) | 233 (190–300) | .86 |
| Serum creatinine (µmol/L) | 103 (73–130) | 84 (70–95) | .10 |
| CRP (mg/L) | 186.3 (129.1–300) | 175 (92.8–240.9) | .11 |
| Serum ferritin (µg/L)b | 1293 (858.5–1847) | 842 (657.5–1304) | .025 |
| ALT (IU/L) | 31.5 (20–59) | 39 (29.5–60) | .52 |
| Management | |||
| Convalescent plasma | 9 (39.1%) | 31 (54.4%) | .32 |
| Invasive mechanical ventilation | 21 (91.3%) | 49 (86.0%) | .72 |
| Vasopressor support | 14 (60.9%) | 28 (49.1%) | .46 |
| Hemodialysis | 5 (21.7%) | 2 (3.5%) | .019 |
| Methylprednisolone | 21 (91.3%) | 45 (78.9%) | .33 |
| Tocilizumab | 21 (91.3%) | 52 (91.2%) | >.99 |
Note: Data are presented as numbers (percentages) or median (interquartile range). p values are based on Fisher's exact or Wilcoxon rank‐sum tests.
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; CRP, C‐reactive protein; ICU, intensive care unit; LDH, lactate dehydrogenase.
Data missing for three patients.
Data missing for eight patients.
Time to improvement in the respiratory support status was not significantly different between the two study groups (p = .99; Figure 1). In the multivariable Cox model, higher APACHE II score (adjusted hazard ratio [aHR], 0.89; 95% confidence interval [CI], 0.83–0.95; p = .001) and receipt of methylprednisolone (aHR, 0.23; 95% CI 0.11–0.50; p < .001) were independently associated with a lower likelihood of achieving improvement in respiratory support status within 28 days. Receipt of convalescent plasma was not associated with time to improvement in the respiratory support status (aHR, 0.87; 95% CI, 0.51–1.49; p = .622; Table 3).
Figure 1.
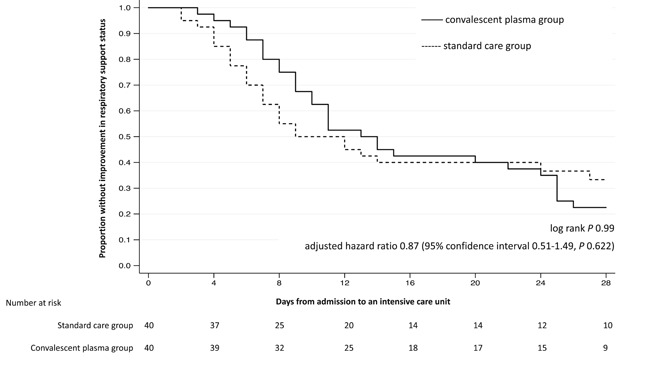
The Kaplan–Meier survival curve for time to improvement in the respiratory support status
Table 3.
Cox proportional hazard for associations with improvement in the respiratory support status
| Variable | Unadjusted hazard ratio (95% confidence interval; p value) | Adjusted hazard ratio (95% confidence interval; p value) |
|---|---|---|
| Convalescent plasma therapy | 0.99 (0.59–1.68; >.999) | 0.87 (0.51–1.49; .622) |
| Male sex | 1.12 (0.53–2.38; .749) | |
| Non‐Arab ethnicity | 1.02 (0.57–1.83; .936) | |
| Body mass index (kg/m2) | 1.00 (0.96–1.04; .856) | |
| Diabetes mellitus | 0.75 (0.44–1.26; .276) | |
| Hypertension | 0.71 (0.41–1.22; .217) | |
| Current or past smoker | 1.09 (0.52–2.32; .808) | |
| Onset of symptoms at ICU admission (days) | 1.08 (0.99–1.18; .083) | |
| Oxygen saturation | 5.11 (0.9–299.44; .432) | |
| APACHE II Score | 0.92 (0.87–0.98; .006) | 0.89 (0.83–0.95; .001) |
| Invasive mechanical ventilation | 0.99 (0.47–2.11; .995) | 2.37 (1.01–5.57; .048) |
| Methylprednisolone | 0.41 (0.21–0.79; .007) | 0.23 (0.11–0.50; <.001) |
| Tocilizumab | 0.63 (0.25–1.59; .331) | |
| Lymphocyte count (×109/L) | 0.74 (0.48–1.12; 153) | |
| Platelet count (×109/L) | 1.00 (0.99–1.00; .506) | |
| CRP (mg/L) | 0.99 (0.99–1.00; .351) | |
| ALT (IU/L) | 1.00 (0.99–1.01; .798) | |
| LDH | 1.00 (0.99–1.00; .576) | |
| d‐dimer | 0.99 (0.97–1.01; .494) | |
| Serum ferritin | 0.99 (0.99–1.00; .991) | |
| Vasopressor support | 0.98 (0.58–1.65; .932) |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; CRP, C‐reactive protein; ICU, intensive care unit; IQR, interquartile range; LDH, lactate dehydrogenase.
4. DISCUSSION
We report 28‐day outcomes for patients with severe COVID‐19 requiring mechanical ventilation in ICU. The administration of convalescent plasma after a median of 10 days from the symptom onset was not associated with an increased likelihood of improvement in the respiratory support status. Our results are consistent with those recently reported in an underpowered randomized clinical trial where the administration of convalescent plasma did not improve time to clinical improvement in patients with critical COVID‐19. 12
There are several possible explanations for our findings. First, the majority of patients in this study (86.2%) required invasive ventilation. Severe COVID‐19 is largely driven by a dysregulated inflammatory response. 13 SARS‐CoV‐2‐neutralizing antibodies may not be adequate to reverse the immunological cascade and result in clinical improvement. 14 Indeed, earlier administration of convalescent plasma for patients with SARS was associated with favorable clinical outcomes. 7 Furthermore, a meta‐analysis of eight studies (1703 patients) found that convalescent plasma for severe influenza within less than 4 days of pneumonia complications was associated with all‐cause mortality rate of 19%, as opposed to 59% in those who started treatment at a later point in their illness. 15 However, five out of six patients with critical COVID‐19 who received convalescent plasma after a median of 21.5 days from molecular confirmation could not survive. 16 To maximize potential clinical benefit, SARS‐CoV‐2 convalescent plasma may need to be administered earlier in the clinical course. To facilitate patient selection, robust COVID‐19 severity prediction tools are required. Examples exist, but none of them have been widely validated. 17
A second possible explanation for the apparent lack of clinical benefit in association with SARS‐CoV‐2 convalescent plasma in this study is the patients' characteristics. The median patient age in this study was 53.5 years, and only 7.5% of patients died within 28 days of follow‐up. This contrasts with experiences reported elsewhere. For example, an Italian report described 1591 patients with critical COVID‐19. The median age was 63 (IQR 56–70) and the overall ICU mortality was 26%. 18 Another study from the United States reported 50% all‐cause mortality in 24 patients with critical COVID‐19 who had a mean age of 64 years. 19 Our substantially lower mortality rate suggests that any clinical benefit with convalescent plasma, if one exists, may be too small to be detected in our setting using our relatively small sample size.
Third, we were unable to establish that our donors' plasma contained adequate SARS‐CoV‐2‐neutralizing antibody titers, a prerequisite for effective convalescent plasma therapy. 20 However, donations were obtained after a median of 37 days (IQR 31–46) after the date of COVID‐19 confirmation by RT‐PCR. It has been shown that immunoglobulin M (IgM) and IgG antibodies against the receptor‐binding domain (anti‐RBD) of SARS‐CoV‐2 are detectable in nearly all COVID‐19 patients after 14 days of the clinical onset. 21 Moreover, the presence of such antibodies strongly correlated with SARS‐CoV‐2‐neutralizing antibody titers. 21 , 22 In one study, SARS‐CoV‐2‐neutralizing antibodies were undetectable in only 5.7% of 175 individuals who recovered from mild COVID‐19. 22 Considering this low percentage and the extended time frame between infection and donation in our study, it is reasonable to suggest that the probability that our donors did not have adequate neutralizing SARS‐CoV‐2 antibodies is low. However, this possibility was not fully excluded.
In this study, the baseline APACHE II score, a well‐established prognostic tool for critically ill patients, was negatively associated with the primary endpoint. In addition, the use of methylprednisolone was independently associated with a lower likelihood of treatment success. Current COVID‐19 management guidelines do not recommend the routine use of systemic corticosteroids. 2 This recommendation is the result of consistent evidence of lack of clinical benefit in settings of viral pneumonia and possible harm including delayed viral clearance and increased mortality. 23 However, dexamethasone at a dose of 6 mg daily for up to 10 days was recently reported to be associated with significant survival benefit in patients with moderate‐to‐severe COVID‐19. 11 It has been suggested that systemic steroids are probably beneficial in severe COVID‐19 when administered at lower doses, but are potentially harmful at higher doses. 24 Our finding emphasizes the importance of carefully selecting COVID‐19 patients for systemic steroid therapy and the need for judicious selection of the specific treatment agent and dosing regimen.
Adverse events occurred in the majority of patients in this study. This is not surprising, given their critical status and the multitude of medical interventions employed for both groups. However, convalescent plasma appeared to be safe and was not associated with excess adverse events. Modern transfusion medicine techniques have substantially improved the safety of blood product therapies. However, there remain some potential risks with convalescent plasma therapy that require careful weighing against any possible benefits. It remains unexplored whether passive immunotherapy for COVID‐19 results in an attenuated natural SARS‐CoV‐2 immune response and post‐recovery susceptibility to SARS‐CoV‐2 re‐infection, and the potential risk of antibody‐dependent enhancement, a detrimental immunological phenomenon previously described with other viral infections. 20 , 25 In addition, the risk of blood product‐derived infections, including currently unrecognized agents, can never be completely eliminated. 26
The limitations of this study include its observational nature. We used multivariate analyses to assess independent associations with the study endpoints, but the risk of residual confounding by indication cannot be eliminated. Nonetheless, existing peer‐reviewed data in this area are mostly limited to uncontrolled case series of two to ten patients and a single prematurely halted randomized trial. 12 , 27 , 28 , 29 , 30 , 31 We, therefore, believe that our report, despite the limitations discussed above, provides useful insight for physicians considering the use of SARS‐CoV‐2 convalescent plasma for the treatment of patients with COVID‐19.
In conclusion, in this retrospective cohort study of critically ill COVID‐19 patients, convalescent plasma therapy was not associated with clinical benefit in terms of improvement in the respiratory support status within 28 days. It is not clear if the administration of convalescent plasma at an earlier stage may prevent the clinical progression of COVID‐19 and result in better clinical outcomes. Randomized clinical trials are urgently required to address these questions.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Conceptualization, methodology, formal analysis, and original draft preparation: Ali S. Omrani. Data curation: Ahmed Zaqout, Anas Baiou, Rand A. Alattar, Dana Bakdach, Hatem Abusriwil, Abdalrahman M. Mostafa, Bassem Alhariri, Naseem Ambra, Ali M. Eldeeb, and Hussam Alsoub. Formal analysis: Joanne Daghfal and Naser Elkum. Resources: Mohamed Khatib, Zeyd Merenkov, Zeinab Fawzi, Saloua M. Hmissi, Ali A. Hssain, Peter V. Coyle, Muna A. Almaslamani, and Abdullatif Alkhal. All authors approved the manuscript and agreed for its submission.
ACKNOWLEDGMENTS
Open access publication of this article was funded by Qatar National Library. No other funding was required.
Omrani AS, Zaqout A, Baiou A, et al. Convalescent plasma for the treatment of patients with severe coronavirus disease 2019: A preliminary report. J Med Virol. 2021;93:1678‐1686. 10.1002/jmv.26537
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. World Health Organization . Coronavirus disease 2019 (COVID‐19) Situation report—165; 3 July 2020. World Health Organization. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200703-covid-19-sitrep-165.pdf?sfvrsn=b27a772e_2. Accessed July 5, 2020.
- 2. World Health Organization . Clinical management of COVID‐19—interim guidance; 27 May 2020. World Health Organization. https://apps.who.int/iris/rest/bitstreams/1278777/retrieve. Accessed July 5, 2020.
- 3. Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid‐19. N Engl J Med. 2020;382(19):1787‐1799. 10.1056/NEJMoa2001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID‐19: a randomised, double‐blind, placebo‐controlled, multicentre trial. Lancet. 2020;395(10236):1569‐1578. 10.1016/S0140-6736(20)31022-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rosenberg ES, Dufort EM, Udo T, et al. Association of treatment with hydroxychloroquine or azithromycin with in‐hospital mortality in patients with COVID‐19 in New York State. JAMA. 2020;323:2493. 10.1001/jama.2020.8630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hung IF, To KK, Lee CK, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52(4):447‐456. 10.1093/cid/ciq106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheng Y, Wong R, Soo YOY, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24(1):44‐46. 10.1007/s10096-004-1271-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rajendran K, Krishnasamy N, Rangarajan J, Rathinam J, Natarajan M, Ramachandran A. Convalescent plasma transfusion for the treatment of COVID‐19: systematic review. J Med Virol. 2020;92(9):1475‐1483. 10.1002/jmv.25961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573‐577. 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 10. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818‐829. [PubMed] [Google Scholar]
- 11. The RECOVERY Collaborative Group . Dexamethasone in hospitalized patients with Covid‐19—preliminary report. N Engl J Med. 2020. 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed]
- 12. Li L, Zhang W, Hu Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life‐threatening COVID‐19: a randomized clinical trial. JAMA. 2020;324(5):460‐470. 10.1001/jama.2020.10044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Casadevall A, Pirofski LA. The convalescent sera option for containing COVID‐19. J Clin Invest. 2020;130(4):1545‐1548. 10.1172/JCI138003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta‐analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med. 2006;145(8):599‐609. 10.7326/0003-4819-145-8-200610170-00139 [DOI] [PubMed] [Google Scholar]
- 16. Zeng QL, Yu ZJ, Gou JJ, et al. Effect of convalescent plasma therapy on viral shedding and survival in COVID‐19 patients. J Infect Dis. 2020;222(1):38‐43. 10.1093/infdis/jiaa228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liang W, Liang H, Ou L, et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID‐19. JAMA Intern Med. 2020;180(8):1081‐1089. 10.1001/jamainternmed.2020.2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS‐CoV‐2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574‐1581. 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City Area. JAMA. 2020;323(20):2052‐2059. 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iwasaki A, Yang Y. The potential danger of suboptimal antibody responses in COVID‐19. Nat Rev Immunol. 2020;20(6):339‐341. 10.1038/s41577-020-0321-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. To KKW, Tsang OTY, Leung WS, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS‐CoV‐2: an observational cohort study. Lancet Infect Dis. 2020;20(5):565‐574. 10.1016/S1473-3099(20)30196-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu F, Wang A, Liu M, et al. Neutralizing antibody responses to SARS‐CoV‐2 in a COVID‐19 recovered patient cohort and their implications. medRxiv. 2020. 10.1101/2020.03.30.20047365 [DOI] [Google Scholar]
- 23. Yang Z, Liu J, Zhou Y, Zhao X, Zhao Q, Liu J. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta‐analysis. J Infect. 2020;81(1):e13‐e20. 10.1016/j.jinf.2020.03.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mahase E. Covid‐19: low dose steroid cuts death in ventilated patients by one third, trial finds. BMJ. 2020;369:m2422. 10.1136/bmj.m2422 [DOI] [PubMed] [Google Scholar]
- 25. Crowe JE Jr, Firestone CY, Murphy BR. Passively acquired antibodies suppress humoral but not cell‐mediated immunity in mice immunized with live attenuated respiratory syncytial virus vaccines. J Immunol. 2001;167(7):3910‐3918. 10.4049/jimmunol.167.7.3910 [DOI] [PubMed] [Google Scholar]
- 26. Busch MP, Bloch EM, Kleinman S. Prevention of transfusion‐transmitted infections. Blood. 2019;133(17):1854‐1864. 10.1182/blood-2018-11-833996 [DOI] [PubMed] [Google Scholar]
- 27. Ahn JY, Sohn Y, Lee SH, et al. Use of convalescent plasma therapy in two COVID‐19 patients with acute respiratory distress syndrome in Korea. J Korean Med Sci. 2020;35(14). 10.3346/jkms.2020.35.e149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID‐19 patients. Proc Natl Acad Sci U S A. 2020;117(17):9490‐9496. 10.1073/pnas.2004168117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID‐19 with convalescent plasma. JAMA. 2020;323(16):1582‐1589. 10.1001/jama.2020.4783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ye M, Fu D, Ren Y, et al. Treatment with convalescent plasma for COVID‐19 patients in Wuhan, China. J Med Virol. 2020;92(10):1890‐1901. 10.1002/jmv.25882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang B, Liu S, Tan T, et al. Treatment with convalescent plasma for critically ill patients with severe acute respiratory syndrome coronavirus 2 infection. Chest. 2020;158(1):e9‐e13. 10.1016/j.chest.2020.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


