Abstract
Background
Patients with human immunodeficiency virus (HIV) infection may be at an increased risk for morbidity and mortality from the coronavirus disease 2019 (COVID‐19). We present the clinical outcomes of HIV patients hospitalized for COVID‐19 in a matched comparison with historical controls.
Methods
We conducted a retrospective cohort study of HIV patients admitted for COVID‐19 between March 2020 and April 2020 to Newark Beth Israel Medical Center. Data on baseline clinical characteristics and hospital course were documented and compared with that of a matched control group of COVID‐19 patients who had no history of HIV. Kaplan–Meier survival curves and the log‐rank tests were used to estimate and compare in‐hospital survival between both unmatched and matched groups.
Results
Twenty‐three patients with HIV were hospitalized with COVID‐19. The median age was 59 years. The rates of in‐hospital death, the need for mechanical ventilation, and intensive care unit (ICU) admission were 13% (n = 3), 9% (n = 2), and 9% (n = 2), respectively. The HIV infection was well‐controlled in all patients except for three patients presented with acquired immune deficiency syndrome (AIDS). All AIDS patients were discharged home uneventfully. A one‐to‐one propensity matching identified 23 COVID‐19 patients who served as a control group. In both pre‐ and post‐match cohorts, survival between HIV and control groups were comparable.
Conclusions
In our cohort of HIV‐infected patients hospitalized for COVID‐19, there was no difference in mortality, ICU admission, and the need for mechanical ventilation when compared with a matched control of COVID‐19 patients with HIV.
Keywords: AIDS, in‐patients, SARS‐CoV‐2
1. INTRODUCTION
Since the declaration of the coronavirus disease 19 (COVID‐19) as a pandemic by the World Health Organization (WHO), the Centers for Disease Control and Prevention (CDC) have cautioned that, compared with the general population, people living with human immunodeficiency virus syndrome (HIV) may be at a higher risk for complications and death associated with COVID‐19. 1
Disease severity in COVID‐19 caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has been described to vary in different demographic populations based on their age, body mass index (BMI), traditional cardiovascular risk factors, and underlying co‐existing conditions. The elderly and people with chronic conditions are more likely to experience worse outcomes from COVID‐19. 2 Patients living with HIV may be at an increased risk for COVID‐19‐related complications due to (i) a higher rate of co‐existing conditions than the general population; (ii) side effects of antiretroviral therapy (ART); and (iii) traditional cardiovascular risk factors such as obesity, alcohol, and tobacco use disorder. 3
An early report suggested that HIV‐related lymphopenia may have a protective role from the severe disease in HIV patients who are susceptible to COVID‐19. 4 Moreover, the role of ART in the outcomes of HIV patients who present with COVID‐19 has been controversial, with some in vitro data showing the activity of ART against SARS coronavirus and SARS‐CoV‐2. 5 , 6
To the best of our knowledge, data on COVID 19 patients with underlying immunodeficiency such as HIV has been limited to a few cases, and clinical outcomes of hospitalized patients have not been well‐characterized. In this report, we sought to describe our experience with HIV patients who were hospitalized for COVID 19. We evaluated their clinical outcomes and compared them to that of a well‐matched control group of patients with no HIV.
2. METHODS
This study is a retrospective review of a prospectively maintained institutional review board (IRB) approved COVID‐19 database in Newark Beth Israel Medical Center, Newark, NJ. The database includes all patients with laboratory‐confirmed COVID‐19 infection, which was defined as a positive result on a reverse‐transcriptase‐polymerase reaction (RT‐PCR) assay (Abbott m2000 RealTime system) of a specimen collected on a nasopharyngeal swab. We queried the database for (a) adults 18 years of age or older, (b) with a history of HIV (confirmed with Architect HIV1/2 antigen–antibody combination fourth‐generation testing) and hospitalized between March 10, 2020 and May 10, 2020, for COVID‐19 infection with follow‐up through May 30, 2020.
There were no clear, strict guidelines used for admission. Hospitalization decision was mainly based on the individual admitting physician's discretion. Some of the criteria used for admission were (a) signs of sepsis or septic shock defined by the 2016 Third International Consensus Definition for sepsis and septic shock 7 and (b) dyspnea requiring a step up in oxygenation therapy to maintain oxygen saturation between 88% and 94%. We collected data on demographic, laboratory, and clinical outcomes from electronic medical records using a standardized data collection protocol. These data points included HIV‐associated characteristics such as most recent CD4+ T cells, CD4/CD8 ratio (obtained by flow cytometry using DD FACSCanto‐II; Becton, Dickinson Company) HIV‐RNA (copies/ml; COBAS® HIV‐1 6800/8800 systems—Roche Molecular Systems, Inc.) and their ART before infection with COVID‐19.
Clinical outcomes of hospitalized patients were compared with that of a propensity‐matched cohort of COVID‐19 patients who had no history of HIV infection. In‐hospital survival was compared between both unmatched and matched groups.
3. STATISTICAL ANALYSIS
All data were summarized by using descriptive statistics, presenting continuous variables as median, interquartile range, and categorical variables as proportions or percentages. We used the Mann–Whitney U test, Chi‐square, or Fisher's exact tests to compare differences when appropriate.
To estimate the effect of HIV on clinical outcomes, we accounted for covariates that predicted the probability of presenting with COVID. Propensity scores from a stepwise logistic regression model with backward selection using the minimum Akaike Information criterion were created. Variables included in the model were age, gender, race, body mass index (BMI), laboratory markers such as white blood cell count (WBC), hemoglobin, platelets, ferritin, C‐reactive protein (CRP), lactate dehydrogensase (LDH), erythrocyte sedimentation rate (ESR), procalcitonin, and albumin. The logit propensity scores were matched using calipers of width equal to 0.2 of their standard deviation. A similar cohort from non‐HIV patients was created in a 1:1 fashion via a greedy matching technique. We used Kaplan–Maier survival curves and the log‐rank and stratified log‐rank tests to estimate the survival and compare their differences between study groups. Statistical significance was considered when a two‐sided α of less than .05 was reached. All analyses were performed using the JMP version 14.0.2 statistical software (SAS Institute Inc.).
4. RESULTS
During the study period, 23 HIV patients were admitted for COVID‐19. The most common presenting symptoms were cough (n = 20), fever (n = 18), and dyspnea (n = 17) over a median duration of 3 days (Figure 1). Shown in Table 1 are the baseline demographic and clinical characteristics of the study cohort. Patients who required hospitalization for COVID‐19 had high rates of co‐existing medical conditions, which included hypertension (65%), chronic kidney disease (48%), or diabetes mellitus (30%). Data on antiretroviral regimen was unavailable for two patients. However, among those with complete data, integrase inhibitors (INIs; 35%), non‐nucleoside reversed transcriptase inhibitors (NNRTIs; 22%), protease inhibitors (PIs) + INI (26%)‐based antiretroviral regimen, and PI‐based regimen (4%), there was only one patient with a combination of nucleoside reversed transcriptase inhibitor (NRTI) with a PI‐based regimen.
Figure 1.
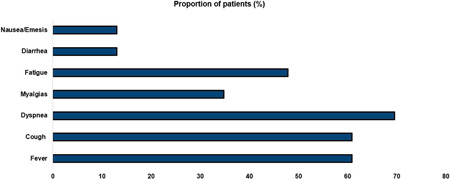
Distributions of symptoms at the time of presentation in HIV patients admitted for COVID‐19. COVID‐19, coronavirus disease 2019; HIV, human immunodeficiency virus
Table 1.
Baseline demographic and clinical characteristics of HIV patients hospitalized for COVID (n = 23)
| Variable | Distribution |
|---|---|
| Age (years) | 59 (51, 67) |
| Sex (M/F; n) (%) | 14/9 |
| Body mass index (kg/m2) | 31 (26, 36) |
| Race (n (%)) | |
| Black/AA | 23 (100) |
| Hispanic | 0 (0) |
| Symptom onset (days) | 4 (1, 7) |
| Comorbidities (n (%)) | |
| Hypertension | 15 (65) |
| Diabetes mellitus | 7 (30) |
| Chronic kidney disease | 11 (48) |
| Dialysis | 5 (22) |
| Coronary artery disease | 2 (9) |
| COPD | 1 (4) |
| White cell count × 103 (per L) | 6.6 (5.8, 8.6) |
| Lymphocytes % 103 (per L) | 16 (9, 23) |
| Hemoglobin (g/dl) | 13 (12, 14) |
| Platelets × 103 (per L) | 310 (260, 330) |
| Antiretroviral regimen (n (%)) | |
| Integrase‐based | 8 (35) |
| NNRTI | 5 (22) |
| PI + integrase‐based | 6 (26) |
| Not available | 2 (9) |
| Protease inhibitor‐based | 1 (4) |
| NNRTI + integrase‐based | 1 (4) |
Abbreviations: AA, African American; COPD, chronic obstructive pulmonary disease; COVID‐19, coronavirus disease 2019; HIV, human immunodeficiency virus; NNRTI, non‐nucleoside reversed transcriptase inhibitor.
Demonstrated in Figure 2 is a schematic representation of the CD4+ count and HIV RNA viral loads (VLs) in hospitalized patients. We had complete data of CD4 and HIV VL in 19 patients. All patients had high CD4 of over 200/µl and undetectable viral load except for three patients who had advanced AIDS. One patient had a CD4 count of 10 cells/µl and HIV RNA viral load 26,900 copies/ml, the other one had a CD4 count of 116 cells/µl and an HIV RNA viral load of >2 million copies ml and the other one had a CD4 of 179 cells/μL. The viral load in the last patient with AIDS was unavailable. All patients were discharged home alive after medical management, which included antimicrobial therapy, administration of steroids, and respiratory support.
Figure 2.
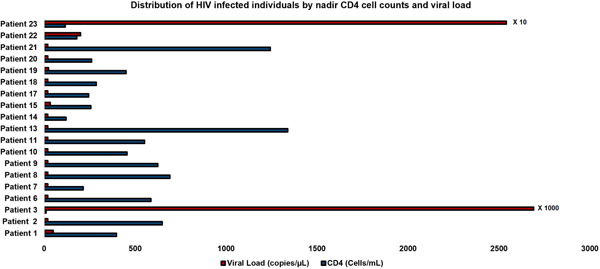
Distributions of HIV‐infected individuals by nadir CD4 cell count and viral load. HIV, human immunodeficiency virus
Given in Table 2 are the demographic, clinical, and outcomes comparison between HIV‐ and non‐HIV‐infected patients before and after 1:1 propensity score matching. A cohort of 254 non‐HIV patients who were hospitalized was compared with 23 HIV patients who were also hospitalized during the same period. Compared with the non‐HIV group, HIV patients were more likely to be of Black/African American ethnicity (p = .008). Other clinical characteristics and laboratory parameters at baseline were comparable in both groups except for the oxygenation saturation, which was worse in the HIV compared with the non‐HIV‐infected patients at the time of presentation (p = .04). For the management of COVID‐19, HIV patients were less likely to receive antimicrobial medicines: hydroxychloroquine (48% vs. 76%; p = .007), ceftriaxone (35% vs. 83%; p < .001) than their no‐HIV counterparts. Similarly, there was a lower incidence of intensive care unit (ICU) admission (9% vs. 40%; p = .001), mechanical ventilation (9% vs. 40%; p = .001), and in‐hospital death (13% vs. 60%; p = .006) in the HIV group than the non‐HIV group. There were three deaths (13%), of whom data on CD4 and HIV VL was available in only two patients. Both patients had undetectable viral loads with high CD4 > 400 cells/µl.
Table 2.
Demographic, clinical, and outcomes comparison between HIV and non‐HIV patients before and after propensity score matching
| Before matching | After 1:1 propensity matching | |||||
|---|---|---|---|---|---|---|
| Variable | HIV (n = 23) | No HIV (n = 254) | p value | HIV (n = 23) | No HIV (n = 23) | p value |
| Age (years) | 59 (51, 67) | 62 (50, 74) | .406 | 59 (51, 67) | 49 (41, 73) | .153 |
| Sex (M/F) | 14/9 | 127/127 | .316 | 14/9 | 8/15 | .760 |
| Body mass index (kg/m2) | 31 (26, 36) | 29 (26, 35) | .642 | 31 (26, 36) | 29 (26, 35) | .903 |
| Race | ||||||
| Black/AA | 23 (100) | 192 (76) | 23 (100) | 23 (100) | ||
| Hispanic | 0 (0) | 40 (16) | 0 (0) | 0 (0) | 1.000 | |
| Caucasian | 0 (0) | 12 (5) | .008 | 0 (0) | 0 (0) | |
| Other | 0 (0) | 8 (4) | 0 (0) | 0 (0) | ||
| Vital signs at presentation | ||||||
| Temperature > 100.4 F | 14 (61) | 142 (56) | .644 | 14 (61) | 13 (56) | 0.765 |
| Heart rate > 100 | 12 (52) | 106 (42) | .335 | 12 (52) | 11 (48) | .685 |
| RR > 20 | 19 (83) | 155 (61) | .030 | 19 (83) | 15 (65) | .176 |
| Oxygen requirements (n (%)) | ||||||
| Ambient air | 17 (74) | 213 (85) | 17 (74) | 20 (87) | ||
| Nasal canula | 2 (9) | 31 (12) | 2 (9) | 2 (9) | ||
| NRB | 3 (13) | 6 (2) | .038 | 3 (13) | 1 (4) | .443 |
| HFNC | 1 (4) | 0 (0) | 1 (4) | 0 (0) | ||
| Comorbidities (n (%)) | ||||||
| Hypertension | 15 (65) | 179 (70) | .235 | 15 (65) | 11 (47) | .258 |
| Diabetes mellitus | 7 (30) | 119 (47) | .124 | 7 (30) | 6 (26) | .743 |
| Dialysis | 5 (22) | 23 (9) | .084 | 5 (22) | 1 (4) | .069 |
| Coronary artery disease | 2 (9) | 56 (22) | .099 | 2 (9) | 1 (4) | .635 |
| COPD | 1 (4) | 24 (9) | .371 | 1 (4) | 2 (9) | .547 |
| Laboratory markers | ||||||
| White cell count × 103 (per L) | 6.6 (5.8, 8.6) | 7.5 (5.3, 9.4) | .428 | 6.6 (5.8, 8.6) | 6.3 (5.3, 8.4) | .629 |
| Hemoglobin (g/dl) | 12.8 (11.6, 14.2) | 12.4 (11.2, 13.9) | .242 | 12.8 (11.6, 14.2) | 13.3 (12.1, 14.5) | .578 |
| Platelets × 103 (per L) | 193 (147, 247) | 212 (161, 267) | .280 | 193 (147, 247) | 184 (144, 243) | .812 |
| d‐dimer (mg/L) | 2.1 (0.8, 13.4) | 1.4 (0.8, 3.1) | .280 | 2.1 (0.8, 13.4) | 1.26 (1.0, 2.9) | .374 |
| Albumin (g/dl) | 3.1 (2.6, 3.3) | 3.0 (2.7, 3.3) | .909 | 3.1 (2.6, 3.3) | 3.2 (2.8, 3.5) | .419 |
| Procalcitonin (ng/ml) | 0.25 (0.07, 0.39) | 0.23 (0.11, 0.77) | .297 | 0.25 (0.07, 0.39) | 0.19 (0.16, 0.52) | .827 |
| Creatinine phosphokinase (U/L) | 240 (106, 1245 | 192 (85, 657) | .442 | 240 (106, 1245) | 100 (83, 598) | .322 |
| LDH (U/L) | 557 (313, 672) | 418 (300, 615) | .220 | 557 (313, 672) | 419 (310, 562) | .355 |
| Management for COVID‐19 (n (%)) | ||||||
| Hydroxychloroquine | 11 (48) | 192 (76) | .007 | 11 (48) | 11 (48) | 1.000 |
| Azithromycin | 9 (39) | 85 (33) | .586 | 9 (39) | 3 (13) | .040 |
| Ceftriaxone | 8 (35) | 210 (83) | <.001 | 8 (35) | 17 (74) | .007 |
| Remdesivir | 0 (0) | 0 (0) | 1.000 | 0 (0) | 0 (0) | 1.000 |
| Steroids | 5 (21) | 36 (14) | .352 | 5 (21) | 5 (21) | 1.000 |
| Tocilizumab | 5 (2 | 44 (17) | .604 | 5 (22) | 3 (13) | .435 |
| Clinical outcomes | ||||||
| Length of stay (days) | 9 (5, 12) | 8 (5, 13) | .939 | 9 (5, 12) | 9 (5, 11) | .810 |
| Mechanical ventilation (n (%)) | 2 (9) | 102 (40) | .001 | 2 (9) | 6 (26) | .113 |
| ICU admission (n (%)) | 2 (9) | 102 (10) | .005 | 2 (9) | 20 (86) | .261 |
| In‐hospital mortality (n (%)) | 3 (13) | 153 (60) | .006 | 3 (13) | 6 (26) | .261 |
Abbreviations: AA, African American; COPD, chronic obstructive pulmonary disease; COVID‐19, coronavirus disease 2019; HFNC, high flow nasal cannula; ICU, intensive care unit; LDH, lactate dehydrogensase; NRB, non‐rebreathing oxygen face mask
A 1:1 propensity matching identified 23 pairs of patients in the HIV vs non‐HIV groups. Demographic and clinical characteristics were comparable after matching for the differences in baseline characteristics. The differences in outcomes in the unmatched cohort were balanced after matching as comparable outcomes for ICU admission (9% vs. 26%; p = .113), mechanical ventilation (9% vs. 26%; p = .261), and in‐hospital death (13% vs. 26%; p = .261) in the HIV group and non‐HIV group were observed.
Figure 3 illustrates the estimated in‐hospital survival between HIV and no‐HIV groups before (A) and after (B) matching. In both pre‐ and post‐match cohort's survival between HIV and non‐HIV groups was comparable. Log‐rank test: p = .057; stratified log‐rank test: p = .318.
Figure 3.
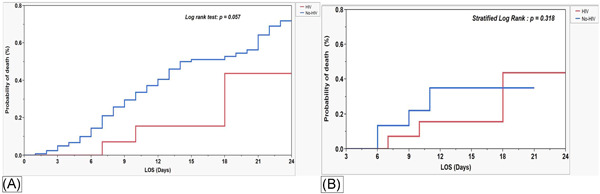
Kaplan–Meier survival curves and survival comparison between HIV and non‐HIV groups before (A) and after (B) propensity matching. HIV, human immunodeficiency virus
5. DISCUSSION
In this study of HIV patients hospitalized for COVID‐19, we found a similarity in clinical outcomes with a matched group of patients with no history of HIV. After a median length of hospitalization of 9 days, 20 patients (13%) survived to discharge and only 2 patients (9%) had severe disease requiring mechanical ventilation or intensive unit level of care. Most of the patients had a well‐controlled HIV infection, evidenced by elevated CD4 count levels and low viral loads.
The development of lymphopenia in COVID‐19 infection seems to be a key factor for the progression of the severity of illness; however, the effect of immunosuppression and the presence of lymphopenia on the clinical progression of COVID 19 has not been well‐established. The activation of the immune system, especially T cells by SARS‐CoV‐2, is associated with the severity of the illness leading to worse clinical outcomes. 8 Romanelli et al. 5 hypothesized that solid‐organ‐transplant patients treated with immunosuppressive medications are possibly protected from the severe manifestations of COVID‐19 by virtue of their anti‐inflammatory activity. Similarly, Mascolo et al. 4 noted that HIV‐related lymphopenia could be a protective feature in preventing severe clinical manifestations of COVID‐19 infection.
On the other hand, CD4 lymphopenia noted in AIDS patients was reported with variable severity of COVID‐19 infection. Guo et al. 9 hypothesized that low CD4 count might protect patients infected with HIV from the development of the cytokine storm, which is part of the COVID‐19 clinical syndrome and potentially reduce some of the severe manifestations of COVID‐19 infection. Another study by Härter et al. 10 on 33 co‐infected patients, of whom 9 were hospitalized, showed high CD4 cell count ≥350 cells in all patients except 4. Of these four patients, two had an undetectable viral load but had severe disease requiring ICU care. However, in a recent study on 51 patients, of which 28 were hospitalized, the authors found that lower CD4 cell count correlated with factors associated with disease severity. 11 Suwanwangse et al. noticed higher mortality in COVID 19 and HIV‐coinfected patients with low CD4 counts. 12 , 13 In our series of patients, we noted CD4 lymphopenia as protective against severe COVID‐19 disease. We noted that only 3 of our 23 admitted patients had CD4 cell values less than 200 µl. Of the three patients with low CD4 cell count, only one had severe disease requiring mechanical ventilation for respiratory support. The other two patients were discharged alive with no COVID‐related complications. The role of CD4 lymphopenia in protection against severe disease cannot be inferred from these present findings.
Wang et al. 14 proposed that coinfection of SARS‐CoV‐2 and HIV may severely impair the immune system based on observation of delayed immunoglobulin M (IgM) antibody response and significantly longer disease course in an HIV patient.
The management of HIV in the setting of COVID‐19 is controversial. In a study by Guo et al., 9 the authors reported no cases of COVID‐19 in 199 HIV patients treated with ritonavir‐boosted lopinavir or INIs but found that 8 out of 947 patients treated with NRTI and NNRTI combination got infected. Moreover, the authors did not find any benefit in previous use of protease inhibitors, INI, or NNRTI in their cohort. Similarly, we did not observe any effect of either class of ART in our patients. In our cohort, 10/23 (43%) of our patients had tenofovir as part of their ART, 4 of our patients had tenofovir disoproxil fumarate (TDF) and 6 had tenofovir alafenamide (TAF). Three of our patients who expired were on three different ARV combinations (one on combination of NRTI/NNRTI; second patient on two‐drug integrase‐based regimen, and the third patient on PI–integrase combination), suggesting no difference in COVID outcomes based on the HIV regimen types. In the study by Vizcarra et al., 11 73% of the patients were treated with either TDF or TAF, and the treatment modality had no effect on the clinical outcomes. Blanco et al. 15 used boosted protease inhibitors on five patients, of them four were sent home while one was still in the hospital at the time of the publication; however, the patients had variable treatment with antibiotics, steroids, and tocilizumab, making it difficult to interpret the role of protease inhibitors.
Systematic review by Cooper et al. 16 on 70 HIV patients from eight studies did show that well‐controlled HIV patients are not associated with poor outcome of COVID‐19 infection than general population which was similar to our observation.
So far, there is no published article on the comparison of HIV patients to matched controls. Our study has several limitations; the study is a retrospective analysis done in a single medical center and therefore, results may not be generalizable to other centers and may also be limited by its small sample size. A larger study from multiple centers will be needed to verify findings from this study. We attempted to account for the differences in clinical characteristics by using a propensity score method to identify the control group and to reduce selection bias. Also, we did not have full data on the immunological profile of all study subjects. A direct association between immunological profile and outcomes can, therefore, not be inferred from the presented data set. Nevertheless, the 100% survival rates of the three patients presented with AIDS may suggest a paradoxical association between a worse immunological profile and improved outcomes in COVID‐19.
6. CONCLUSION
In this cohort of HIV patients hospitalized for COVID‐19, we found a similarity in morbidity and mortality to that of historical controls. In‐hospital survival was 87%, and 9% required ICU admission. All patients had well‐controlled HIV infection except for three who had presented with AIDS. As the science regarding the management of COVID‐19 evolves, more extensive studies are needed to understand better the prognosis of HIV patients who present with COVID.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Sandhya R. Nagarakanti: patient data collection, manuscript preparation, and edits. Alexis K. Okoh: helped with manuscript preparation, edits, statistics, methods, and results. Sagy Grinberg: helped with patient data collection and data entry. Eliahu Bishburg: manuscript preparation and editing.
Nagarakanti SR, Okoh AK, Grinberg S, Bishburg E. Clinical outcomes of patients with COVID‐19 and HIV coinfection. J Med Virol. 2021;93:1687‐1693. 10.1002/jmv.26533
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. CDC . Coronavirus disease 2019 (COVID‐19) in people with HIV [Internet]. Centers for Disease Control and Prevention; 2020. https://www.cdc.gov/coronavirus/2019-ncov/specific-groups/hiv.html. Accessed March 23, 2020.
- 2. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aberg JA. Aging, inflammation, and HIV infection. Top Antivir Med. 2012;20:101. [PMC free article] [PubMed] [Google Scholar]
- 4. Mascolo S, Romanelli A, Carleo MA, Esposito V. Could HIV infection alter the clinical course of SARS‐CoV‐2 infection? When less is better [published online ahead of print April 15, 2020]. J Med Virol. 2020. 10.1002/jmv.25881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Romanelli A, Mascolo S. Immunosuppression drug‐related and clinical manifestation of coronavirus disease 2019: a therapeutical hypothesis. Am J Transplant. 2020;20:1947‐1948. 10.1111/ajt.15905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen F, Chan KH, Jiang Y, et al. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J Clin Virol. 2004;31:69‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis‐3). JAMA. 2016;315:801‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhu F, Cao Y, Xu S, Zhou M. Co‐infection of SARS‐CoV‐2 and HIV in a patient in Wuhan city, China. J Med Virol. 2020;92:529‐530. 10.1002/jmv.25732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guo W, Ming F, Dong Y, et al. A survey for COVID‐19 among HIV/AIDS patients in two districts of Wuhan, China. Preprint, March 13, 2020. https://papers.ssrn.com/sol3/papers
- 10. Härter G, Spinner CD, Roider J, et al. COVID‐19 in people living with human immunodeficiency virus: a case series of 33 patients [published online ahead of print, 2020 May 11]. Infection. 2020;1(1):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vizcarra P, Perez‐Elias M, Quereda C, et al. Description of COVID‐19 in HIV‐infected individuals a single‐center, prospective cohort. Lancet HIV. 2020;3018(20):30164‐30168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kanwugu ON, Adadi PHIV/SARS‐CoV‐2 coinfection: a global perspective [published online ahead of print July 21, 2020]. J Med Virol. 2020. 10.1002/jmv.26321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mirzaei H, McFarland W, Karamouzian M, Sharifi H. COVID‐19 among people living with HIV: a systematic review [published online ahead of print July 30, 2020]. AIDS Behav. 2020;1‐8. 10.1007/s10461-020-02983-2 [DOI] [PMC free article] [PubMed]
- 14. Wang M, Luo L, Bu H, Xia H. One case of coronavirus disease 2019 (COVID‐19) in a patient co‐infected by HIV with a low CD4+ T‐cell count. Int J Infect Dis. 2020;96:148‐150. 10.1016/j.ijid.2020.04.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blanco JL, Ambrosioni J, Garcia F, et al. COVID‐19 in patients with HIV: clinical case series. Lancet HIV. 2020;7(5):e314‐e316. 10.1016/S2352-3018(20)30111-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cooper TJ, Woodward BL, Alom S, Harky A. Coronavirus disease 2019 (COVID‐19) outcomes in HIV/AIDS patients: a systematic review. HIV Med. 2020;21(9):567‐577. 10.1111/hiv.12911 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


