Abstract
Background
Data regarding breast cancer epidemiology, treatment and survival in Africa are scarce. We aimed to assess the distribution of breast cancer subtypes in Mozambique and its impact on patients’ treatment and survival. The concordance of biomarker assessment between cytological and histological samples was also evaluated.
Methods
Prospective cohort study including 210 patients diagnosed between January 2015 and August 2017, followed to November 2019. Clinicopathological characteristics, treatment, 3-year overall survival (OS) and disease-free survival (DFS) were compared across classic tumour subtypes (oestrogen receptor (ER)-positive/human epidermal growth factor receptor 2 (HER2)-negative, HER2-positive and triple-negative breast cancer (TNBC)) and surrogate intrinsic subtypes (St. Gallen classification). Concordance was measured using Cohen’s κ statistics.
Results
A total of 51% of patients had ER-positive/HER2-negative tumours, 24% HER2-positive and 25% TNBC. Concordance between cytological and histological samples regarding ER and HER2 status was substantial (κ=0.762 and κ=0.603, respectively). There were no significant differences across subtypes regarding clinical characteristics and treatment, except for HIV positivity and high histological grade (more prevalent among TNBC) or endocrine therapy (higher use among ER-positive/HER2-negative and HER2-positive patients). Three-year OS was 52.5% (95% CI, 44.3% to 60.0%), being higher in ER-positive/HER2-negative (61.1%) compared with HER2-positive (53.2%) and TNBC (31.9%) patients. Adjusted HRs were 1.96 (95% CI, 1.13 to 3.39) among HER2-positive and 3.10 (95% CI, 1.81 to 5.31) among TNBC versus ER-positive/HER2-negative patients. Three-year DFS was 46.6% (95% CI, 38.0% to 54.8%), being lower among TNBC versus ER-positive/HER2-negative patients (HR 2.91; 95% CI, 1.64 to 5.16). Results were similar between surrogate intrinsic subtypes.
Conclusion
There was a high proportion of HER2-positive and TNBC among Mozambican patients and their survival was poor compared with ER-positive/HER2-negative patients, partly due to the limited treatment options. A systematic assessment of ER, PR and HER2 status is feasible and may help tailoring and optimise the treatment of patients with breast cancer in low-resource settings, potentially leading to survival gains in this underserved population.
Keywords: breast neoplasms, biomarkers, survival analysis, sub-Saharan Africa, global health
Key questions.
What is already known about this subject?
Breast cancer incidence and mortality rates have been increasing over the last decades in developing countries, including sub-Saharan Africa. Part of this high mortality has been attributed to the large proportion of cases of triple-negative breast cancer (TNBC) and lower proportion of the oestrogen receptor (ER)-positive/human epidermal growth factor receptor 2 (HER2)-negative subtype among African populations compared with Western countries. Nonetheless, there is a paucity of data regarding treatment and survival according to the different breast cancer subtypes in Africa.
What does this study add?
Nearly half of patients with breast cancer in Mozambique had HER2-positive or TNBC and their treatment was similar across subtypes. Part of these subtypes were determined in cytological samples and we demonstrated, for the first time in Africa, that this is a feasible method for their assessment. Survival was poor, especially among HER2-positive and TNBC patients, who had a twofold and threefold increase in the risk of death versus ER-positive/HER2-negative patients, respectively.
How might this impact on clinical practice?
Our study has clinical and health policy implications for the management of breast cancer in Africa. Our results highlight the need and the feasibility of the universal assessment of ER, PR and HER2 status on breast tumours, even in low-resource settings, as this may optimise the use of systemic treatments, potentially leading to important survival gains in this underserved population.
Introduction
Breast cancer incidence rates have been increasing over the last decades, especially in developing countries.1 In Mozambique, it is now the second most incident cancer among Maputo City women, with a crude incidence rate of 8.6/100 000 women.2 Furthermore, age-standardised mortality rates are greater in low/medium-income than in high-income countries (14.9 versus 11.6/100 000 women, respectively).1 In sub-Saharan Africa, these differences have been attributed to the high proportion of patients with locally advanced/metastatic disease3 and to poor access to diagnosis and treatments.4
However, breast cancer is a heterogeneous entity, comprising four molecular subtypes (luminal A, luminal B, human epidermal growth factor receptor 2 (HER2) overexpression, basal), with different clinical courses, prognosis and treatment.5 6 As this molecular determination is not widely available, a surrogate classification based on immunohistochemistry and in situ hybridisation (ISH) assessments of the oestrogen receptor (ER), the progesterone receptor (PR), HER2 and Ki67 biomarkers was proposed at the St. Gallen conference.7 Nonetheless, in clinical practice and trials, breast tumours are still classified according to the ‘classic’ definition of ‘ER-positive/HER2-negative’, ‘HER2-positive’ and triple-negative breast cancer (TNBC; ER-negative/PR-negative/HER2-negative).
These biomarker determinations are usually carried out on surgically excised specimens or core needle biopsies.8 9 Nevertheless, in developing countries, fine needle aspiration cytology (FNAC) may be an appropriate resource to deal with the difficulties of inadequate pathology services, as it is a cheaper and less invasive diagnostic method.10 Additionally, studies from high-income countries demonstrated that biomarker assessment can be performed on cell blocks taken from FNAC, with concordances around 96%–98% for ER and 96% for HER2 when compared with histological samples.11 12
A meta-analysis showed that there is a higher proportion of cases of TNBC (21%) and a lower proportion of the ER-positive/HER2-negative subtype (52%) among African populations13 compared with Western countries, where ER-positive/HER2-negative tumours represent more than 70% of cases.14 Nonetheless, there is a wide variation in these proportions among different African studies.13 Additionally, there is a paucity of data regarding breast cancer treatment and survival according to the different subtypes in African countries.15–19 This knowledge may allow for a better management and organisation of healthcare services in this low-resource setting, translating into improved outcomes for patients with breast cancer.
Thus, this study aimed to assess the distribution of breast cancer subtypes in Mozambique, and its impact on treatment and survival. The concordance between biomarker and subtype assessment in cell blocks and histological samples was also evaluated.
Methods
Setting
Mozambique is a low-income country in eastern sub-Saharan Africa, with 28 million inhabitants.20 Around 13% of adults aged 15–49 years are infected by HIV,21 but the country is now facing an increase in non-communicable diseases, such as cardiovascular disease and cancer.22 The free-of-charge public health system is the largest healthcare provider, but has few resources for cancer care.
Until 2016, there were only three Pathology Departments in the country, one in each of the Central Hospitals: in Maputo (the capital city), Beira and Nampula. The Pathology Department of the Maputo Central Hospital (MCH) is the national reference department and it has a dedicated FNAC clinic since 1996.23 This department also centralises the performance of immunohistochemistry, but there are frequent ruptures in reagent supplies. Thus, a research grant allowed for the acquisition of most reagents for the assessment of ER, PR, HER2 and Ki67 that were used in this study.
There are two Medical Oncology Units (in Nampula and Maputo), where patients have access to anthracyclines, cyclophosphamide, taxanes, methotrexate and tamoxifen, although with occasional interruptions in supply. Trastuzumab and aromatase inhibitors are not available. The Radiotherapy Unit opened in August 2019 and a multidisciplinary tumour board meeting for breast cancer was created in March 2016. Treatment decisions at the multidisciplinary tumour board are usually based on the European Society for Medical Oncology breast cancer guidelines,6 although adapted to the available resources.
Study design
The prospective Moza-BC cohort study included consecutive incident cases of breast cancer, with a pathological diagnosis performed in one of the three Central Hospitals of Mozambique, from January 2015 to August 2017 (online supplementary figure 1). Data on sociodemographic, clinicopathological characteristics, treatment and survival of patients followed in the Oncology Unit of the MCH were prospectively collected until July 2018. Survival data were updated through the MCH Cancer Registry, health records and telephone interviews in November 2019. Patients without treatment/follow-up data were mostly from the Centre/North of the country, but there were no significant differences in clinicopathological characteristics in relation to those followed at the MCH (online supplementary table 1).
esmoopen-2020-000829supp001.pdf (563.3KB, pdf)
Breast tissue samples were collected by FNAC, surgical biopsy and breast surgery, and infrequently by core needle biopsy. Handling of histological specimens was standardised in the three hospitals according to the College of American Pathologists’ recommendations.8 Cell blocks were created from the aspirated FNAC material using HistoGel (Thermo Scientific, USA), according to the manufacturer’s instructions. Cell blocks were sent to the MCH and those with adequate cellularity (≥100 cells) were selected for biomarker assessment. Immunostaining of both cell blocks and histological samples was manually performed at the MCH, using the UltraVision Detection System anti-Polyvalent, horseradish peroxidase (HRP) (ready-to-use, Thermo Scientific) and Quanto Detection System HRP DAB (Thermo Scientific). Anti-ER, anti-PR, anti-HER2 and anti-Ki67 antibodies (clones SP1, SP2, SP3 and SP6, respectively; Thermo Scientific) were used. Expression of ER, PR, HER2 and Ki67 were assessed as described in the literature.8 9 24 Cases with a HER2 immunohistochemistry score of 2+ (HER2 equivocal) were submitted to silver-ISH at the Centro Hospitalar Universitário de São João, Portugal. Two pathologists from the MCH determined the pathology diagnosis and biomarker assessment independently. Approximately 10% of cell blocks were sent for quality control to Portugal, where they were restained and reassessed by a third pathologist.
Tumours were grouped according to the ‘classic’ classification into ER-positive/HER2-negative, HER2-positive (ER-positive/HER2-positive and ER-negative/HER2-positive) and TNBC. Tumours were also classified into surrogate intrinsic subtypes, according to the 2015 St. Gallen Consensus7: luminal A-like (ER-positive, PR positivity ≥20%, HER2-negative and Ki67 ≤29%), luminal B-like (ER-positive/HER2-negative and either PR positivity <20% or Ki67 >29%, or ER-positive/HER2-positive), HER2-enriched (ER-negative/PR-negative/HER2-positive) and basal-like (ER-negative/PR-negative/HER2-negative). Due to the absence of validated local reference data, the Ki67 cut-off of 29% was used based on the international recommendations.7 Among cases with both histological and cytological samples, in case of disagreement in subtype classification, the sample with the largest expression of biomarkers was selected, as it dictated treatment. Histological grade was assessed according to the Elston-Ellis definition.25
Staging was classified by the American Joint Committee on Cancer tumour, node, metastasis 7th edition.26 Clinical staging was usually performed using physical examination, mammography/breast ultrasound, chest X-ray and abdominal ultrasound.
Statistical analysis
Baseline patient, tumour and treatment characteristics were compared across subtypes using t-test and analysis of variance for continuous variables, and χ2 and Fisher exact tests for categorical variables. Overall survival (OS) was defined as time from diagnosis (date of pathological confirmation of breast cancer) until death by any cause. Among patients with early stage disease (stage I–III), disease-free survival (DFS) was defined as time from diagnosis until locoregional or distant relapse, second primary malignancy or death by any cause. Survival analyses were performed using the Kaplan-Meier estimator. Comparisons of survival across subtypes were accomplished through log-rank tests, and adjusted HRs and the corresponding 95% CIs computed using Cox proportional hazards regression.
Agreement of biomarker assessment between cell blocks and histological samples was measured using Cohen’s κ statistics and classified as fair (0.21–0.40), moderate (0.41–0.60), substantial (0.61–0.80) and almost perfect (0.81–1).27Spearman’s rank order coefficients were calculated for the correlation of ER and PR expressions in percentage for cell blocks versus histological samples. Analyses were carried out in STATA V.15 (Stata, College Station, Texas, USA). All tests were two-sided and a p-value of <0.05 was considered significant. Changes in subtype classification were illustrated using Sankey diagrams created in R (V,3.6.0) with the flipPlots package (V.1.2.0). The STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) consensus was followed for the reporting of this manuscript.28
Results
Among the 262 patients included in the Moza-BC cohort, 210 had tissue available for biomarker assessment (online supplementary figure 1): 108 (51%) had ER-positive/HER2-negative disease, 50 (24%) HER2-positive tumours and 52 (25%) TNBC. Using the St. Gallen classification of surrogate intrinsic subtypes, 28 (13%) patients had luminal A-like, 103 (49%) luminal B-like, 27 (13%) HER2-enriched and 52 (25%) basal-like tumours. Overall, 62% of tumours were ER-positive.
Patients were young (median age: 48.0 years), mostly premenopausal (53%), overweight/obese (62%), black (98%) and lived in the South of Mozambique (87%). There were 45 (25%) HIV-positive patients and 74% were diagnosed with stage III/IV disease (table 1). HIV-positive patients had a higher proportion of TNBC compared with HIV-negative patients (33% versus 20% respectively, p=0.048). TNBC tumours were more likely grade 3, but there were no differences in stage distribution. Results were similar across surrogate intrinsic subtypes (online supplementary table 2).
Table 1.
Patients’ baseline characteristics according to each classic breast cancer subtype
| ER-positive/HER2-negative | HER2-positive | TNBC | P value | |
| n=108 | n=50 | n=52 | ||
| Age in years (n, %) | 0.702 | |||
| <40 | 25 (23.8) | 13 (26.0) | 14 (26.9) | |
| 40–49 | 32 (30.5) | 12 (24.0) | 12 (23.1) | |
| 50–59 | 23 (21.9) | 14 (28.0) | 9 (17.3) | |
| ≥60 | 25 (23.8) | 11 (22.0) | 17 (32.7) | |
| Missing | 3 | 0 | 0 | |
| Race (n, %) | 0.603 | |||
| Black | 105 (98.1) | 49 (98.0) | 52 (100) | |
| Other* | 2 (1.9) | 1 (2.0) | 0 | |
| Missing | 1 | 0 | 0 | |
| Education in years (n, %) | 0.253 | |||
| 0 | 20 (27.8) | 7 (17.9) | 5 (14.3) | |
| 1–4 | 9 (12.5) | 3 (7.7) | 7 (20.0) | |
| >4 | 43 (59.7) | 29 (74.4) | 23 (65.7) | |
| Missing | 36 | 11 | 17 | |
| Place of residence (n, %) | 0.178 | |||
| South (including Maputo) | 90 (89.1) | 43 (89.6) | 37 (78.7) | |
| Centre/North | 11 (10.9) | 5 (10.4) | 10 (21.3) | |
| Missing | 7 | 2 | 5 | |
| Menopausal status (n, %) | 0.916 | |||
| Premenopausal | 48 (52.7) | 24 (54.5) | 20 (50.0) | |
| Postmenopausal | 43 (47.3) | 20 (45.5) | 20 (50.0) | |
| Missing | 17 | 6 | 12 | |
| Body mass index (n, %) | 0.255 | |||
| Under/normal weight (<25 kg/m2) | 36 (42.9) | 15 (38.5) | 10 (27.0) | |
| Overweight/obese (≥25 kg/m2) | 48 (57.1) | 24 (61.5) | 27 (73.0) | |
| Missing | 24 | 11 | 15 | |
| HIV status (n, %)† | 0.043 | |||
| Negative/unknown | 67 (73.6) | 38 (86.4) | 25 (62.5) | |
| Positive | 24 (26.4) | 6 (13.6) | 15 (37.5) | |
| Missing | 17 | 6 | 12 | |
| Tumour characteristics (clinical staging) (n, %) | 0.513 | |||
| cT1 | 3 (2.8) | 3 (6.1) | 1 (1.9) | |
| cT2 | 27 (25.2) | 15 (30.6) | 14 (26.9) | |
| cT3 | 29 (27.1) | 17 (34.7) | 18 (34.6) | |
| cT4 | 48 (44.9) | 14 (28.6) | 19 (36.5) | |
| Missing | * | * | 0 | |
| Lymph node status (clinical staging) (n, %) | 0.095 | |||
| cN0 | 27 (27.6) | 20 (44.4) | 12 (26.7) | |
| cN+ | 71 (72.4) | 25 (55.6) | 33 (73.3) | |
| Missing | 10 | 5 | 7 | |
| Tumour characteristics (pathological staging) (n, %) | 0.672 | |||
| (y)pT0/Tis | 4 (5.1) | 1 (2.4) | 2 (5.9) | |
| (y)pT1 | 15 (19.2) | 8 (19.0) | 7 (20.6) | |
| (y)pT2 | 31 (39.7) | 21 (50.0) | 10 (29.4) | |
| (y)pT3 | 15 (19.2) | 7 (16.7) | 11 (32.4) | |
| (y)pT4 | 13 (16.7) | 5 (11.9) | 4 (11.8) | |
| Missing | 30 | 8 | 8 | |
| Lymph node status (pathological staging) (n, %) | 0.512 | |||
| (y)pN0 | 13 (21.7) | 11 (29.7) | 9 (31.0) | |
| (y)pN1 | 26 (43.3) | 10 (27.0) | 11 (37.9) | |
| (y)pN2 | 15 (25.0) | 9 (24.3) | 4 (13.8) | |
| (y)pN3 | 6 (10.0) | 7 (18.9) | 5 (17.2) | |
| Missing | 48 | 13 | 23 | |
| Median tumour size at surgery in millimetres (median, range) | 40 (0–180) | 40 (2.5–134) | 45 (0–180) | 0.115 |
| Missing | 31 | 8 | 18 | |
| Multifocal tumours at surgery (n, %) | 0.974 | |||
| No | 64 (88.9) | 35 (89.7) | 28 (90.3) | |
| Yes | 8 (11.1) | 4 (10.3) | 3 (9.7) | |
| Missing | 36 | 11 | 21 | |
| Lymphovascular invasion at surgery (n, %) | 0.596 | |||
| No | 14 (22.6) | 12 (30.8) | 9 (30.0) | |
| Yes | 48 (77.4) | 27 (69.2) | 21 (70.0) | |
| Missing | 46 | 11 | 22 | |
| Neural invasion at surgery (n, %) | 0.642 | |||
| No | 47 (75.8) | 29 (74.4) | 25 (83.3) | |
| Yes | 15 (24.2) | 10 (25.6) | 5 (16.7) | |
| Missing | 46 | 11 | 22 | |
| Histological grade at surgery (n, %) | 0.001 | |||
| 1 | 14 (19.2) | 16 (40.0) | 5 (16.1) | |
| 2 | 42 (57.5) | 13 (32.5) | 9 (29.0) | |
| 3 | 17 (23.3) | 11 (27.5) | 17 (54.8) | |
| Missing | 35 | 10 | 21 | |
| Histological subtype at surgery (n, %) | 0.749 | |||
| No residual tumour/in situ carcinoma | 4 (5.2) | 1 (2.4) | 2 (5.7) | |
| Invasive ductal carcinoma (NST) | 64 (82.1) | 36 (87.8) | 31 (88.6) | |
| Other invasive subtypes‡ | 10 (12.8) | 4 (9.8) | 2 (5.7) | |
| Missing | 30 | 9 | 17 | |
| Stage at diagnosis (n, %) | 0.153 | |||
| I | 1 (1.1) | 1 (2.3) | 1 (2.5) | |
| II | 14 (15.4) | 15 (34.1) | 12 (30.0) | |
| III | 58 (63.7) | 23 (52.3) | 18 (45.0) | |
| IV | 18 (19.8) | 5 (11.4) | 9 (22.5) | |
| Missing§ | 17 | 6 | 12 |
P values in bold are considered to be statistically significant (<0.05).
*Includes mixed and Indian race.
†Seven patients had unknown HIV status; among HIV-positive patients, 31 (69%) had been previously diagnosed; the median time since HIV diagnosis was 3.93 years (range: 0.1–11.7); 41 (91%) patients were under ART when starting chemotherapy, mostly with the TDF+3TC+EFV regimen (28 patients); the median time under ART was 2 years (range 0.1–11.7); the median CD4+ cell count was 448 cells/µL (range 43–1104 cells/µL) and 39 (87%) patients had a CD4+ cell count >200/μL.
‡Includes lobular, mixed, papillary, squamous cell carcinoma, metaplastic and mucinous breast cancer.
§Includes the 35 patients for whom there is available cT/N and/or (y)pT/N status, but without information regarding the presence of metastases.
ART, antiretroviral treatment; cT/N, clinical tumor status and clinical lymph node status; ER, oestrogen receptor; HER2, human epidermal growth factor receptor 2; NST, no special type;TDF+3TC+EFV, tenofovir + lamivudine + efavirenz; TNBC, triple-negative breast cancer; (y)pT/N, pathological tumor status and pathological lymph node status.
Clinical management
There were no differences in terms of time from symptoms to diagnosis, the type of first treatment received or in time from diagnosis to treatment across classic subtypes (table 2). More than 80% of patients underwent surgery and the proportion was higher among those with HER2-positive tumours. Surgical treatments were mostly mastectomies, as only 5% of patients had access to radiotherapy.
Table 2.
Breast cancer clinical management according to each classic subtype
| ER-positive/HER2-negative | HER2-positive | TNBC | P value | |
| n=108 | n=50 | n=52 | ||
| Time from first symptom until diagnosis (n, %) | 0.468 | |||
| <180 days | 30 (50.8) | 13 (48.1) | 13 (65.0) | |
| ≥180 days | 29 (49.2) | 14 (51.9) | 7 (35.0) | |
| Missing | 49 | 23 | 32 | |
| Timing of diagnosis (n, %) | 0.729 | |||
| Pre-MTB implementation | 46 (50.5) | 22 (50.0) | 23 (57.5) | |
| Post-MTB implementation | 45 (49.5) | 22 (50.0) | 17 (42.5) | |
| Not applicable* | 17 | 6 | 12 | |
| Type of first treatment received (n, %) | 0.069 | |||
| No treatment received | 1 (1.1) | 0 | 2 (5.0) | |
| Surgery | 17 (18.9) | 14 (31.8) | 14 (35.0) | |
| Chemotherapy/endocrine therapy† | 72 (80.0) | 30 (68.2) | 24 (60.0) | |
| Missing | 18 | 6 | 12 | |
| Time from diagnosis until first treatment (n, %) | 0.083 | |||
| <45 days | 36 (40.4) | 26 (59.1) | 21 (55.3) | |
| ≥45 days | 53 (59.6) | 18 (40.9) | 17 (44.7) | |
| Missing | 19 | 6 | 14 | |
| Surgery (ever) (n, %) | 0.030 | |||
| No | 17 (18.3) | 1 (2.2) | 6 (15.0) | |
| Yes | 76 (81.7) | 45 (97.8) | 34 (85.0) | |
| Missing | 15 | 4 | 12 | |
| Surgical intent (n, %)‡ | 0.971 | |||
| Diagnostic | 3 (3.9) | 1 (2.2) | 1 (2.9) | |
| Curative | 61 (80.3) | 37 (82.2) | 28 (82.4) | |
| Palliative | 8 (10.5) | 4 (8.9) | 2 (5.9) | |
| Unknown | 4 (5.3) | 3 (6.7) | 3 (8.8) | |
| Type of breast surgery (n, %)§ | ||||
| Total mastectomy | 70 (92.1) | 41 (91.1) | 33 (97.1) | 0.553 |
| Tumourectomy | 6 (7.9) | 4 (8.9) | 1 (2.9) | |
| Status of surgical margins (n, %) | 0.431 | |||
| Clean | 64 (91.4) | 36 (92.3) | 26 (83.9) | |
| Positive | 6 (8.6) | 3 (7.7) | 5 (16.1) | |
| Missing | 6 | 6 | 3 | |
| Axillary surgery—type (n, %)¶ | ||||
| Axillary dissection | 66 (98.5) | 40 (88.9) | 31 (100) | 0.586 |
| Sentinel lymph node biopsy | 1 (1.5) | 0 | 0 | |
| Not done/missing | 9 | 5 | 3 | |
| Axillary surgery—completeness (n, %)** | 0.710 | |||
| Not done/no isolated lymph nodes | 9 (11.8) | 5 (11.9) | 3 (9.1) | |
| Incomplete | 21 (27.6) | 7 (16.7) | 9 (27.3) | |
| Complete | 46 (60.5) | 30 (71.4) | 21 (63.6) | |
| Missing | 0 | 3 | 1 | |
| Chemotherapy (ever) (n, %) | 0.413 | |||
| No | 4 (4.4) | 2 (4.5) | 4 (10.0) | |
| Yes | 87 (95.6) | 42 (95.5) | 36 (90.0) | |
| Missing | 17 | 6 | 12 | |
| Intent of first-line CT (n, %)†† | 0.075 | |||
| Neoadjuvant only | 16 (18.4) | 2 (4.8) | 2 (5.6) | |
| Neoadjuvant+adjuvant | 42 (48.3) | 25 (59.5) | 16 (44.4) | |
| Adjuvant only | 12 (13.8) | 10 (23.8) | 10 (27.8) | |
| Palliative | 17 (19.5) | 5 (11.9) | 8 (22.2) | |
| Neoadjuvant CT—outcome (n, %) | 0.017 | |||
| Same stage | 14 (24.1) | 13 (48.1) | 10 (55.6) | |
| Upstaging | 7 (12.1) | 6 (22.2) | 1 (5.6) | |
| Downstaging | 26 (44.8) | 8 (29.6) | 6 (33.3) | |
| Unknown‡‡ | 11 (19.0) | 0 | 1 (5.6) | |
| pCR rate after neoadjuvant CT (n, %) | 0.825 | |||
| No pCR | 55 (93.2) | 26 (96.3) | 16 (88.9) | |
| pCR only in the breast (ypT0/is) | 2 (3.4) | 1 (3.7) | 1 (5.6) | |
| pCR in the breast and lymph nodes (ypT0/is, ypN0) | 2 (3.4) | 0 | 1 (5.6) | |
| Type of first-line CT regimen (n, %)†† | 0.086 | |||
| Anthracycline-based only | 43 (49.4) | 13 (31.0) | 20 (55.6) | |
| Anthracyclines+taxanes based | 40 (46.0) | 28 (66.7) | 15 (41.7) | |
| Other§§ | 4 (4.6) | 1 (2.4) | 1 (2.8) | |
| First-line CT dose intensity (n, %)†† | 0.263 | |||
| <85% | 47 (57.3) | 20 (50.0) | 24 (68.6) | |
| ≥85% | 35 (42.7) | 20 (50.0) | 11 (31.4) | |
| Missing | 5 | 2 | 1 | |
| Cumulative dose of doxorubicin in mg/m2 (median, range) | 240 (60–420) | 240 (120–360) | 240 (120–360) | 0.75 |
| Endocrine therapy (ever) (n, %) | <0.001 | |||
| No | 30 (33.3) | 19 (43.2) | 31 (79.5) | |
| Yes | 60 (66.7) | 25 (56.8) | 8 (20.5) | |
| Missing | 18 | 6 | 13 | |
| Radiotherapy (ever) (n, %) | 0.358 | |||
| No | 86 (95.6) | 40 (90.9) | 39 (97.5) | |
| Yes | 4 (4.4) | 4 (9.1) | 1 (2.5) | |
| Missing | 18 | 6 | 12 |
P values in bold are considered to be statistically significant (<0.05).
*Not applicable as patients were not treated/followed at the Maputo Central Hospital and, therefore, were not discussed by the multidisciplinary tumour board.
†One patient received endocrine therapy as first treatment, who had a luminal B-like tumour.
‡Patients submitted to a surgical biopsy with diagnostic intent followed by a tumourectomy or a mastectomy with curative intent were included in the ‘Curative’ intent group.
§Patients submitted to a tumourectomy followed by a mastectomy were included in the ‘Mastectomy’ group.
¶One patient received a sentinel lymph node biopsy followed by an axillary dissection and was, therefore, included in the ‘Axillary dissection’ group.
**Among patients receiving any type of breast surgery (n=155). Not done: not done or no isolated lymph nodes; incomplete: 1–5 isolated lymph nodes (in case of axillary lymph node dissection); complete: ≥6 isolated lymph nodes (in case of axillary lymph node dissection) or ≥1 isolated lymph nodes with ≤2 positive lymph nodes (in case of sentinel lymph node biopsy).
††First line of chemotherapy that the patient received includes neoadjuvant, adjuvant or palliative treatment. If the patient received part of chemotherapy as neoadjuvant (eg, AC regimen), and another part as adjuvant chemotherapy (eg, paclitaxel), the type of regimen and dose intensity refer to the entire scheme (neoadjuvant plus adjuvant).
‡‡Cases in whom there were missing data regarding clinical staging or patient-abandoned treatment.
§§Includes: taxane-based CT (three patients), non-anthracycline/non-taxane-based CT (two patients) and unknown regimen (one patient). The preferred anthracycline-containing regimen was AC (cyclophosphamide 600 mg/m2 and doxorubicin 60 mg/m2 every 3 weeks) and the preferred taxane used was paclitaxel (175 mg/m2 every 3 weeks); dose-dense regimens were not used due to the unpredictable availability of granulocyte-colony stimulating factors.
CT, chemotherapy; ER, oestrogen receptor; HER2, human epidermal growth factor receptor 2; MTB, multidisciplinary tumour board; pCR, pathological complete response; TNBC, triple-negative breast cancer.
More than 90% of patients received chemotherapy, including patients with ER-positive/HER2-negative tumours (96%) and luminal A-like tumours (96%; online supplementary table 3). Among early patients with breast cancer, 104 (73%) received neoadjuvant chemotherapy, but only 3 (2%) achieved a pathological complete response (pCR) in the breast and lymph nodes (ypT0/is, ypN0). TNBC and HER2-positive tumours were less likely to be downstaged under neoadjuvant chemotherapy compared with ER-positive/HER2-negative tumours. Among ER-positive/HER2-negative patients with de novo stage IV disease (n=18), one patient did not receive any treatment and all the others received chemotherapy as the first-line systemic treatment; among them, only eight patients ever received endocrine therapy. In ER-negative patients, 19 (31%) were given endocrine therapy, as biomarker results were not yet available at the time of prescription.
Survival
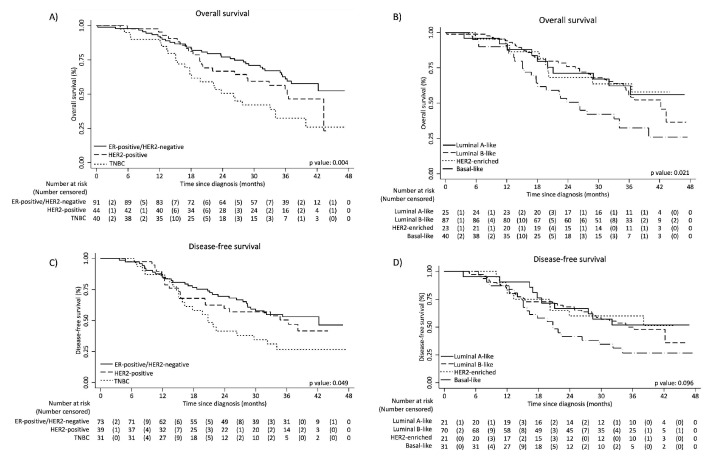
After a median follow-up of 38.3 months, only 16 (9%) patients were lost to follow-up as their last contact with the hospital was >12 months before the survival cut-off date, but they were still included in the analysis. Globally, 3-year OS was 52.5% (95% CI, 44.3% to 60.1%), being higher in the ER-positive/HER2-negative subgroup (61.1%; 95% CI, 49.5% to 70.9%) compared with HER2-positive (53.1%; 95% CI, 36.5% to 67.3%) and TNBC (32.4%, 95% CI, 17.8% to 47.9%) (figure 1). Adjusting for prognostic factors, OS was significantly worse among HER2-positive (HR 1.96; 95% CI, 1.13 to 3.39) and TNBC (HR 3.10; 95% CI, 1.81 to 5.31) versus ER-positive/HER2-negative patients (table 3). Patients with luminal A-like and HER2-enriched subtypes appeared to have a better 3-year OS (62.3% and 63.6%, respectively) than those with luminal B-like (55.9%) or basal-like (32.4%) disease, but no significant differences were observed in the adjusted analysis.
Figure 1.
Kaplan-Meier curves for overall survival and disease-free survival according to classic subtypes (panels A and C) and surrogate intrinsic subtypes (panels B and D). ER, oestrogen receptor; HER2, human epidermal growth factor receptor 2; TNBC, triple-negative breast cancer.
Table 3.
Overall survival and disease-free survival estimates and multivariable analysis, according to classic and surrogate intrinsic subtypes
| n | 3-year overall survival (95% CI) | P value* | Adjusted HR (95% CI)† | |
| 3-year overall survival | ||||
| Classic subtypes | ||||
| ER-positive/HER2-negative | 91 | 61.1 (49.5 to 70.9) | 0.004 | 1 |
| HER2-positive | 44 | 53.1 (36.5 to 67.3) | 1.96 (1.13 to 3.39) | |
| TNBC | 40 | 32.4 (17.8 to 47.9) | 3.10 (1.81 to 5.31) | |
| Surrogate intrinsic subtypes | ||||
| Luminal A-like | 25 | 62.3 (39.8 to 78.4) | 0.021 | *1 |
| Luminal B-like | 87 | 55.9 (43.8 to 66.3) | 0.69 (0.33 to 1.44) | |
| HER2-enriched | 23 | 63.6 (40.3 to 79.9) | 1.09 (0.43 to 2.74) | |
| Basal-like | 40 | 32.4 (17.8 to 47.9) | 1.96 (0.93 to 4.12) | |
| n | 3-year disease-free survival‡ | P value* | AdjustedHR (95% CI)† | |
| Classic subtypes | ||||
| ER-positive/HER2-negative | 73 | 53.1 (40.7 to 64.0) | 0.049 | *1 |
| HER2-positive | 39 | 50.5 (33.2 to 65.5) | 1.61 (0.91 to 2.85) | |
| TNBC | 31 | 26.7 (12.2 to 43.6) | 2.91 (1.64 to 5.16) | |
| Surrogate intrinsic subtypes | ||||
| Luminal A-like | 21 | 52.0 (29.1 to 70.6) | 0.096 | *1 |
| Luminal B-like | 70 | 49.9 (37.1 to 61.4) | 0.91 (0.44 to 1.88) | |
| HER2-enriched | 21 | 60.0 (35.7 to 77.6) | 1.15 (0.44 to 3.02) | |
| Basal-like | 31 | 26.7 (12.2 to 43.6) | 2.38 (1.10 to 5.13) |
Values in bold are considered to be statistically significant.
*p value for the univariate survival analysis.
†Adjusted for age (<40 versus 40–49 versus 50–59 versus ≥60 years), HIV status (negative/unknown versus positive), stage at diagnosis (0–II versus III versus IV) and date of diagnosis (pre- versus post-multidisciplinary tumour board implementation).
‡There were 75 disease-free survival events, consisting of locoregional relapse (n=27), distant relapse (n=12), both locoregional and distant relapse (n=8), second primary cancer (n=1) and death (n=27).
ER, oestrogen receptor; HER2, human epidermal growth factor receptor 2; TNBC, triple-negative breast cancer.
There were 75 DFS events, consisting of locoregional relapse (n=27), distant relapse (n=12), both locoregional and distant relapse (n=8), second primary cancer (n=1) and death (n=27). Three-year DFS was 46.7% (95% CI, 38.1% to 54.9%), being significantly lower among patients with TNBC (26.7%; 95% CI, 12.2% to 43.6%) compared with ER-positive/HER2-negative (53.1%; 95% CI, 40.7% to 64.0%) or HER2-positive patients (50.5%; 95% CI, 33.2% to 65.5%) (figure 1). Results were similar across surrogate intrinsic subtypes.
When separately analysing patients with stage I–II and stage III–IV diseases, OS and DFS differences across subtypes were more pronounced among those with stage III–IV breast cancer (online supplementary table 4).
Biomarker/subtype concordance and quality control
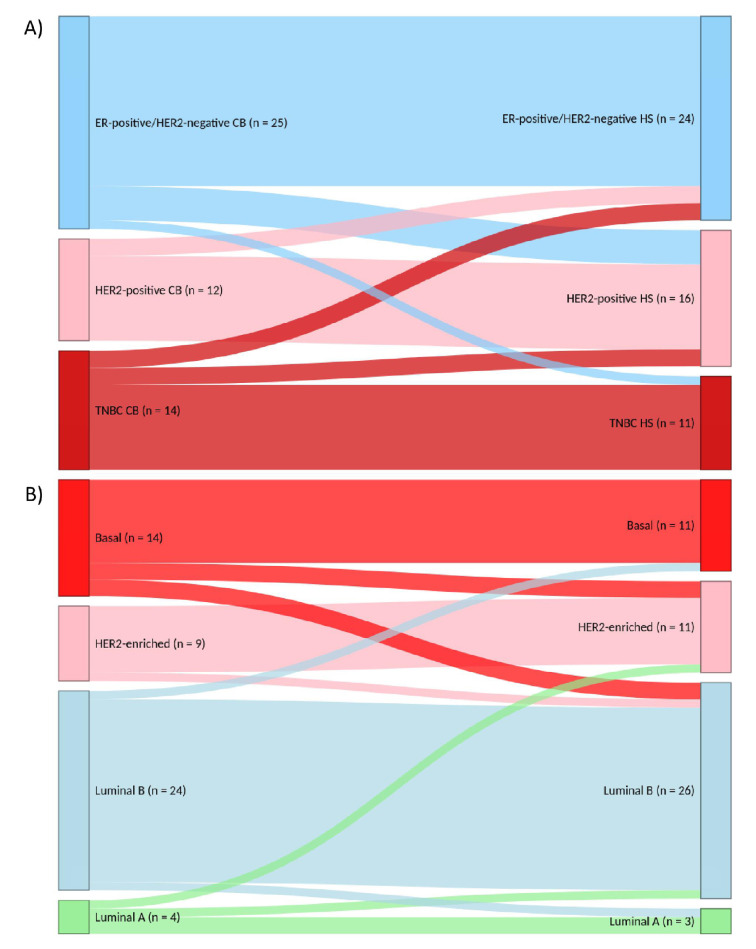
Among the 51 patients with paired cell blocks/histological samples, the observed concordance was 88.2% for ER (κ=0.762), 80.4% for PR (κ=0.574), 83.7% for HER2 (κ=0.603) and 76.1% for Ki67 status (κ=0.271) (online supplementary table 5). Spearman’s correlation σ was 0.749 for ER expression and 0.625 for PR expression in percentage, from 0 to 100%. Concordance was 78.4% (κ=0.661) for the classic subtype classification and 82.4% (κ=0.732) for the surrogate intrinsic subtype classification (figure 2).
Figure 2.
Sankey diagrams showing the reclassification of classic subtypes (panel A) and surrogate intrinsic subtypes (panel B) between CBs versus paired histological specimens (n=51). CB, cell blocks; ER: oestrogen receptor; HER2, human epidermal growth factor receptor 2; HS, histological samples; TNBC, triple-negatives breast cancer.
Out of the 109 cell blocks, 15 (14%) were sent to quality control. Concordance was 93.3% for ER and PR status (κ=0.842 and κ=0.857, respectively), 80.0% (κ=0.541) for HER2 immunohistochemical score and 50.0% (κ=0.248) for Ki67 (online supplementary table 6).
Discussion
This prospective study showed that nearly half of patients with breast cancer in Mozambique had TNBC or HER2-positive disease and that only 62% of tumours were ER-positive. Part of these subtypes were determined in cell blocks and we demonstrated, for the first time in Africa, that this is a feasible method for their assessment.
There were no striking differences in baseline characteristics or treatment across subtypes, except for HIV status, histological grade or endocrine therapy. The association between TNBC and high histological grade is well-known29; however, it is intriguing between TNBC and HIV-positive status. TNBC is the most immunogenic breast cancer subtype30 31 and, therefore, patients with a compromised immune system may be more susceptible to its development. Nonetheless, a previous meta-analysis has not demonstrated a significant difference in TNBC proportion among HIV-positive versus HIV-negative patients.32
Neoadjuvant chemotherapy was prescribed to 73% of early patients with breast cancer, which is much higher than what has been reported in South Africa (35%–62%),16 17 Rwanda (48%)33or stage III patients from the USA (42%).34 A report from South Africa showed that 64% of patients receiving neoadjuvant chemotherapy had a clinically significant response,16 which is better than our results. In our study, the pCR rate was also low: 2% in this analysis and 7% if considering patients from the Moza-BC cohort having pCR but not included in this analysis (online supplementary figure 1). These disappointing results may be explained by the overall low chemotherapy dose intensity, absence of targeted anti-HER2 therapy (ie, trastuzumab) and the generalised use of ‘sandwich’ chemotherapy regimens (neoadjuvant plus adjuvant).
Prognosis was dismal, as almost 50% of patients had died within 3 years following diagnosis. This is in line with a pooled analysis from several sub-Saharan African cancer registries showing an overall 3-year relative survival of 66%.35 Furthermore, we observed that despite the global poor prognosis, survival was even worse among patients with HER2-positive tumours and TNBC, who had a twofold and threefold increase in the risk of death versus ER-positive/HER2-negative patients, respectively.
Our cohort study has many advantages compared with other African reports.15–19 We used ISH to assess HER2-equivocal cases and evaluated Ki67, and as such were able to categorise surrogate intrinsic subtypes according to the St. Gallen classification, which is often used to determine early breast cancer treatment.6 7 Many African series did not use ISH and classified HER2-equivocal cases as ‘HER2-negative’ or excluded them, which may have underestimated the prevalence of the HER2-positive subtype.15 16 18 36 37 Additionally, most African series did not assess Ki67, and thus luminal A-like was classified as ‘ER-positive/HER2-negative’ and luminal B-like as ‘ER-positive/HER2-positive’,13 36 37 which differs from the St. Gallen definition.7 Moreover, we not only assessed biomarkers in histological samples, but also in cytological samples, which were frequently excluded from other series.19 37 38 Although only 109 out of 159 cell blocks (69%) had sufficient cellularity for immunohistochemistry assessment, we found that concordance between cell blocks and histological samples was substantial for ER and HER2 status, supporting its use in assessing breast cancer biomarkers in low-resource settings. The exception was the low concordance for Ki67; however, its low reproducibility is well-known.39 Nonetheless, further research is still needed for a better understanding of the reliability of ER and HER2 immunohistochemistry evaluation in breast cancer cell blocks in low-resource settings.
By classifying our tumour samples according to international standardised methods, we could reliably compare clinicopathological characteristics, treatment and survival of patients across the different breast cancer subtypes. Furthermore, we enriched our cohort with patients with stage III/IV tumours by using cell blocks, making our sample more representative of the ‘real’ breast cancer population. Moreover, unlike retrospective series with high losses to follow-up (up to 48%),15–19 only 9% of our patients were considered lost.
Nonetheless, our study has limitations. This is a hospital-based study; however, OS was similar to African population-based estimates,35 and this allowed for the description of survival according to breast tumour subtypes, which has not yet been estimated at a population-level in Africa. Despite the fact that we included patients from the three largest hospitals in Mozambique, detailed follow-up data were only available for patients treated at the MCH. Yet, this single-centre subcohort is similar to other African series in terms of young age at diagnosis,40 high prevalence of HIV infection,15 long delays between symptoms and diagnosis41 and a large proportion of stage III/IV.3 The limited staging options available (chest X-ray and abdominal ultrasound) may have led to an underestimation of the real incidence of stage IV disease, which may partly explain the low survival of our patients. Nonetheless, these are the staging examinations usually available in most African countries4 and, therefore, our findings may be compared with the other African series.
When performing HER2 determination in cell blocks, there may be a risk of false positivity due to the tumour’s in situ component. However, only 2 out of 49 cases switched from ‘HER2-positive’ in the cell block to ‘HER2-negative’ in the surgical sample. Nonetheless, both patients were submitted to neoadjuvant chemotherapy, which may have led to HER2 expression loss. In a large series from the USA, such potentially false-positive HER2 results on cell blocks were not observed.11
Our study has clinical and health policy implications for the management of breast cancer in Africa. Clinicians and stakeholders should move from a ‘homogeneous’ view of this disease and understand the importance of determining breast tumour subtypes before starting treatment. This is especially relevant in a setting with such a high proportion of stage III/IV, in which systemic therapy has a more predominant role. The WHO has recently included ER/PR and HER2 overexpression tests in the list of essential diagnostic tools.42 Here, we demonstrated that this assessment can be made in low-resource settings, using cell blocks from FNAC, which are usually available and much less expensive than core needle biopsies.23
International societies increasingly recommend the use of neoadjuvant therapy, especially for HER2-positive tumours and TNBC.6 Therefore, if the physician has access to biomarker determination and is aware that the patient has TNBC, neoadjuvant chemotherapy combining anthracyclines, taxanes and platinum salts could be recommended, as the addition of platinum salts increases the chance of achieving a pCR.43 Even if there is no demonstrated long-term survival benefit from the addition of platinum, these inexpensive drugs could still be used to increase the chance of tumour downsizing, improving the proportion of clean surgical margins and breast-conserving surgeries. Moreover, it would be important to enhance chemotherapy dose intensity, by using adequate supportive treatments.
Over a quarter of our patients with early disease had HER2-positive tumours. The survival of these patients has substantially improved in Western countries, due to anti-HER2 therapy.44 However, in Mozambique, like in most African countries, patients do not have access to these drugs,45 which also contributes to their dismal prognosis. With the appearance of trastuzumab biosimilars,46 it may now be possible to use them in the neoadjuvant setting to increase the likelihood of tumour downsizing and pCR, with impact in long-term survival.47 Then, in the adjuvant setting, administration of trastuzumab for 9 weeks48 to 6 months49 may also be considered, instead of the standard 12-month regimen,44 as it still might improve survival when compared with the absence of trastuzumab treatment.
Almost two-thirds of our patients had ER-positive tumours. However, if the tumour’s ER/PR status is unknown, the physician may act ‘on the safe side’ and prefer chemotherapy over endocrine therapy for (neo)adjuvant/palliative treatment. This partly explains the very high use of chemotherapy among ER-positive/HER2-negative patients in our series (96%), as in most cases biomarker results were only available after systemic treatment had been already started. On the other hand, before this study, following chemotherapy, all patients with unknown ER status would receive endocrine therapy, which is a common situation across sub-Saharan Africa.4 Therefore, it is paramount to test for ER/PR to adequately select patients who benefit from endocrine therapy—especially in the African setting, where the proportion of ER/PR-positive tumours is lower than in Western countries.13 Thus, if the tumour’s ER/PR status is known, this may lead to substantial savings, as an important proportion of patients would be spared from endocrine therapy and/or chemotherapy.
Conclusion
In this prospective cohort study, we found a high proportion of HER2-positive disease and TNBC among Mozambican patients. We demonstrated that biomarker assessment is feasible, even in patients undergoing FNAC. Globally, the 3-year OS was 52.5%, being even worse among patients with HER2-positive disease or TNBC. Our results highlight the need for the universal assessment of ER, PR and HER2 status on breast tumours, as this may optimise the use of systemic treatment, potentially leading to important survival gains in this underserved population.
Acknowledgments
We posthumously thank Dr João Fumane, former head of the Oncology Unit (2000–2011) and Director of the MCH (from 2012 until his death, in 2017), for his continuous support to this cohort study. We thank the Calouste Gulbenkian Foundation (Portugal) for funding the short-term training programme of Assucena Guisseve at the Centro Hospitalar Universitário de São João, under the Project “Atenção Integrada ao Doente Oncológico”. We thank the staff of the Oncology Unit and the Pathology Department of the MCH for ensuring the favourable environment in which this research was conducted. We thank the Department of Pathology of Centro Hospitalar Universitário de São João for performing the immunohistochemical restaining of a subset of our samples for quality control. We thank Dr Danai Fimereli, from the Institut Jules Bordet, for creating the Sankey diagrams. We thank Dr Samantha Morais, from the Instituto de Saúde Pública, Universidade do Porto, for proof-reading the manuscript.
Footnotes
Twitter: @MarianaBrandao0
MB and AG contributed equally.
CC and NL contributed equally.
Contributors: Conceptualisation and methodology: MB, AG, CC, NL. Data curation: MB, AG, GB, CG, CZ, CL, FS, AG-M, ST, CC. Formal analysis: MB, NL. Investigation: all authors. Supervision: CC, NL. Writing—original draft: MB, AG. Writing—review and editing: all authors.
Funding: We acknowledge the funding support given to the Moza-BC cohort study by the Beginning Investigator Grant for Catalytic Research (BIG Cat) programme, an African Organisation for Research and Training in Cancer (AORTIC) programme with support from the US National Cancer Institute (grant no 59-210-6-004). The funding source had no involvement in the analysis, interpretation of data, writing of the report or decision to submit the manuscript for publication.
Competing interests: MB: travel grant and speaker honoraria from Roche/GNE; research grants for her institute from Radius, AstraZeneca, Lilly, MSD, GSK/Novartis, Roche/GNE, Synthon, Servier and Pfizer.
Patient consent for publication: Not required.
Ethics approval: The National Health Bioethical Committee of Mozambique approved this study (reference number 226/CNBS/15). All participants provided written informed consent.
Provenance and peer review: Commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request. The datasets generated and analysed during the current study are not publicly available due to the fact that the included patients did not specifically provide their consent for public sharing of their data and that anonymisation, even if possible, is partially impaired by the fact that the majority of patients were treated in the same institution and diagnosed within a restricted period of time (January 2015–August 2017), with some of the groups being small. Nonetheless, data may be available from the corresponding author on reasonable request.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Carrilho C, Fontes F, Tulsidás S, et al. Cancer incidence in Mozambique in 2015-2016: data from the Maputo central Hospital cancer registry. Eur J Cancer Prev 2019;28:373–6. 10.1097/CEJ.0000000000000457 [DOI] [PubMed] [Google Scholar]
- 3.Jedy-Agba E, McCormack V, Adebamowo C, et al. Stage at diagnosis of breast cancer in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob Health 2016;4:e923–35. 10.1016/S2214-109X(16)30259-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vanderpuye V, Grover S, Hammad N, et al. An update on the management of breast cancer in Africa. Infect Agent Cancer 2017;12:13. 10.1186/s13027-017-0124-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000;406:747–52. 10.1038/35021093 [DOI] [PubMed] [Google Scholar]
- 6.Cardoso F, Kyriakides S, Ohno S, et al. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2019;30:1194–220. 10.1093/annonc/mdz173 [DOI] [PubMed] [Google Scholar]
- 7.Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies--improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 2015;26:1533–46. 10.1093/annonc/mdv221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammond MEH, Hayes DF, Dowsett M, et al. American Society of clinical Oncology/College of American pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 2010;28:2784–95. 10.1200/JCO.2009.25.6529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolff AC, Hammond MEH, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of clinical Oncology/College of American pathologists clinical practice guideline update. J Clin Oncol 2013;31:3997–4013. 10.1200/JCO.2013.50.9984 [DOI] [PubMed] [Google Scholar]
- 10.Field AS, Raymond WA, Rickard M, et al. The International Academy of cytology Yokohama system for reporting breast fine-needle aspiration biopsy cytopathology. Acta Cytol 2019;63:257–73. 10.1159/000499509 [DOI] [PubMed] [Google Scholar]
- 11.Vohra P, Buelow B, Chen Y-Y, et al. Estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 expression in breast cancer fna cell blocks and paired histologic specimens: a large retrospective study. Cancer Cytopathol 2016;124:828–35. 10.1002/cncy.21745 [DOI] [PubMed] [Google Scholar]
- 12.Puccetti M, Ravaioli S, Tumedei MM, et al. Are fine-needle aspiration biopsy-derived cell blocks a useful surrogate for tissue samples in breast cancer? Histopathology 2018;73:801–8. 10.1111/his.13694 [DOI] [PubMed] [Google Scholar]
- 13.Eng A, McCormack V, dos-Santos-Silva I. Receptor-defined subtypes of breast cancer in Indigenous populations in Africa: a systematic review and meta-analysis. PLoS Med 2014;11:e1001720. 10.1371/journal.pmed.1001720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howlader N, Altekruse SF, Li CI, et al. Us incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst 2014;106:dju055. 10.1093/jnci/dju055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cubasch H, Dickens C, Joffe M, et al. Breast cancer survival in Soweto, Johannesburg, South Africa: a receptor-defined cohort of women diagnosed from 2009 to 11. Cancer Epidemiol 2018;52:120–7. 10.1016/j.canep.2017.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruff P, Cubasch H, Joffe M, et al. Neoadjuvant chemotherapy among patients treated for nonmetastatic breast cancer in a population with a high HIV prevalence in Johannesburg, South Africa. Cancer Manag Res 2018;10:279–86. 10.2147/CMAR.S148317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Neil DS, Nietz S, Buccimazza I, et al. Neoadjuvant chemotherapy use for nonmetastatic breast cancer at five public South African hospitals and impact on time to initial cancer therapy. Oncologist 2019;24:933–44. 10.1634/theoncologist.2018-0535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas AS, Kidwell KM, Oppong JK, et al. Breast cancer in Ghana: demonstrating the need for population-based cancer registries in low- and middle-income countries. J Glob Oncol 2017;3:765–72. 10.1200/JGO.2016.006098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galukande M, Wabinga H, Mirembe F. Breast cancer survival experiences at a tertiary hospital in sub-Saharan Africa: a cohort study. World J Surg Oncol 2015;13:220. 10.1186/s12957-015-0632-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.GDP per capita (current US$) - Mozambique Data. Available: https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=MZ&most_recent_value_desc=false [Accessed 10 Mar 2020].
- 21.MISAU, INE, ICF Inquérito de Indicadores de Imunização, Malária E HIV/SIDA em Moçambique (IMASIDA) 2015, 2018. Available: https://dhsprogram.com/publications/publication-ais12-ais-final-reports.cfm [Accessed 26 Mar 2020].
- 22.MISAU Doenças Crónicas e Não Transmissíveis em Moçambique - Relatório Nacional 2018. Maputo, Mozambique: MISAU, 2018. https://static1.squarespace.com/static/55d4de6de4b011a1673a40a6/t/5b36457388251bc29f1b1b8b/1530283379635/Relatorio+Final_Portugues.pdf [Google Scholar]
- 23.Carrilho C, Ismail M, Lorenzoni C, et al. Fine needle aspiration cytology in Mozambique: report of a 15-year experience. Diagn Cytopathol 2019;47:166–71. 10.1002/dc.24062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dowsett M, Nielsen TO, A'Hern R, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in breast cancer Working group. J Natl Cancer Inst 2011;103:1656–64. 10.1093/jnci/djr393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 1991;19:403–10. 10.1111/j.1365-2559.1991.tb00229.x [DOI] [PubMed] [Google Scholar]
- 26.Edge S, Byrd D, Compton C, et al. AJCC cancer staging manual. 7th ed New York: Springer, 2010. [Google Scholar]
- 27.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–74. [PubMed] [Google Scholar]
- 28.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453–7. 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 29.Metzger-Filho O, Tutt A, de Azambuja E, et al. Dissecting the heterogeneity of triple-negative breast cancer. J Clin Oncol 2012;30:1879–87. 10.1200/JCO.2011.38.2010 [DOI] [PubMed] [Google Scholar]
- 30.Denkert C, von Minckwitz G, Darb-Esfahani S, et al. Tumour-Infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol 2018;19:40–50. 10.1016/S1470-2045(17)30904-X [DOI] [PubMed] [Google Scholar]
- 31.Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature 2012;490:61. 10.1038/nature11412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brandão M, Bruzzone M, Franzoi MA, et al. Abstract P6-11-03: characteristics and survival outcomes of HIV-positive breast cancer patients: a systematic review and meta-analysis. Cancer Res 2020;80:P6-11-03. [Google Scholar]
- 33.O’Neil DS, Keating NL, Dusengimana JMV, et al. Quality of breast cancer treatment at a rural cancer center in Rwanda. JGO 2017:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mougalian SS, Soulos PR, Killelea BK, et al. Use of neoadjuvant chemotherapy for patients with stage I to III breast cancer in the United States. Cancer 2015;121:2544–52. 10.1002/cncr.29348 [DOI] [PubMed] [Google Scholar]
- 35.Joko-Fru WY, Miranda-Filho A, Soerjomataram I, et al. Breast cancer survival in sub-Saharan Africa by age, stage at diagnosis and human development index: a population-based registry study. Int J Cancer 2020;146:1208–18. 10.1002/ijc.32406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aman NA, Doukoure B, Koffi KD, et al. Her2 overexpression and correlation with other significant clinicopathologic parameters in Ivorian breast cancer women. BMC Clin Pathol 2019;19:1. 10.1186/s12907-018-0081-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sengal AT, Haj-Mukhtar NS, Elhaj AM, et al. Immunohistochemistry defined subtypes of breast cancer in 678 Sudanese and Eritrean women; hospitals based case series. BMC Cancer 2017;17:804. 10.1186/s12885-017-3805-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hadgu E, Seifu D, Tigneh W, et al. Breast cancer in Ethiopia: evidence for geographic difference in the distribution of molecular subtypes in Africa. BMC Womens Health 2018;18:40. 10.1186/s12905-018-0531-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dowsett M, Nielsen TO, A'Hern R, A’Hern R, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in breast cancer Working group. J Natl Cancer Inst 2011;103:1656–64. 10.1093/jnci/djr393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ssentongo P, Lewcun JA, Candela X, et al. Regional, racial, gender, and tumor biology disparities in breast cancer survival rates in Africa: a systematic review and meta-analysis. PLoS One 2019;14:e0225039. 10.1371/journal.pone.0225039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grosse Frie K, Kamaté B, Traoré CB, et al. Factors associated with time to first healthcare visit, diagnosis and treatment, and their impact on survival among breast cancer patients in Mali. PLoS One 2018;13:e0207928. 10.1371/journal.pone.0207928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.WHO Second who model list of essential in vitro diagnostics. Geneva: WHO, 2019. https://www.who.int/medical_devices/publications/Standalone_document_v8.pdf?ua=1 [Google Scholar]
- 43.Poggio F, Bruzzone M, Ceppi M, et al. Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: a systematic review and meta-analysis. Ann Oncol 2018;29:1497–508. 10.1093/annonc/mdy127 [DOI] [PubMed] [Google Scholar]
- 44.Cameron D, Piccart-Gebhart MJ, Gelber RD, et al. 11 years' follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the Herceptin adjuvant (HERA) trial. Lancet 2017;389:1195–205. 10.1016/S0140-6736(16)32616-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vanderpuye VDNK, Olopade OI, Huo D. Pilot survey of breast cancer management in sub-Saharan Africa. J Glob Oncol 2017;3:194–200. 10.1200/JGO.2016.004945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barbier L, Declerck P, Simoens S, et al. The arrival of biosimilar monoclonal antibodies in oncology: clinical studies for trastuzumab biosimilars. Br J Cancer 2019;121:199–210. 10.1038/s41416-019-0480-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014;384:164–72. 10.1016/S0140-6736(13)62422-8 [DOI] [PubMed] [Google Scholar]
- 48.Joensuu H, Bono P, Kataja V, et al. Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: final results of the FinHer trial. JCO 2009;27:5685–92. 10.1200/JCO.2008.21.4577 [DOI] [PubMed] [Google Scholar]
- 49.Earl HM, Hiller L, Vallier A-L, et al. 6 versus 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (Persephone): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial. Lancet 2019;393:2599–612. 10.1016/S0140-6736(19)30650-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2020-000829supp001.pdf (563.3KB, pdf)