Abstract
Cancer cells can evade immune surveillance in the body. However, immune checkpoint inhibitors can interrupt this evasion and enhance the antitumor activity of T cells. Other mechanisms for promoting antitumor T-cell function are the targeting of costimulatory molecules expressed on the surface of T cells, such as 4-1BB, OX40, inducible T-cell costimulator and glucocorticoid-induced tumor necrosis factor receptor. In addition, CD40 targets the modulation of the activation of antigen-presenting cells, which ultimately leads to T-cell activation. Agonists of these costimulatory molecules have demonstrated promising results in preclinical and early-phase trials and are now being tested in ongoing clinical trials. In addition, researchers are conducting trials of combinations of such immune modulators with checkpoint blockade, radiotherapy and cytotoxic chemotherapeutic drugs in patients with advanced tumors. This review gives a comprehensive picture of the current knowledge of T-cell agonists based on their use in recent and ongoing clinical trials.
Keywords: T-lymphocytes, review, receptors, immunologic, immunotherapy, costimulatory and Inhibitory T-cell receptors
Introduction
Antitumor immune responses are complex, involving multiple steps and various types of cells, and depend on the interplay of innate and adaptive immune systems. Immunotherapies targeting innate, adaptive immune cells or molecules have demonstrated therapeutic efficacy for a broad range of human malignancies.1–5 Most recently, immunotherapies targeting the adaptive immune system, specifically, T cells, have improved tumor control.4 5 Full T-cell activation requires three signals: T-cell receptor (TCR) signaling, costimulatory signaling and cytokine support.6 TCR signaling occurs through TCR recognition of a neoantigen uniquely expressed on tumor cells. Neoantigens are encoded by the mutated DNA of tumor cells, and their peptide epitopes are distinct from those derived from the normal human genome.7 They are processed and then displayed in major histocompatibility complexes on the surfaces of tumor cells and antigen-presenting cells (APCs).8 These neoantigen peptide-major histocompatibility complexes can be recognized by the TCRs of antigen-specific T cells. Therapies manipulating TCR signaling, such as chimeric antigen receptor T-cell therapy, are already used in the clinic.5
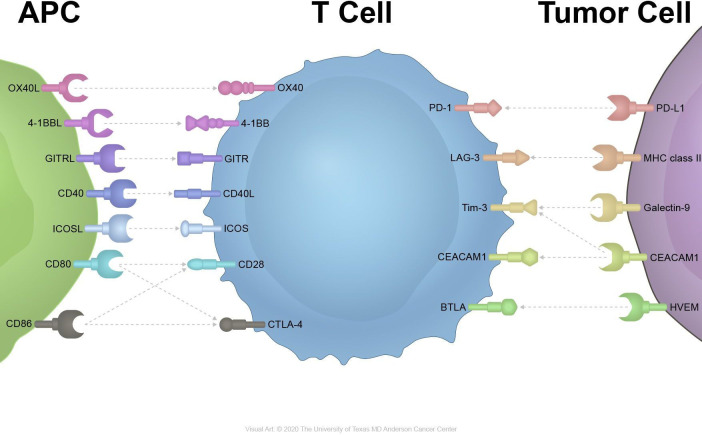
Multiple costimulatory pathways can result in the activation of T cells (figure 1).9 CD80/CD86-CD28 signaling is a major costimulatory signaling cascade contributing to T-cell activation and cytokine release.10 And the T-cell checkpoint inhibitor cytotoxic T-lymphocyte-associated protein 4 competitively binds to CD80/CD86 with a higher affinity and leads to T-cell suppression.11 Inducible T-cell costimulator (ICOS), which interacts with the ICOS ligand, is an inducible costimulatory receptor expressed on activated T cells.12 4-1BB, OX40, glucocorticoid-induced tumor necrosis factor (TNF) receptor (GITR) and other receptors in the TNF superfamily can synergize with TCR signaling to enhance T-cell responses and survival.13
Figure 1.
Inhibitory and stimulatory receptors on immune cells and cancer cells. APC, antigen-presenting cells; GITR, glucocorticoid-induced tumor necrosis factor receptor; ICOS, inducible T-cell costimulator.
Despite the success of checkpoint inhibitors in the treatment of cancer, more than 80% of patients do not respond to treatment or eventually experience resistance. Therefore, the focus of efforts to improve T cells’ antitumor responses has shifted to modifying signal through the use of agonistic antibodies targeting these molecules to boost antitumor T-cell responses. Common targets include ICOS, 4-1BB, OX40 and GITR. In addition, costimulatory receptors on APCs such as CD40 provide another means of improving T cells’ antitumor responses because they induce the expression of costimulatory ligands and the secretion of cytokines that drive antitumor activity.14
In this review, we discuss the current use of T-cell agonists in cancer immunotherapy, challenges regarding the timing of agonistic drug delivery and optimal combinations of checkpoint inhibitors, chemotherapy and/or radiotherapy.
OX40
OX40 (CD134), a member of the TNF receptor superfamily 4, is expressed mostly on activated CD4+ and CD8+ T cells and Foxp3+CD4+ regulatory T cells (Tregs). Intratumoral Tregs have particularly high levels of OX40 expression. The expression of OX40 is driven by T-cell activation and is transient, peaking 24–48 hours after T-cell activation and typically lasting 3–4 days.15 In contrast, the ligand of OX40 (CD252) is expressed on activated APCs, specifically dendritic cells (DCs), B cells and macrophages.15 16 OX40 is expressed frequently in breast cancer, melanoma, head and neck cancer, colon cancer, and B cell lymphoma cells.17–19
The signals from the binding of OX40 and its ligand promote effector T-cell expansion and survival by enhancing the expression of cyclin A, cyclin-dependent kinases, Bcl-2 antiapoptotic molecules, multiple cytokines and related receptors like interleukin (IL)-2.20 In addition, OX40 signaling promotes the generation of memory T cells and inhibits the function of Tregs.16 Several in vivo studies have demonstrated that OX40 antagonizes Foxp3+ induction in naïve CD4 T cells and inhibits IL-10 expression in inducible Tregs. Moreover, agonistic OX40 antibodies help deplete tumor-infiltrating Tregs that express OX40 via the antibody-dependent cell cytotoxicity that myeloid and natural killer cells induce after interacting with Tregs.21 In murine tumor models, an agonistic OX40 antibody, when combined with a transforming growth factor-β1 antagonist, increased the expression of interferon (IFN)-γ, downregulated IL-4 production and blocked transforming growth factor-β1-mediated Treg induction.22 However, there is evidence that the effects of agonistic OX40 antibodies can be changed under certain situations, such as when IFN-γ and IL-4 are absent. In these circumstances, Tregs could proliferate on OX40 signaling. Thus, OX40 affects the ability of Tregs to suppress immune reactions in both positive and negative directions and on the basis of the cytokine milieu.23
Thirty patients with advanced cancer (most common subtypes: melanoma and gastrointestinal, renal cell and prostate cancers) were included in the first phase 1 trial of an OX40 agonist as an anti-9B12 murine antibody.24 The most common toxic effects were lymphopenia, fatigue, rash and flu-like symptoms with fever and chills. The maximum tolerated dose (MTD) was never reached during the trial. Twelve of 30 patients had tumor shrinkage. The longest interval of stable disease (SD) lasted 470 days in a patient with renal cancer. The proliferation rate for both CD4+ and CD8+ T cells increased substantially in a dose-dependent manner, as determined by flow cytometric analysis. In contrast, the level of expression of CD4+/Foxp3+ did not increase and upregulation of OX40 was greater in tumor interstitial Tregs than in peripheral blood Tregs. However, high levels of the human antibody neutralizing this murine antibody limited the treatment to only one cycle, not multiple cycles (table 1).
Table 1.
Completed clinical trials
| Target | Drug | Trial number | Cancer type | ORR and control rate | Treatment-related adverse events (%, if available) | |
| OX40 | Monotherapy | 9B12 | NCT01644968 | Solid tumors (melanoma, gastrointestinal, renal cell, prostate, etc) | No CR or PR 40% with SD |
Lymphopenia, fatigue, rash, pruritus, fever, chills |
| MEDI0562 | NCT02318394 | Solid tumors (head and neck, bladder) | 4% with PR | Fatigue, infusion-related reaction, fever | ||
| Combination therapy | MOXR0916 given with atezolizumab | NCT02410512 | Solid tumors | 4% with PR | Mostly grade 3, one related grade 3 of pneumonitis | |
| 4-1BB | Monotherapy | Urelumab (BMS-663513) |
NCT00309023 until 2008. NCT01471210 thereafter |
Solid tumors (renal cell, ovarian, etc) Solid tumors (melanoma, renal cell, ovarian) and non-Hodgkin's lymphoma |
No CR or PR, 2.6% with SD N/A |
Grade 3–4 neutropenia (5%), fatigue (all grade) (26%), grade 3–4 transaminitis (11%), two hepatotoxicity-related deaths in each trial |
| Utomilumab (PF-05082566) | NCT01307267 | Solid tumors (Meckel cell carcinoma, and colon, gastric, and pancreatic cancers) | 5.3% with ORR 24.5% with SD |
Fatigue, fever, decreased appetite, dizziness, and rash (<10% of patients) | ||
| Combination therapy | Utomilumab plus pembrolizumab | NCT02179918 | Solid tumors (non-small cell lung, renal cell, and head and neck cancers) | 26.1% with PR or CR | Fatigue (34.8%), rash, decreased appetite (13%), nausea | |
| Urelumab plus nivolumab | NCT02253992 | Advanced solid tumor including melanoma, non-small cell lung cancer, and diffuse larger B cell lymphoma | 50% in evaluable melanoma patients. 3% in other cohorts |
Fatigue (31%), ALT increase (11%), anemia (10%) and AST increase (9%) | ||
| GITR | Monotherapy | TRX-518 | NCT01239134 | Solid tumors (melanoma, non- small cell lung and colorectal cancers) | 14% with irSD | Cough and fatigue (28% each); vomiting, abdominal pain, and nausea (18% each); dyspnea and anorexia (15% each) |
| AMG228 | NCT02437916 | Solid tumors (colorectal, head and neck, urothelial and non-small cell lung cancer) | No CR or PR; 23%with irSD |
Fatigue (13%), infusion-related reaction (7%), fever (7%), decreased appetite (7%), and hypophosphatemia (7%) | ||
| MEDI1873 | NCT02583165 | non-small cell lung cancer, head and neck squamous cell carcinoma, colorectal cancer | 42.5% with SD | Headache (25%) and infusion- related reaction, Grade three nausea/vomiting, and ST elevation myocardial infarction | ||
| Monotherapy and combination therapy | MK-4166 with or without pembrolizumab | NCT02132754 | Solid tumors and melanoma expansion cohorts | 9% for the combination therapy groups. 69% with ICI-naïve melanoma patients in the expansion |
Fatigue, infusion-related reaction, nausea, abdominal pain, and pruritus, DLT of bladder perforation | |
| CD40 | Monotherapy | rhuCD40L | Solid tumors and non-Hodgkin's lymphoma | 6% with PR, 38% with SD |
Transient elevations of serum liver transaminases | |
| CP-870,893 | NCT02225002 | Solid tumors (melanoma, non-small cell lung cancer, sarcoma, cholangiocarcinoma) | 14% of all patients and 27% of melanoma patients with PR | CRS, transient hematologica; abnormalities, LFT abnormalities | ||
| Combination therapy | CP-870,893 plus gemcitabine | Pancreatic cancer | 19% with PR, 52% with SD |
CRS, fatigue, nausea, vomiting, hematologic events | ||
| CP-870 to 893 plus paclitaxel and carboplatin | NCT00607048 | Solid tumors (mostly melanoma, renal cell carcinoma, sarcoma) | 20% with PR; in 30 evaluable patients, 40% with SD |
Fatigue (81%), neuropathy (46%), Cytokine release syndrome (42%), alopecia (42%), constipation (38%), nausea (37%), neutropenia (38%), 1 DLT of Cardiovascular syndrome |
||
| CP-870,893 plus pemetrexed and cisplatin | *ACTRN12609000294257 | Mesothelioma | 40% with PR | CRS (80%), hematological adverse events (consistent with reported chemotherapy toxicities), Grade four splenic infarction, and 1 grade 3 confusion and hyponatremia. |
*Conducted in Australia and New Zealand.
ALT, alanine aminotransferase; AST, aspartate transaminase; CR, complete response; CRS, cytokine release syndrome; DLT, dose-limiting toxicity; ICI, immune checkpoint inhibitor; irSD, immune-related stable disease; LFT, liver function test; N/A, not applicable; ORR, overall response rate; PR, partial response; SD, stable disease.
The OX40 agonist MEDI0562 was tested in a phase 1 trial of patients with advanced solid tumors. As of April 1 2016, 55 patients had received MEDI0562, with 96% of the patients experiencing adverse events (AEs).25 Sixty-seven per cent of these AEs were treatment-related, but the researchers detected no serious treatment-related AEs (TRAEs) or immune-related AEs, including dose-limiting toxicity (DLT). Paired biopsy analyzes suggested accrual of PD-L1 expression and upregulation of CD8+ T-cell infiltration in two of three evaluable patients. One patient with head and neck squamous cell carcinoma had a partial response (PR) of at least 3.7 months,26 and updated results published in 2018 reported that two patients had had PRs (table 1).25
When given in combination with other immune-modulating agents, OX40 agonists and PD-1 blockade together have led to greater effects than has either agent alone in some murine models.27 In an orthotopically transplanted murine mammary tumor virus polyoma middle T murine model of mammary cancer refractory to PD-1 blockade, OX40 stimulation delayed tumor growth.27 Of note, delayed PD-1 administration increased the durable responses of CD4+ and CD8+ T cells to tumors. A phase 1 trial of the OX40 agonist MOXR0916 given with atezolizumab included 44 patients with advanced solid tumors who were placed in dose-escalation and serial biopsy groups. The majority of the patients had tolerable safety profiles with mostly grade 1 and no grade 4 or 5 toxicity.28 However, only 2 of 51 patients in the extended trial had responses to the drug and the sponsor decided to discontinue the trial (table 1).
Another OX40 agonist, PF-04518600, is currently being tested alone and in combination with other drugs.29 In a phase 1 clinical trial involving 52 patients with melanoma, hepatocellular carcinoma, head and neck squamous cell carcinomaor renal cell carcinoma, DLT was not reported at doses up to 10 mg/kg; thus, safety was confirmed and a dose expansion cohort for the recommended dose for phase 2 was enrolled from the hepatocellular carcinoma group. Thirty-three (63%) of these patients had no cancer progression after treatment with PF-04518600.30 Other trials of OX40 agonists, such as BMS-986178 given with or without nivolumab or ipilimumab (NCT02737475) and GSK-3174998 given alone or in combination with pembrolizumab (NCT02528357), are ongoing and results are pending (table 2).
Table 2.
Selected ongoing and/or recruiting clinical trials in advanced malignant tumor
| Target | Trial no | Regimen | Disease | Phase |
| OX40 | NCT02528357 | GSK3174998 alone or with pembrolizumab | Advanced solid tumor | 1 |
| NCT02737475 | BMS-986178 alone or in combination With nivolumab and/or Ipilimumab | Advanced solid tumor | 1/2 | |
| NCT03092856 | Axitinib With or Without PF-04518600 | Metastatic kidney cancer | 2 | |
| NCT04198766 | INBRX-106 (Hexavalent OX40 Agonist) and pembrolizumab | Locally advanced or metastatic solid tumor | 1 | |
| NCT03831295 | SD-101 and BMS-986178 | Locally advanced or metastatic solid tumor | 1 | |
| NCT03894618 | SL-279252 (PD1-Fc- OX40L) | Locally advanced or metastatic solid tumor or lymphoma | 1 | |
| NCT03971409 | Avelumab with binimetinib, utomilumab or PF-04518600 | Stage IV or unresectable or recurrent triple negative breast cancer | 1 | |
| NCT03217747 | Avelumab, utomilumab, PF-04518600 and radiation therapy | Advanced solid tumor | 1/2 | |
| NCT03782467 | ATOR-1015 (Bispecific human monoclonal antibody targeting cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and OX40) | Advanced solid tumor | 1 | |
| NCT03447314 | Combination of GSK1795091 and Immunotherapies (GSK1795091Drug: GSK3174998Drug: GSK3359609Drug: pembrolizumab) | Advanced solid tumor | 1 | |
| 4-1BB | NCT02845323 | Neoadjuvant nivolumab with and without urelumab | Cisplatin ineligible or chemorefractory muscle invasive urothelial carcinoma | 2 |
| NCT03364348 | 4-1BB agonist monoclonal antibody PF-05082566 with trastuzumab emtansine or trastuzumab | Advanced HER2-positive breast cancer | 1 | |
| NCT03217747 | Avelumab, utomilumab, PF-04518600 (Anti OX40) and radiation therapy | Advanced solid tumor | 1/2 | |
| NCT04009460 | ES101 (PD-L1×4–1 BB bispecific antibody) | Advanced solid tumor | 1 | |
| NCT03650348 | PRS-343 in combination with atezolizumab | HER2-positive solid tumor | 1 | |
| NCT04144842 | ATOR-1017 | Advanced solid tumor | 1 | |
| NCT04121676 | AGEN2373 | Advanced cancer | 1 | |
| NCT03881488 | CTX-471 post-PD-1/PD-L1 | Locally advanced solid tumor/metastatic cancer | 1 | |
| GITR | NCT02598960 | BMS-986156 alone or in combination with nivolumab | Advanced solid tumor | 1/2 |
| NCT04021043 | MS-986156, Ipilimumab, and nivolumab with or without stereotactic body radiation therapy | Advanced or metastatic lung/chest or liver cancer | 1/2 | |
| NCT03799003 | ASP1951 with or without pembrolizumab | Advanced solid tumor | 1 | |
| NCT03123783 | APX005M in combination with nivolumab | Non-small cell lung cancer and melanoma | 1 | |
| NCT02740270 | GWN323 alone and in combination with PDR001 | Advanced malignancies and lymphomas | 1 | |
| NCT03126110 | INCAGN01876 combined with immune therapies | Locally advanced solid tumor/metastatic cancer | 1/2 | |
| CD40 | NCT03123783 | APX055M in combination with nivolumab | Non-small cell lung cancer/metastatic melanoma | 1/2 |
| NCT02706353 | APX005M in combination with pembrolizumab | Metastatic melanoma | 1/2 | |
| NCT02376699 | SEA-CD40 | Advanced malignancy | 1 | |
| NCT03852511 | NG-350A oncolytic virus | Metastatic cancer | 1 | |
| NCT02705196 | LOAd704 oncolytic virus | Pancreatic cancer | 1/2 | |
| NCT03329950 | CDX-1140 as monotherapy or in combination | Advanced malignancies | 1 | |
| ICOS | NCT03989362 | Vopratelimab (JTX-2011) and a CTLA-4 inhibitor | Non-small cell lung cancer/urothelial cancer | 2 |
| NCT03219224 | Vopratelimab alone and in combination with anti-PD-1 or anti-CTLA-4 | Advanced solid tumor | 1/2 | |
| NCT03829501 | KY1044 and atezolizumab | Advanced cancer | 1/2 | |
| NCT02723955 | GSK3359609 | Advanced solid tumor | 1 | |
| NCT03693612 | GSK3359609 plus tremelimumab | Advanced solid tumor | 2 |
Combining OX40 agonist-based treatment with radiotherapy controls tumor growth in murine models, and researchers are currently determining the optimal timing of the two treatments.31 32 In one trial, mice injected with CT26 colorectal tumor cells received radiation plus anti-OX40 antibodies via multiple methods. In this trial, giving anti-OX40 prior to radiotherapy was the most effective treatment method and the optimal time for administering anti-OX40 antibodies was 1 day before radiotherapy.33
4-1BB
The costimulatory molecule 4-1BB is found on both CD4+ and CD8+ T cells in mice. In humans, 4-1BB is expressed on activated CD8+ T cells, activated natural killer cells, natural killer T cells, Tregs, DCs and other myeloid-lineage cells. The ligand of 4-1BB is 4-1BBL, which is inducibly expressed on activated APCs, myeloid progenitor and hematopoietic stem cells.34 35 4-1BB expression levels peak about 12 hours after stimulation and decline within 72 hours.36
On conjunction with its ligand CD137L, 4-1BB forms a heterotrimer with the TNF-associated factors TRAF1 and TRAF2, resulting in downstream activation of the nuclear factor kappa-light-chain-enhancer of activated B cells, extracellular signal-regulated kinase, c-Jun N-terminal kinase and mitogen-activated protein kinase signaling pathways.37 Ultimately, this enhances the cytotoxic T-cell effect via upregulation of the antiapoptotic molecules Bcl-xL and Bfl-1 via TNF-α and IFN-γ secretion.35 38 4-1BB signaling also prolongs cytotoxic T-cell survival.
With regard to Tregs, the role of 4-1BB is controversial. 4-1BB activation is reported to inhibit the formation of inducible Tregs via downregulation of Foxp3 and to possibly inhibit Treg suppression via activation of the Akt pathway.39 On the other hand, scientists also reported that a soluble form of 4-1BBL promotes Treg expansion and immune-inhibitory function and that IL-2 upregulates 4-1BB on Tregs.35 40 As for natural killer cells, Fc receptor engagement-upregulated 4-1BB expression enhances antibody-dependent cell-mediated cytotoxicity.41
In addition to being a target for immunotherapy, 4-1BB signaling has shown tremendous promise in the generation of the generation of chimeric antigen receptors.42 43 To increase T cells’ persistence, activity and ability to expand, chimeric antigen receptor T-cell therapy needs a costimulatory domain incorporated with the CD3ξ chain’s cytoplasmic domain. 4-1BB is a candidate costimulatory domain, and CD19 chimeric antigen receptor T-cell therapy with 4-1BB has shown promising results in the treatment of hematological malignancies.44 45 Several studies have combined targeting 4-1BB with chimeric antigen receptor T-cell therapy.46 47
In preclinical studies, 4-1BB agonists demonstrated antitumor efficacy in several solid tumor models.48 49 For example, in a CT26 colorectal cancer mouse model, a 4-1BB agonist demonstrated dose-dependent suppression of tumor proliferation.50 Based on this preclinical study, researchers developed fully human monoclonal antibodies against 4-1BB, such as urelumab (BMS-663513) and utomilumab (PF-05082566).51
Urelumab is a fully human IgG4 with a point mutation of S228P. In phase 1 and 2 trials of urelumab, 115 patients with advanced or metastatic melanoma, renal cell carcinoma or ovarian cancer were randomized to receive different dose levels of urelumab. Overall, fatigue, reversible grades 3–4 transaminitis and grades 3–4 neutropenia were the most common AEs. The optimal dose of urelumab was not identified, but three patients experienced SD.52 Likewise, a biomarker study of urelumab demonstrated increased levels of the activation molecules of peripheral CD8 T cells and IFN-inducible genes in peripheral blood.52 In a phase 2 trial of urelumab, patients with metastatic melanoma were placed in four groups and given treatment; however, the trial was terminated because of a high incidence of grade 4 hepatotoxicity. Eventually, enrolment in the trial was ended following two hepatotoxicity-related deaths in December 2008.53 A phase 1b monotherapy trial of urelumab resumed in 2012. In this study, two deaths occurred at the higher doses given to patients (1 and 5 mg/kg). A dosage of 0.1 mg/kg given every 3 weeks was considered safe, although 16% of patients given this dose experienced fatigue and 13% experienced nausea.53
Utomilumab, another fully human engineered IgG2 antibody against 4-1BB, was studied in a phase 1 monotherapy trial of 55 patients with advanced solid cancers (Meckel cell carcinoma of the skin, soft tissue sarcoma, and colorectal, gastric, pancreatic, lung, hepatobiliary and breast cancers) or lymphoma.54 A 3+3 dose escalation model was used, and the primary endpoint for the first two cycles was DLT. As a result, utomilumab was tolerated by all patients. The most common AEs were fatigue, fever, decreased appetite and dizziness, and none of the patients experienced liver toxicity. The objective response rate was 3.8%, and patients with Merkel cell carcinoma had a higher response rate, including one complete response (CR), than did patients with other cancers. The best overall response, SD, was seen in 24.5% of patients across all dose-level groups.54
A great deal of evidence demonstrates that 4-1BB agonists induce synergism with other immunotherapy or cytotoxic chemotherapy treatments. In a melanoma cell line model in which a 4-1BB agonist was combined with pembrolizumab, the combination increased antitumor activity in melanoma cells. Specifically, it elevated the CD8+/Treg ratio and increased the activity of tumor-specific cytotoxic T lymphocytes.55 In an ovarian cancer cell model, dual targeting of 4-1BB and PD-1 receptors improved survival and increased effector CD8+ T-cell density but decreased the number of Tregs and myeloid-derived suppressor cells.56 A study in which 4-1BB was given in combination with radiotherapy to mice with implanted tumor cells had similar results.57 In these murine models, the combination led to significant growth retardation in breast tumors. In addition, antitumor activity increased in a lung tumor model, although only at the highest evaluated radiation dose.57 Finally, other studies have shown that administering 4-1BB agonist with chemotherapeutic drugs, including 5-fluorouracil, DNA-alkylating platinum-containing derivatives and cyclophosphamide, led to tumor progression and increased survival rates in mouse models with certain types of solid tumors, such as renal cancer and colorectal cancer.58–60
On the basis of preclinical data, Tolcher et al61 conducted a phase 1b study of utomilumab plus pembrolizumab for the treatment of advanced solid tumors, including non-small cell lung cancer, renal cell cancer, and head and neck squamous cell carcinoma. Twenty-three patients participated in the trial and were given utomilumab (0.45–5.00 mg/kg) and pembrolizumab (2 mg/kg) every 3 weeks. No patients experienced DLT, and TRAEs were manageable and grade 1 or 2. The objective response rate was 26% (6 of 23 patients), and one complete remission occurred in a patient with small cell lung cancer. In five of the six responders, the responses were maintained for more than 6 months.
A phase 1/2 study reported by Massarelli et al62 in 2016 demonstrated the safety of the combination of urelumab and nivolumab in patients with advanced malignancies. In patients given urelumab alone, TRAEs occurred in only 7% of patients and led to discontinuation of the study in 5% of them. Six patients in the response group had lymphoma. In 104 patients with melanoma, non-small cell lung cancer, head and neck cancer squamous cell carcinoma, or diffuse large B cell lymphoma, only 7% of the patients had TRAEs, and TRAEs led to study discontinuation (the primary end point) in 6% of the patients. The objective response rate was 10.5% (9 of 86 patients). In this study, adding nivolumab to urelumab did not produce substantial additive/synergistic benefits at the evaluated dose levels. Several trials of 4-1BB agonists are currently recruiting patients (table 1).
Glucocorticoid-induced tumor necrosis factor receptor
GITR (CD357), a member of TNF receptor superfamily 18, is highly expressed on Tregs—which contributes to Tregs’ expansion and differentiation63—and at low levels on naïve and memory T cells.64 It peaks after 2–3 days of single administration and declines by day 5. The complementary ligand is GITR ligand, which is expressed at low levels in APCs such as DCs, macrophages and B cells. GITR boosts T-lymphocyte activity after suboptimal TCR stimulation by upregulating IL-2 and IFN-γ.65 66 It also enhances T-cell survival by inhibiting TCR activation-induced apoptosis.67 Preclinical data on GITR monotherapy demonstrated that the agonist anti-GITR antibody DTA-1 was efficacious against cancer cells in B16 melanoma mouse models.68 It stimulates both CD4+ and CD8+ T cells and inhibits intratumoral Tregs by altering their stability.
A phase 1 study of the GITR monoclonal antibody of TRX-518 was performed in 40 patients with metastatic solid tumors such as melanoma and non-small cell lung and colorectal cancers.69 The patients tolerated TRX-518 well and did not experience DLT. The incidence rate for TRAEs was 15%, and the most common events were cough and fatigue (both 28%). Four of 28 patients had immune-related SD, although the efficacy data in the study were limited.69 Also, a phase 1 study of AMG228, another GITR agonist, involved 30 patients with advanced solid tumors, including colorectal, head and neck squamous cell, urothelial and non-small cell lung cancers.70 The most common TRAEs were fatigue, infusion-related reactions, fever and decreased appetite. Three fatal AEs, consisting of pneumonitis, acute hypoxemic respiratory failure and progressive disease were reported, but in the remaining patients, the MTD was not reached. Twenty-seven patients were evaluable by using immune-related response criteria and seven had SD. However, T-cell activation and antitumor activity were not correlated with GITR coverage in tumors or peripheral blood. Another GITR agonist, MEDI1873, was investigated in a phase 1 study.71 Forty patients with advanced tumors (including non-small cell lung, head and neck squamous cell and colorectal cancers) were enrolled, 82.5% of whom experienced drug-related AEs. Three patients experienced DLT, and one presented with non-ST-elevation myocardial infarction at the maximum dose of MEDI1873; however, the MTD was not reached. The best overall response was SD, occurring in 42.5% of the patients, and SD was maintained in three patients for more than 52 weeks. In an associated translational research study, MEDI1873 was involved in GITR expression on CD4+ T cells and increased it in CD4+/Ki67+ T cells at doses greater than 25 mg. Responses in intratumoral T cells were similar to those in extratumoral T cells.71
The combination of a GITR agonist with other treatments was explored as well. In a preclinical study with an ID8 murine ovarian cancer model, the combination of a GITR agonist and a PD-1 inhibitor inhibited peritoneal ID8 tumor growth by increasing the responses of memory immune cells to tumor cells and the frequencies of IFN-γ-producing effector T cells. In addition, it suppressed Tregs and myeloid-derived suppressor cells by shifting an immunosuppressive tumor milieu to an immunostimulatory state.65 72 Furthermore, adding chemotherapy (cisplatin and paclitaxel) to the GITR agonist and PD-1 inhibitor enhanced the synergy of the treatments.72
An active study of the anti-GITR agonist BMS-986156 and nivolumab suggested that the incidence rate for grade 1 TRAEs was 70% in patients receiving combination therapy compared with 59% in those receiving monotherapy; no patients in either group experienced DLT (table 1).73 A comparison study of MK-1248 monotherapy and MK-1248 in combination with pembrolizumab in 37 patients showed that 17 patients (45.9%) had at least one TRAE and that most AEs were manageable and not accompanied by DLT. The incidence of serious AEs did not differ markedly between the two groups: 6 of 20 patients receiving monotherapy and 5 of 17 receiving combination treatment. In terms of efficacy, of the patients in the combination treatment group, one had a CR and two had a PR.74 A phase 1 study of MK4166 (NCT02132754) with and without pembrolizumab demonstrated a similar safety profile but higher response rates in immune checkpoint inhibitor-naïve melanoma patients.75
CD40
CD40 is a member of TNF receptor superfamily 5. It is expressed on DCs, B lymphocytes, monocytes, and vascular and epithelial cells. Its ligand, CD40L (CD154), is expressed transiently on activated T lymphocytes.76 77 CD40 signaling in DCs promotes proinflammatory cytokine release, upregulates costimulatory molecules and facilitates the cross-presentation of antigens.78 CD40 ligation of B cells also increases B cells’ antigen-presentation capacity and promotes immunoglobulin class switching.79 80 CD40 is not a direct T-cell agonist. However, its increased antigen presentation and immunoglobulin class-switching enhances T-cell activation indirectly. CD40 also affects the expression of proapoptotic and antiapoptotic genes on different types of cells. In normal and certain low-grade malignant B cells, CD40 ligation rapidly rescues cells from apoptosis via the nuclear factor-κB pathway, whereas CD40 ligation causes the apoptosis of breast carcinoma cells by upregulating Bax expression.76 Macrophages activated by CD40 can become tumoricidal, facilitate the depletion of tumor stroma, and cause tumor regression independent of T cells.
A preclinical study of CD40 agonists in pancreatic ductal carcinoma suggested that these agonistic antibodies activate macrophages, directly causing tumor regression, and that this process is independent of T cells.81 Based on this scientific rationale, a CD40 agonistic antibody from multimeric versions of CD40L was evaluated in 32 patients with solid tumors or lymphoma.82 Two patients had objective responses. In addition, the researchers conducting the study determined that serum liver transaminase was a scale for defining MTD. In another study, CP-870,893, another fully selective CD40 agonist, was given to 29 patients with advanced tumors (mostly melanomas), in whom manageable CPS was the most common AE. Four patients with melanoma had objective responses at restaging.83 CDX-1140, APX005M and SEA-CD40 are other candidate agonistic CD40 monoclonal antibodies for cancer immunotherapy, but they resulted in minor antitumor responses in early-phase studies (table 1).
Studies of some preclinical tumor models have shown that combination therapies are promising as means of overcoming immune refractoriness. In a pancreatic carcinoma model, T-cell immunity induced by a CD40 agonist overcame resistance to PD-L1 or CTLA-4 blockage.84
Based on the assumption that CP-870,893 has a synergic effect with chemotherapy when given at the single-agent MTD, it has been extensively tested in combination with cytotoxic chemotherapy. In a study of 22 patients with treatment-naïve advanced pancreatic cancer, patients were given gemcitabine and CP-870,893.85 The drug was well tolerated. One patient experienced DLT (a grade 4 cerebrovascular accident) at a dose that was regarded as the MTD. The most common TRAE was cytokine release syndrome. Patients given combination therapy had better prognoses than did those given monotherapy, with four patients having PRs and 11 patients having SD. CP-870, 893 has also shown good results when combined with paclitaxel/cisplatin and administered to cohorts of mostly melanoma patients.86 In other studies, CP-870, 893 combined with cisplatin and pemetrexed in patients with malignant pleural mesothelioma had tolerable toxicity profiles and tumor control effects (overall response rate, 40%, respectively)87 (table 1).
An active phase 1b trial of the anti-CD40 antibody APX005M given to patients with pancreatic cancer in combination with gemcitabine and nab-paclitaxel with or without nivolumab (NCT03214250) showed favorable results.88 In the DLT-evaluable population (24 patients), the overall response rate was 54%. In the subgroup who received nivolumab, the overall response rate was 67%. A phase 2 study of APX005M is now underway, as are other combination trials of APX005M with PD-1, PD-L1 or Flt3 ligand (table 2).
ICOS (CD278)
ICOS is a member of the CD28 superfamily of costimulatory molecules. Like other members of this superfamily, its expression can be induced rapidly by T-cell activation and delivers secondary costimulatory signals inside T cells.89 90 The ligand of ICOS is ICOS ligand, which is mostly expressed on B cells, macrophages and DCs. The expression of ICOS ligand can be induced by IFN-γand TNF-α.91 ICOS ligand-ICOS signaling modestly promotes T-cell proliferation and differentiation and substantially enhances the production of cytokines such as IL-4, IL-5, IL-10, IFN-γ and TNF-α. It also induces Foxp3 transcription and suppresses Tregs.92 93
The efficacy of JTX2011, a cross-reactive humanized ICOS agonist, was tested first in murine cancer models. The antibody showed activity when given as monotherapy and in combination with anti-PD-L1 antibodies, and its level of activity corresponded with the level of ICOS expression on tumor interstitial Tregs.94 This study also demonstrated that non-small cell lung cancer and head and neck squamous cell carcinoma have higher percentages of ICOS-expressing cell infiltrates as demonstrated by an integrated expression analysis of human tumors. Subsequent, active phase 1/2 trials in patients with advanced malignancies have tested JTX2011 when given as monotherapy and in combination with immune checkpoint inhibitors.95 The study demonstrated that JTX2011-based therapy was tolerable, but it did not demonstrate promising results in terms of response. Of the 67 patients given JTX2011 monotherapy, only 1 (1.5%) had a PR. Of the 106 patients who received JTX2011 in combination with nivolumab, 8 (7.5%) had PRs (table 2).
The anti-ICOS monoclonal antibody KY1044 targets depletion of intratumoral Tregs and stimulation of T effectors.96 A phase 1 trial of KY1044 as monotherapy or in combination with atezolizumab is underway (NCT03829501).97 A first-in-human study of the ICOS agonist GSK3359609 was performed with the agonist given as monotherapy and in combination with pembrolizumab.98 The primary basket trial enrolled 98 patients with metastatic or relapsed invasive cancer and demonstrated drug safety in both monotherapy and combination therapy groups without reaching the MTD. In an expansion cohort of patients with head and neck squamous cell carcinoma, 51 patients received either monotherapy or combination therapy with pembrolizumab.99 The overall response rate was 8% (1 of 8 patients) for the monotherapy group and 28% (8 of 29 patients) for the combination therapy group. The combination treatment showed promising antitumor activity and a manageable safety profile. A phase 3 trial of KY1044 and atezolizumab in patients with PD-L1-positive head and neck squamous cell carcinoma is ongoing (NCT04128696) (table 1).
Challenges associated with targeting costimulatory pathways to improve antitumor immunity
Although checkpoint blockade immunotherapy advances cancer treatment, the resulting tumor control outcomes vary among patients. Various factors contribute to resistance to checkpoint blockade immunotherapy, such as changes in tumor mutations, T-cell infiltration, suppressive tumor microenvironments, antigen presentation deficiencies and other non-specific issues.100 Likewise, therapies targeting costimulatory molecule pathways are also complicated by tumor subtypes, expression pattern of the target molecules, and how to combine with other immune check point inhibitor or chemotherapy. In particular, the use of a single agonist drug has shown a lack of response in early-phase trials, and the overall response to these agonists is, contrary to researchers’ expectations, very low (less than 10%).101 However, when combined with other treatment methods, synergism has been observed. Overall, a deeper understanding of the impact of these agents in vivo is needed to help identify logical combination approaches that can be tested in the clinic
Regarding subtypes of cancers, patients treated with urelumab with nivolumab and MK-4166 with pembrolizumab in melanoma patients have had overall response rates of more than 50%. Moreover, patients with pancreatic cancer—a disease known to respond poorly to immunotherapy—who were treated with CD40 with chemotherapy and immune checkpoint inhibitors.85 88 In other studies, PRs have been seen in patients with head and neck squamous cell cancer who were treated with the OX40 agonist MEDI0562 urelumab plus nivolumab, or utomilumab plus pembrolizumab (one case each).25 61 62 CRs and PRs were also seen in patients with renal cell carcinoma who were treated with utomilumab plus pembrolizumab.61 In addition, PRs were seen in patients with non-small cell lung cancer who received 4-1BB agonist with anti PD-L1.61 62 Patients with small cell lung cancer, bladder cancer, and anaplastic thyroid cancer have also responded to combination therapy.25 61
The main function of a T-cell agonist is to enhance T-cell proliferation and survival without driving Treg expansion. However, most costimulatory molecules targeted are expressed for only a brief time following stimulation. Another hurdle to this form of therapy is that repeated T cell stimulation by agonistic approaches can led to exhaustion.102 Therefore, the concept of treatment duration has been examined during long-term follow-up for immune modulator trials. In fact, some studies have shown that T-cell activation decreases after a few cycles of the agonistic agent demonstrating that this may be a critical challenge in this approach. Two major avenues are being explored to address overcome the T-cell exhaustion hypothesis. The first avenue is to examine the impact of the duration of treatment. How many cycles are needed to induce a properly activated T-cell response without resulting in exhaustion? For example, the OX40 agonist as an anti-9B12 murine antibody has an effect within only one cycle, not multiple cycle.24
The second avenue is the sequencing of drug administration in agonist combination studies (ie, agonist followed by antagonist vs antagonist followed by agonist vs concurrent administration). In the orthotopically transplanted murine mammary tumor virus polyoma middle T murine model of mammary cancer refractory to PD-1 blockade, OX40 stimulation followed by PD-1 blockage delayed tumor growth and increased the durable responses of CD4+ and CD8+ T cells to tumors.62
Therefore, the concept of treatment duration has been examined in long-term follow-up in immune modulator trials.103 In combination of OX40 and concurrent anti-PD-L1 blockade led to rapid, intense intratumoral T-cell proliferation in patients with solid cancer but also induced exhaustion of T-cell immunoglobulin and mucin-domain-containing-3+CD8+ cells in the later stages of immune response.27 In contrast, an encouraging finding regarding T-cell agonists is that sequential administration of these agonists with PD-1 or PD-L1 can reverse T-cell exhaustion and maintain its effect, even in mammary tumor virus polyoma middle T mammary cancer models resistant to PD-1 blockage.
Several studies have demonstrated that radiotherapy can be synergistic with immune check point inhibitor treatment.104–107 When polyomavirus middle T mammary cancer murine models with anti-PD-L1 agent resistance were treated with sequential OX40 activation and radiotherapy, the antitumor activity of the OX40 agonist was profoundly enhanced.27 It has also been shown that 4-1BB administered with radiation therapy enhances antitumor activity.57 Interestingly, treatment with both CTLA-4 and OX40 antibody after radiation may be optimal due to the ability of this combination treatment to increase antigen expression after radiation. The advantage of sequential therapy may be related to the exhaustion of Tregs, release of tumor antigens and enhanced proliferation of CD4 and CD8 cells. Thus, questions emerge about the timing of T-cell agonist treatment when it is combined with other treatment methods.
To improve the efficacy of agonist therapies, it is important to understand the expression level of the costimulatory receptor within the tumor microenvironment by examining expression on tumor infiltrating lymphocytes. Because inefficient T-cell infiltration may be due to a reduced influx of tumor-infiltrating APCs, presence of suppressive or immature APC subsets or dysfunctional tumor vasculature.100 108 If the number of tumor-infiltrating APCs is adequate, T-cell infiltration may possibly be increased through the use of immunotherapies that increase chemokine release by APCs or radiotherapies or chemotherapies that can create an immune-permissive tumor microenvironment by inducing inflammatory immune responses. In situations in which the influx of APCs is reduced at a tumor site, radiotherapy or chemotherapy may be given before the administration of T-cell-agonist-based reagents or checkpoint inhibitors to release chemokines such as CCL2 and CCL5 that attract DCs to the tumor.109 110
The timing of drug administration should be calculated based on drug half-lives and the expression peaks for the agonist target. However, this method of calculating administration time needs further refinement.27 Based on the lack of accuracy in pharmacokinetics, agonists might be used in suboptimal settings and lead to failure in early-phase trials. Unfortunately, researchers have yet to determine the optimal timing, number of doses, and methods to use when delivering multiple treatment modalities.
The control of immune-related AEs can be problematic in immunotherapy. Agonists of T cells can boost the original immune effects of cancer, have unexpected off-target effects, or aggravate pre-existing autoimmune disease. More than half of cancer patients given previously approved immune checkpoint inhibitors experience toxic effects, including dermatologic and gastrointestinal effects.111 Most toxic effects are manageable with proper care. However, severe toxicity precludes patients from further drug administration and can even lead to death. Sufficient data to establish complete safety profiles for T-cell agonists are lacking. Therefore, investigators should always take into consideration both expected and unexpected TRAEs. The majority of AEs greater than grade 2 are pneumonitis, transaminitis and hematological toxic effects.28 52 Some agents cause DLT, including non-ST-elevation myocardial infarctions, at doses below the MTD.71
Finally, we do not have any useful biomarkers with which to gage responses to immune agonists. In the case of immune check-point inhibitors, PD-L1 expression and the tumor mutation burden are now used as biomarkers of response to immunotherapy.112 113 However, these markers are insufficient and there are no known biomarkers for responses to T-cell agonists. Hence, one of the reasons that researchers have not seen strong signals of success in agonist trials might be that all patients were enrolled without stratification based on useful biomarkers.
Summary
With the development of immunotherapy, more methods than ever are available to promote the immune system’s resistance to tumor cells. The currently approved immunotherapies enhance the immune system primarily by targeting immune checkpoint molecules related to immune-escape mechanisms. T-cell activation requires not only a TCR signal but also a second costimulatory signal. OX40, 4-1BB, GITR and ICOS are costimulatory molecules essential to complete T-cell activation. Targeting CD40 on B cells and DCs results in a secondary boost in T-cell activation via antigen presentation and cytokine secretion. Agonists of costimulatory molecules may lead to improved T-cell activation and tumor control. In preclinical studies, some agents have demonstrated clinical efficacy in tumor control. A number of these agonistic drugs have proceeded to first-in-human clinical trials. Although some agents have failed to fulfill their intended goals, others have demonstrated promising results in early phase I trials. In particular, the combination of immune checkpoint inhibitors and chemotherapy or radiotherapy has also inhibited tumor progression, and a potential synergistic mechanism of combination therapy may play a role in the positive results seen in both animal and human models. Based on these potential benefits of combination therapy, combination trials have been under development, especially those involving checkpoint inhibitors. Most agents demonstrated their safety in preclinical or early-phase studies without reaching the DLT. Investigators expect these agents to demonstrate clinical benefits in ongoing last-phase trials, and phase 2 and 3 trials of these drugs for various types of solid tumors are ongoing. Moreover, other types of immunotherapy, such as oncolytic virus therapy and chimeric antigen receptor T-cell therapy, can be combined, and neoantigen vaccines can potentiate responses to T-cell agonists. More studies of combination treatments are warranted.
Nevertheless, some challenges must be managed before T-cell agonists can be widely used in cancer immunotherapy. Inadequate T cell infiltration and activation, reduced influx and activity of APCs, and depressive tumor microenvironments impede the antitumor function of costimulatory agonists. In addition, finding ways to incorporate T-cell agonist-based treatments with other types of cancer treatment and to alleviate concerns about possible immune deletion due to constant T-cell stimulation are important issues. The sequence and dose of treatments is related to enhanced responses and determining optimal treatment delivery methods will be critical in planning combination trials. Lastly, an important challenge is finding a way to manage immune-related AEs. More than half of patients receiving immune checkpoint inhibitors experience more than one kind of AE. T-cell agonists enhance the same immune responses as those enhanced by immune checkpoint inhibitors, so they also may cause AEs. Moreover, combination therapy trials involving T-cell agonists may induce serious AEs, as well. The discovery of potential biomarkers is important to the prediction of benefits from immune-agonist treatment.
Immune costimulatory agonists are powerful candidates for future immunotherapy and have provided promising results in early-phase trials. T-cell agonists will show promising ways against cancer.
Acknowledgments
The authors would like to thank Sunita Patterson and Laura L. Russell from the Scientific Publications Team, Research Medical Library at The University of Texas MD Anderson Cancer Center, who helped with scientific editing of this manuscript.
Footnotes
Twitter: @AnaingMD
YC and YS contributed equally.
Contributors: YC and YS drafted the manuscript. CLH and AN were involved in the writing and critical review of the manuscript. JH and GC participated in the critical review of the content of the manuscript. All authors read and provided final approval of the version to be published.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: AN declaresResearch funding from NCI; EMD Serono; MedImmune; Healios Onc. Nutrition; Atterocor/Millendo; Amplimmune; ARMO BioSciences; Karyopharm Therapeutics; Incyte; Novartis; Regeneron; Merck; BMS; Pfizer, CytomX Therapeutics; Neon Therapeutics; Calithera Biosciences; TopAlliance Biosciences; Eli Lilly; Kymab; PsiOxus; Arcus Biosciences;· On advisory board of CytomX Therapeutics, Novartis and Genome & Company· Travel and accommodation expense from ARMO BioSciences. JH declares Research funding: Immune Deficiency Foundation, Jeffery Modell Foundation and chao physician-scientist. Baxalta Advisory board: Takeda, CSL-BehringWe will provide COI disclosure form from all authors if published.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Wylie B, Macri C, Mintern JD, et al. . Dendritic cells and cancer: from biology to therapeutic intervention. Cancers 2019;11:521. 10.3390/cancers11040521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quaranta V, Schmid MC. Macrophage-Mediated subversion of anti-tumour immunity. Cells 2019;8:747. 10.3390/cells8070747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu W, Wang G, Huang D, et al. . Cancer immunotherapy based on natural killer cells: current progress and new opportunities. Front Immunol 2019;10:1205. 10.3389/fimmu.2019.01205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egen JG, Ouyang W, Wu LC. Human anti-tumor immunity: insights from immunotherapy clinical trials. Immunity 2020;52:36–54. 10.1016/j.immuni.2019.12.010 [DOI] [PubMed] [Google Scholar]
- 5.Mchayleh W, Bedi P, Sehgal R, et al. . Chimeric antigen receptor T-cells: the future is now. J Clin Med 2019;8:207. 10.3390/jcm8020207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol 2009;27:591–619. 10.1146/annurev.immunol.021908.132706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science 2015;348:69–74. 10.1126/science.aaa4971 [DOI] [PubMed] [Google Scholar]
- 8.Neefjes J, Jongsma MLM, Paul P, et al. . Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat Rev Immunol 2011;11:823–36. 10.1038/nri3084 [DOI] [PubMed] [Google Scholar]
- 9.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol 2013;13:227–42. 10.1038/nri3405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esensten JH, Helou YA, Chopra G, et al. . Cd28 costimulation: from mechanism to therapy. Immunity 2016;44:973–88. 10.1016/j.immuni.2016.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alegre ML, Frauwirth KA, Thompson CB. T-Cell regulation by CD28 and CTLA-4. Nat Rev Immunol 2001;1:220–8. 10.1038/35105024 [DOI] [PubMed] [Google Scholar]
- 12.Dong C, Juedes AE, Temann UA, et al. . Icos co-stimulatory receptor is essential for T-cell activation and function. Nature 2001;409:97–101. 10.1038/35051100 [DOI] [PubMed] [Google Scholar]
- 13.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol 2009;9:271–85. 10.1038/nri2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elgueta R, Benson MJ, de Vries VC, et al. . Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev 2009;229:152–72. 10.1111/j.1600-065X.2009.00782.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aspeslagh S, Postel-Vinay S, Rusakiewicz S, et al. . Rationale for anti-OX40 cancer immunotherapy. Eur J Cancer 2016;52:50–66. 10.1016/j.ejca.2015.08.021 [DOI] [PubMed] [Google Scholar]
- 16.Croft M, So T, Duan W, et al. . The significance of OX40 and OX40L to T-cell biology and immune disease. Immunol Rev 2009;229:173–91. 10.1111/j.1600-065X.2009.00766.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie F, Wang Q, Chen Y, et al. . Costimulatory molecule OX40/OX40L expression in ductal carcinoma in situ and invasive ductal carcinoma of breast: an immunohistochemistry-based pilot study. Pathol Res Pract 2010;206:735–9. 10.1016/j.prp.2010.05.016 [DOI] [PubMed] [Google Scholar]
- 18.Vetto JT, Lum S, Morris A, et al. . Presence of the T-cell activation marker OX-40 on tumor infiltrating lymphocytes and draining lymph node cells from patients with melanoma and head and neck cancers. Am J Surg 1997;174:258–65. 10.1016/S0002-9610(97)00139-6 [DOI] [PubMed] [Google Scholar]
- 19.Sawada R, Arai Y, Sagawa Y, et al. . High blood levels of soluble OX40 (CD134), an immune costimulatory molecule, indicate reduced survival in patients with advanced colorectal cancer. Oncol Rep 2019;42:2057–64. [DOI] [PubMed] [Google Scholar]
- 20.Croft M. Control of immunity by the TNFR-related molecule OX40 (CD134). Annu Rev Immunol 2010;28:57–78. 10.1146/annurev-immunol-030409-101243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marabelle A, Kohrt H, Sagiv-Barfi I, et al. . Depleting tumor-specific Tregs at a single site eradicates disseminated tumors. J. Clin. Invest. 2013;123:2447–63. 10.1172/JCI64859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garrison K, Hahn T, Lee W-C, et al. . The small molecule TGF-β signaling inhibitor SM16 synergizes with agonistic OX40 antibody to suppress established mammary tumors and reduce spontaneous metastasis. Cancer Immunol Immunother 2012;61:511–21. 10.1007/s00262-011-1119-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruby CE, Yates MA, Hirschhorn-Cymerman D, et al. . Cutting edge: OX40 agonists can drive regulatory T cell expansion if the cytokine milieu is right. J Immunol 2009;183:4853–7. 10.4049/jimmunol.0901112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curti BD, Kovacsovics-Bankowski M, Morris N, et al. . Ox40 is a potent immune-stimulating target in late-stage cancer patients. Cancer Res 2013;73:7189–98. 10.1158/0008-5472.CAN-12-4174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glisson B, Leidner R, Ferris RL, et al. . Safety and clinical activity of MEDI0562, a humanized OX40 agonist monoclonal antibody, in adult patients with advanced solid tumors. Annals of Oncology 2018;29:viii410 10.1093/annonc/mdy288.025 [DOI] [PubMed] [Google Scholar]
- 26.Glisson BS, Leidner R, Ferris RL, et al. . Phase 1 study of MEDI0562, a humanized OX40 agonist monoclonal antibody (mAb), in adult patients (PTS) with advanced solid tumors. Annals of Oncology 2016;27:vi361 10.1093/annonc/mdw378.07 [DOI] [PubMed] [Google Scholar]
- 27.Messenheimer DJ, Jensen SM, Afentoulis ME, et al. . Timing of PD-1 blockade is critical to effective combination immunotherapy with Anti-OX40. Clin Cancer Res 2017;23:6165–77. 10.1158/1078-0432.CCR-16-2677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Infante JR, Hansen AR, Pishvaian MJ, et al. . A phase Ib dose escalation study of the OX40 agonist MOXR0916 and the PD-L1 inhibitor atezolizumab in patients with advanced solid tumors. Journal of Clinical Oncology 2016;34:101 10.1200/JCO.2016.34.15_suppl.101 [DOI] [Google Scholar]
- 29.Mayes PA, Hance KW, Hoos A. The promise and challenges of immune agonist antibody development in cancer. Nat Rev Drug Discov 2018;17:509–27. 10.1038/nrd.2018.75 [DOI] [PubMed] [Google Scholar]
- 30.El-Khoueiry AB, Hamid O, Thompson JA, et al. . The relationship of pharmacodynamics (PD) and pharmacokinetics (pK) to clinical outcomes in a phase I study of OX40 agonistic monoclonal antibody (mAb) PF-04518600 (PF-8600). Journal of Clinical Oncology 2017;35:3027 10.1200/JCO.2017.35.15_suppl.3027 [DOI] [Google Scholar]
- 31.Gough MJ, Crittenden MR, Sarff M, et al. . Adjuvant therapy with agonistic antibodies to CD134 (OX40) increases local control after surgical or radiation therapy of cancer in mice. J Immunother 2010;33:798–809. 10.1097/CJI.0b013e3181ee7095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yokouchi H, Yamazaki K, Chamoto K, et al. . Anti-OX40 monoclonal antibody therapy in combination with radiotherapy results in therapeutic antitumor immunity to murine lung cancer. Cancer Sci 2008;99:361–7. 10.1111/j.1349-7006.2007.00664.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Young KH, Baird JR, Savage T, et al. . Optimizing timing of immunotherapy improves control of tumors by Hypofractionated radiation therapy. PLoS One 2016;11:e0157164 10.1371/journal.pone.0157164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang C, Lin GHY, McPherson AJ, et al. . Immune regulation by 4-1BB and 4-1BBL: complexities and challenges. Immunol Rev 2009;229:192–215. 10.1111/j.1600-065X.2009.00765.x [DOI] [PubMed] [Google Scholar]
- 35.Bartkowiak T, Curran MA. 4-1Bb agonists: multi-potent potentiators of tumor immunity. Front Oncol 2015;5:117. 10.3389/fonc.2015.00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheuk ATC, Mufti GJ, Guinn B-ann. Role of 4-1BB:4-1BB ligand in cancer immunotherapy. Cancer Gene Ther 2004;11:215–26. 10.1038/sj.cgt.7700670 [DOI] [PubMed] [Google Scholar]
- 37.Martinez-Forero I, Azpilikueta A, Bolaños-Mateo E, et al. . T cell costimulation with Anti-CD137 monoclonal antibodies is mediated by K63–Polyubiquitin-Dependent signals from endosomes. J.i. 2013;190:6694–706. 10.4049/jimmunol.1203010 [DOI] [PubMed] [Google Scholar]
- 38.So T, Croft M. Regulation of PI-3-kinase and Akt signaling in T lymphocytes and other cells by TNFR family molecules. Front Immunol 2013;4:139 10.3389/fimmu.2013.00139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.So T, Lee S-W, Croft M. Immune regulation and control of regulatory T cells by OX40 and 4-1BB. Cytokine Growth Factor Rev 2008;19:253–62. 10.1016/j.cytogfr.2008.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elpek KG, Yolcu ES, Franke DDH, et al. . Ex vivo expansion of CD4+CD25+FoxP3+ T regulatory cells based on synergy between IL-2 and 4-1BB signaling. J Immunol 2007;179:7295–304. 10.4049/jimmunol.179.11.7295 [DOI] [PubMed] [Google Scholar]
- 41.Houot R, Kohrt H, Levy R. Boosting antibody-dependant cellular cytotoxicity against tumor cells with a CD137 stimulatory antibody. Oncoimmunology 2012;1:957–8. 10.4161/onci.19974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao Z, Condomines M, van der Stegen SJC, et al. . Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell 2015;28:415–28. 10.1016/j.ccell.2015.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guedan S, Posey AD, Shaw C, et al. . Enhancing CAR T cell persistence through ICOS and 4-1BB costimulation. JCI Insight 2018;3 10.1172/jci.insight.96976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ying Z, He T, Wang X, et al. . Parallel comparison of 4-1BB or CD28 Co-stimulated CD19-Targeted CAR-T cells for B cell non-Hodgkin's lymphoma. Mol Ther Oncolytics 2019;15:60–8. 10.1016/j.omto.2019.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maude SL, Laetsch TW, Buechner J, et al. . Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 2018;378:439–48. 10.1056/NEJMoa1709866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mardiana S, John LB, Henderson MA, et al. . A multifunctional role for adjuvant Anti-4-1BB therapy in augmenting antitumor response by chimeric antigen receptor T cells. Cancer Res 2017;77:1296–309. 10.1158/0008-5472.CAN-16-1831 [DOI] [PubMed] [Google Scholar]
- 47.Claus C, Ferrara C, Lang S, et al. . Abstract 3634: a novel tumor-targeted 4-1BB agonist and its combination with T-cell bispecific antibodies: an off-the-shelf cancer immunotherapy alternative to CAR T-cells. Cancer Research 2017;77:3634. [Google Scholar]
- 48.Gauttier V, Judor J-P, Le Guen V, et al. . Agonistic anti-CD137 antibody treatment leads to antitumor response in mice with liver cancer. Int J Cancer 2014;135:2857–67. 10.1002/ijc.28943 [DOI] [PubMed] [Google Scholar]
- 49.Li B, Lin J, VanRoey M, et al. . Established B16 tumors are rejected following treatment with GM-CSF-secreting tumor cell immunotherapy in combination with anti-4-1BB mAb. Clinical Immunology 2007;125:76–87. 10.1016/j.clim.2007.07.005 [DOI] [PubMed] [Google Scholar]
- 50.Segal NH, Gopal AK, Bhatia S, et al. . A phase 1 study of PF-05082566 (anti-4-1BB) in patients with advanced cancer. Journal of Clinical Oncology 2014;32:3007 10.1200/jco.2014.32.15_suppl.3007 [DOI] [Google Scholar]
- 51.Fisher TS, Kamperschroer C, Oliphant T, et al. . Targeting of 4-1BB by monoclonal antibody PF-05082566 enhances T-cell function and promotes anti-tumor activity. Cancer Immunol Immunother 2012;61:1721–33. 10.1007/s00262-012-1237-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sznol M, Hodi FS, Margolin K, et al. . Phase I study of BMS-663513, a fully human anti-CD137 agonist monoclonal antibody, in patients (PTS) with advanced cancer (Ca). Journal of Clinical Oncology 2008;26:3007 10.1200/jco.2008.26.15_suppl.3007 [DOI] [Google Scholar]
- 53.Segal NH, Logan TF, Hodi FS, et al. . Results from an integrated safety analysis of Urelumab, an agonist Anti-CD137 monoclonal antibody. Clin Cancer Res 2017;23:1929–36. 10.1158/1078-0432.CCR-16-1272 [DOI] [PubMed] [Google Scholar]
- 54.Segal NH, He AR, Doi T, et al. . Phase I study of single-agent Utomilumab (PF-05082566), a 4-1BB/CD137 agonist, in patients with advanced cancer. Clin Cancer Res 2018;24:1816–23. 10.1158/1078-0432.CCR-17-1922 [DOI] [PubMed] [Google Scholar]
- 55.Chen S, Lee L-F, Fisher TS, et al. . Combination of 4-1BB agonist and PD-1 antagonist promotes antitumor effector/memory CD8 T cells in a poorly immunogenic tumor model. Cancer Immunol Res 2015;3:149–60. 10.1158/2326-6066.CIR-14-0118 [DOI] [PubMed] [Google Scholar]
- 56.Wei H, Zhao L, Hellstrom I, et al. . Dual targeting of CD137 co-stimulatory and PD-1 co-inhibitory molecules for ovarian cancer immunotherapy. Oncoimmunology 2014;3:e28248 10.4161/onci.28248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi W, Siemann DW. Augmented antitumor effects of radiation therapy by 4-1BB antibody (BMS-469492) treatment. Anticancer Res 2006;26:3445–53. [PubMed] [Google Scholar]
- 58.SA J, Cheon SH, Park SM, et al. . Eradication of established renal cell carcinoma by a combination of 5-fluorouracil and anti-4-1BB monoclonal antibody in mice. Int J Cancer 2008;122:2784–90. [DOI] [PubMed] [Google Scholar]
- 59.Kim YH, Choi BK, Kim KH, et al. . Combination therapy with cisplatin and anti-4-1BB: synergistic anticancer effects and amelioration of cisplatin-induced nephrotoxicity. Cancer Res 2008;68:7264–9. 10.1158/0008-5472.CAN-08-1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim YH, Choi BK, Oh HS, et al. . Mechanisms involved in synergistic anticancer effects of anti-4-1BB and cyclophosphamide therapy. Mol Cancer Ther 2009;8:469–78. 10.1158/1535-7163.MCT-08-0993 [DOI] [PubMed] [Google Scholar]
- 61.Tolcher AW, Sznol M, Hu-Lieskovan S, et al. . Phase Ib study of Utomilumab (PF-05082566), a 4-1BB/CD137 agonist, in combination with pembrolizumab (MK-3475) in patients with advanced solid tumors. Clin Cancer Res 2017;23:5349–57. 10.1158/1078-0432.CCR-17-1243 [DOI] [PubMed] [Google Scholar]
- 62.Massarelli E, Segal N, Ribrag V. Clinical safety and efficacy assessment of the CD137 agonist urelumab alone and in combination with nivolumab in patients with hematologic and solid tumor malignancies. J Immunother Cancer 2016;4:O7. [Google Scholar]
- 63.Ephrem A, Epstein AL, Stephens GL, et al. . Modulation of Treg cells/T effector function by GITR signaling is context-dependent. Eur J Immunol 2013;43:2421–9. 10.1002/eji.201343451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Knee DA, Hewes B, Brogdon JL. Rationale for anti-GITR cancer immunotherapy. Eur J Cancer 2016;67:1–10. 10.1016/j.ejca.2016.06.028 [DOI] [PubMed] [Google Scholar]
- 65.Riccardi C, Ronchetti S, Nocentini G. Glucocorticoid-Induced TNFR-related gene (GITR) as a therapeutic target for immunotherapy. Expert Opin Ther Targets 2018;22:783–97. 10.1080/14728222.2018.1512588 [DOI] [PubMed] [Google Scholar]
- 66.Schaer DA, Murphy JT, Wolchok JD. Modulation of GITR for cancer immunotherapy. Curr Opin Immunol 2012;24:217–24. 10.1016/j.coi.2011.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nocentini G, Giunchi L, Ronchetti S, et al. . A new member of the tumor necrosis factor/nerve growth factor receptor family inhibits T cell receptor-induced apoptosis. Proc Natl Acad Sci U S A 1997;94:6216–21. 10.1073/pnas.94.12.6216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cohen AD, Schaer DA, Liu C, et al. . Agonist anti-GITR monoclonal antibody induces melanoma tumor immunity in mice by altering regulatory T cell stability and intra-tumor accumulation. PLoS One 2010;5:e10436 10.1371/journal.pone.0010436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koon HB, Shepard DR, Merghoub T, et al. . First-In-Human phase 1 single-dose study of TRX-518, an anti-human glucocorticoid-induced tumor necrosis factor receptor (GITR) monoclonal antibody in adults with advanced solid tumors. Journal of Clinical Oncology 2016;34:3017 10.1200/JCO.2016.34.15_suppl.3017 [DOI] [Google Scholar]
- 70.Tran B, Carvajal RD, Marabelle A, et al. . Dose escalation results from a first-in-human, phase 1 study of glucocorticoid-induced TNF receptor-related protein agonist AMG 228 in patients with advanced solid tumors. J Immunother Cancer 2018;6:93. 10.1186/s40425-018-0407-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Denlinger CS, Infante JR, Aljumaily R, et al. . A phase I study of MEDI1873, a novel GITR agonist, in advanced solid tumors. Annals of Oncology 2018;29:viii411 10.1093/annonc/mdy288.027 [DOI] [Google Scholar]
- 72.Lu L, Xu X, Zhang B, et al. . Combined PD-1 blockade and GITR triggering induce a potent antitumor immunity in murine cancer models and synergizes with chemotherapeutic drugs. J Transl Med 2014;12:36. 10.1186/1479-5876-12-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Siu LL, Steeghs N, Meniawy T, et al. . Preliminary results of a phase I/IIa study of BMS-986156 (glucocorticoid-induced tumor necrosis factor receptor–related gene [GITR] agonist), alone and in combination with nivolumab in pts with advanced solid tumors. Journal of Clinical Oncology 2017;35:104 10.1200/JCO.2017.35.15_suppl.104 [DOI] [Google Scholar]
- 74.Geva R, Voskoboynik M, Beebe AM, et al. . First-In-Human phase 1 study of MK-1248, an anti-human glucocorticoid-induced tumor necrosis factor receptor (GITR) monoclonal antibody, as monotherapy or in combination with pembrolizumab in patients with advanced solid tumors. Journal of Clinical Oncology 2018;36:3029 10.1200/JCO.2018.36.15_suppl.3029 [DOI] [PubMed] [Google Scholar]
- 75.Papadopoulos KP, Autio KA, Golan T, et al. . Phase 1 study of MK-4166, an anti-human glucocorticoid-induced tumor necrosis factor receptor (GITR) antibody, as monotherapy or with pembrolizumab (pembro) in patients (PTS) with advanced solid tumors. Journal of Clinical Oncology 2019;37:9509 10.1200/JCO.2019.37.15_suppl.9509 [DOI] [Google Scholar]
- 76.Vonderheide RH. Prospect of targeting the CD40 pathway for cancer therapy. Clin Cancer Res 2007;13:1083–8. 10.1158/1078-0432.CCR-06-1893 [DOI] [PubMed] [Google Scholar]
- 77.Elgueta R, Benson MJ, de Vries VC, et al. . Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev 2009;229:152–72. 10.1111/j.1600-065X.2009.00782.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Quezada SA, Jarvinen LZ, Lind EF, et al. . CD40/CD154 interactions at the interface of tolerance and immunity. Annu Rev Immunol 2004;22:307–28. 10.1146/annurev.immunol.22.012703.104533 [DOI] [PubMed] [Google Scholar]
- 79.Allen RC, Armitage RJ, Conley ME, et al. . Cd40 ligand gene defects responsible for X-linked hyper-IgM syndrome. Science 1993;259:990–3. 10.1126/science.7679801 [DOI] [PubMed] [Google Scholar]
- 80.Ferrari S, Giliani S, Insalaco A, et al. . Mutations of CD40 gene cause an autosomal recessive form of immunodeficiency with HyPer IgM. Proc Natl Acad Sci U S A 2001;98:12614–9. 10.1073/pnas.221456898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Beatty GL, Chiorean EG, Fishman MP, et al. . Cd40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 2011;331:1612–6. 10.1126/science.1198443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vonderheide RH, Dutcher JP, Anderson JE, et al. . Phase I study of recombinant human CD40 ligand in cancer patients. J Clin Oncol 2001;19:3280–7. 10.1200/JCO.2001.19.13.3280 [DOI] [PubMed] [Google Scholar]
- 83.Vonderheide RH, Flaherty KT, Khalil M, et al. . Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. Journal of Clinical Oncology 2007;25:876–83. 10.1200/JCO.2006.08.3311 [DOI] [PubMed] [Google Scholar]
- 84.Winograd R, Byrne KT, Evans RA, et al. . Induction of T-cell immunity overcomes complete resistance to PD-1 and CTLA-4 blockade and improves survival in pancreatic carcinoma. Cancer Immunology Research 2015;3:399–411. 10.1158/2326-6066.CIR-14-0215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Beatty GL, Torigian DA, Chiorean EG, et al. . A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clinical Cancer Research 2013;19:6286–95. 10.1158/1078-0432.CCR-13-1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vonderheide RH, Burg JM, Mick R, et al. . Phase I study of the CD40 agonist antibody CP-870,893 combined with carboplatin and paclitaxel in patients with advanced solid tumors. Oncoimmunology 2013;2:e23033 10.4161/onci.23033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nowak AK, Cook AM, McDonnell AM, et al. . A phase 1B clinical trial of the CD40-activating antibody CP-870,893 in combination with cisplatin and pemetrexed in malignant pleural mesothelioma. Ann Oncol 2015;26:2483–90. 10.1093/annonc/mdv387 [DOI] [PubMed] [Google Scholar]
- 88.O'Hara MH, O'Reilly EM, Rosemarie M, et al. . Abstract CT004: a phase Ib study of CD40 agonistic monoclonal antibody APX005M together with gemcitabine (GEM) and nab-paclitaxel (NP) with or without nivolumab (Nivo) in untreated metastatic ductal pancreatic adenocarcinoma (PdaC) patients. Cancer Research 2019;79:CT004–CT. [Google Scholar]
- 89.Sharpe AH, Freeman GJ. The B7–CD28 superfamily. Nat Rev Immunol 2002;2:116–26. 10.1038/nri727 [DOI] [PubMed] [Google Scholar]
- 90.Wikenheiser DJ, Stumhofer JS. Icos co-stimulation: friend or foe? Front Immunol 2016;7:304. 10.3389/fimmu.2016.00304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Swallow MM, Wallin JJ, Sha WC. B7h, a novel costimulatory homolog of B7.1 and B7.2, is induced by TNFalpha. Immunity 1999;11:423–32. 10.1016/S1074-7613(00)80117-X [DOI] [PubMed] [Google Scholar]
- 92.Chen Q, Mo L, Cai X, et al. . Icos signal facilitates FOXP3 transcription to favor suppressive function of regulatory T cells. Int J Med Sci 2018;15:666–73. 10.7150/ijms.23940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.JF T, Ding YH, Ying XH, et al. . Regulatory T cells, especially ICOS(+) FOXP3(+) regulatory T cells, are increased in the hepatocellular carcinoma microenvironment and predict reduced survival. Sci Rep 2016;6:35056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Michaelson JS, Harvey C, Elpek K, et al. . Abstract SY03-02: preclinical assessment of JTX-2011, an agonist antibody targeting ICOS, supports evaluation in iconic clinical trial. Cancer Research 2017;77:SY03–2-SY-2. [Google Scholar]
- 95.Yap TA, Burris HA, Kummar S, et al. . Iconic: biologic and clinical activity of first in class ICOS agonist antibody JTX-2011 +/- nivolumab (nivo) in patients (PTS) with advanced cancers. Journal of Clinical Oncology 2018;36:3000 10.1200/JCO.2018.36.15_suppl.3000 [DOI] [Google Scholar]
- 96.Sainson RCA, Thotakura AK, Kosmac M, et al. . A novel antibody targeting ICOS increases intratumoural cytotoxic to regulatory T cell ratio and induces tumour regression. bioRxiv 2019;771493. [DOI] [PubMed] [Google Scholar]
- 97.Quaratino S, Sainson R, Thotakura A, et al. . A first-in-human study of KY1044, a fully human anti-ICOS IgG1 antibody as monotherapy and in combination with atezolizumab in patients with selected advanced malignancies. Journal of Clinical Oncology 2019;37:TPS2644–TPS. 10.1200/JCO.2019.37.15_suppl.TPS2644 [DOI] [Google Scholar]
- 98.Hansen A, Bauer TM, Moreno V, et al. . First in human study with GSK3359609 [GSK609], inducible T cell co-stimulator (ICOS) receptor agonist in patients [Pts] with advanced, solid tumors: Preliminary results from INDUCE-1. Annals of Oncology 2018;29:viii404 10.1093/annonc/mdy288.011 [DOI] [Google Scholar]
- 99.Rischin D, Groenland SL, Lim AML, et al. . Inducible T cell costimulatory (ICOS) receptor agonist, GSK3359609 (GSK609) alone and in combination with pembrolizumab (pembro): preliminary results from INDUCE-1 expansion cohorts (EC) in head and neck squamous cell carcinoma (HNSCC). Annals of Oncology 2019;30:v454–5. 10.1093/annonc/mdz252.011 [DOI] [Google Scholar]
- 100.Zhao X, Subramanian S. Intrinsic resistance of solid tumors to immune checkpoint blockade therapy. Cancer Res 2017;77:817–22. 10.1158/0008-5472.CAN-16-2379 [DOI] [PubMed] [Google Scholar]
- 101.Han X, Vesely MD. Stimulating T cells against cancer with agonist immunostimulatory monoclonal antibodies. Int Rev Cell Mol Biol 2019;342:1–25. 10.1016/bs.ircmb.2018.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bantia S, Choradia N. Treatment duration with immune-based therapies in cancer: an enigma. J Immunother Cancer 2018;6:143. 10.1186/s40425-018-0465-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bantia S, Choradia N. Correction to: treatment duration with immune-based therapies in cancer: an enigma. J Immunother Cancer 2019;7:56. 10.1186/s40425-018-0486-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rodríguez-Ruiz ME, Vanpouille-Box C, Melero I, et al. . Immunological mechanisms responsible for radiation-induced Abscopal effect. Trends Immunol 2018;39:644–55. 10.1016/j.it.2018.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Antonia SJ, Villegas A, Daniel D, et al. . Overall survival with Durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018;379:2342–50. 10.1056/NEJMoa1809697 [DOI] [PubMed] [Google Scholar]
- 106.Gray JE, Villegas A, Daniel D, et al. . Three-Year overall survival with Durvalumab after chemoradiotherapy in stage III NSCLC-Update from Pacific. J Thorac Oncol 2020;15:288–93. 10.1016/j.jtho.2019.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Theelen WSME, Peulen HMU, Lalezari F, et al. . Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol 2019. 10.1001/jamaoncol.2019.1478. [Epub ahead of print: 11 Jul 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bonaventura P, Shekarian T, Alcazer V, et al. . Cold tumors: a therapeutic challenge for immunotherapy. Front Immunol 2019;10:168. 10.3389/fimmu.2019.00168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Marcuzzi E, Angioni R, Molon B, et al. . Chemokines and chemokine receptors: orchestrating tumor metastasization. Int J Mol Sci 2018;20:96. 10.3390/ijms20010096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Connolly KA, Belt BA, Figueroa NM, et al. . Increasing the efficacy of radiotherapy by modulating the CCR2/CCR5 chemokine axes. Oncotarget 2016;7:86522–35. 10.18632/oncotarget.13287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Melero I, Grimaldi AM, Perez-Gracia JL, et al. . Clinical development of immunostimulatory monoclonal antibodies and opportunities for combination. Clin Cancer Res 2013;19:997–1008. 10.1158/1078-0432.CCR-12-2214 [DOI] [PubMed] [Google Scholar]
- 112.Boumber Y. Tumor mutational burden (TMB) as a biomarker of response to immunotherapy in small cell lung cancer. J Thorac Dis 2018;10:4689–93. 10.21037/jtd.2018.07.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Davis AA, Patel VG. The role of PD-L1 expression as a predictive biomarker: an analysis of all US food and drug administration (FDA) approvals of immune checkpoint inhibitors. J Immunother Cancer 2019;7:278. 10.1186/s40425-019-0768-9 [DOI] [PMC free article] [PubMed] [Google Scholar]