Abstract
In this issue, Michelucci et al. report the existence of specific sites acting as Ca2+ entry units (CEUs) in fast skeletal muscle of mice lacking calsequestrin (CASQ1), the major Ca2+ binding protein of the SR. The CEU provides constitutive and store-operated Ca2+ entry (SOCE) and resistance to force decline resulting from SR Ca2+ depletion during repetitive muscle activity.
Calcium ions can rapidly transmit information from the surface of cells to the cytoplasm, conducted by plasma membrane channels, or can be released from intracellular stores. Many invertebrate muscle types and vertebrate heart and smooth muscle use both the external and internal Ca2+ source for excitation-contraction coupling (ECC) in the mechanism of calcium-induced calcium release (CICR). Here, a short and small Ca2+ entry impulse generated by voltage-activated channels triggers a larger Ca2+ efflux from the SR (Ríos, 2018). In skeletal muscle, the voltage-activated Ca2+ current could be ruled out as a trigger for ECC (Dayal et al., 2017); nevertheless, muscle function depends on external Ca2+ in several ways. In recent years, store-operated Ca2+ entry (SOCE), which sets in when the internal stores of cells get depleted (Prakriya and Lewis, 2015), has progressively attracted the interest of ECC research (Dirksen, 2009). In the present issue of the Journal of General Physiology, Michelucci and coworkers (Michelucci et al., 2020) report structural and functional characteristics of SOCE in skeletal muscle of a mouse with constitutively compromised Ca2+ storing capabilities. The work provides novel insights into the mechanism by which the extracellular Ca2+ source is used to mitigate loss of Ca2+ from the SR.
Excitation-calcium release coupling in skeletal muscle
In skeletal muscle, a dense network of thin transverse tubules (TTs) conducts the action potential from the cell surface to the terminal cisternae of the SR (Franzini-Armstrong, 2018). The triad, consisting of one TT flanked on two sides by a terminal cisterna, is the location of excitation-Ca2+ release coupling. In the TT membrane, a specialized voltage-dependent calcium channel (CaV1.1; Bannister and Beam, 2013), commonly known as dihydropyridine receptor (DHPR), communicates the electrical signal to the calcium release channel of the SR (ryanodine receptor; RyR1). The coupling is based on a unique conformational handshake between CaV1.1 and RyR1 (Hernández-Ochoa and Schneider, 2018; Shishmarev, 2020) in a contact structure termed “calcium release unit” (CRU) or “couplon” (Franzini-Armstrong, 1999; Ríos et al., 2015).
The resting free-Ca2+ concentration within the SR has been estimated at ∼0.3 mM (Rudolf et al., 2006). It is roughly four orders of magnitude higher than in the myoplasm and about fourfold lower than in the extracellular space (∼1.2 mM; Walser, 1961). The steep gradient across the SR membrane, i.e., the driving force for the depolarization-activated Ca2+ release, is achieved by the SR/ER calcium ATPase (SERCA), densely packed in the nonjunctional regions of the SR membrane. Much more than the free Ca2+ can rapidly be released from the SR because most is loosely bound to the two isoforms of the acidic protein calsequestrin (MacLennan and Wong, 1971), mainly CASQ1 in fast muscle, and other less abundant Ca2+ binding proteins. Ca2+-bound CASQ is a very large multi-subunit complex that forms a dense network of polymeric strings (Rossi et al., 2020). These structures may even act as rails for efficient one-dimensional surface diffusion toward the Ca2+ releasing channels (MacLennan and Reithmeier, 1998). Calsequestrin depolymerizes when free [Ca2+] is reduced (Park et al., 2003), thus lowering its affinity and allowing more Ca2+ to dissociate (Royer and Ríos, 2009). This regenerative dissociation process is a proper mechanism to prevent the driving force for Ca2+ release from declining too rapidly under depleting conditions (Manno et al., 2017). In addition to its role as an adaptive Ca2+ buffer, calsequestrin and its Ca2+-dependent structural changes seem to influence RyR1 permeability, in mammalian muscle apparently by favoring RyR1 closure during depletion (Sztretye et al., 2011).
The role of extracellular calcium in skeletal muscle ECC
Early studies, using radioactive isotopes, demonstrated that Ca2+ enters muscle cells during electrical excitation (Bianchi and Shanes, 1959). One entry pathway for external Ca2+ is the DHPR itself. Even though its primary role is acting as a voltage sensor to control the open state of RyR1, CaV1.1 permits Ca2+ to pass from the lumen of the TT to the myoplasm (L-type Ca2+ current), providing a flux that is more than two orders of magnitude smaller than the peak release flux from the SR in mouse skeletal muscle (Ursu et al., 2005). Its slow time course of activation during step depolarization made it seem unlikely that individual action potentials would open the CaV1.1 pore at all. Nevertheless, Ca2+ entry associated with action potentials could be detected (Launikonis et al., 2009) and, at least in part, attributed to the DHPR (Bannister et al., 2009). Recent experiments on mice expressing nonconducting CaV1.1 (Dayal et al., 2017) corroborated earlier studies indicating that the L-type Ca2+ current is irrelevant for EC coupling and is probably not required for SR Ca2+ loading either. Nonetheless, irrespective of Ca2+ entry, the external Ca2+ is not without influence on contraction and the mechanism that couples voltage to Ca2+ release (Lüttgau and Spiecker, 1979; Brum et al., 1988b; Melzer et al., 1995). If it is removed, the voltage-sensor gating charges of the DHPR are shifted to an altered mode of mobility (termed charge 2) exhibiting slower kinetics in a more negative voltage range. In this mode, voltage sensing is no longer coupled to calcium release (Brum et al., 1988a; Ferreira Gregorio et al., 2017).
Another Ca2+ influx pathway, distinct from the CaV1.1, attracted attention in muscle research rather late. As in many nonmuscle cell types, a SOCE is present in skeletal muscle (Kurebayashi and Ogawa, 2001; Launikonis and Ríos, 2007). It is associated with the stromal interaction molecule STIM1 that senses the intraluminal Ca2+ concentration of the SR and activates a Ca2+-conducting channel, Orai1 (originally termed “calcium release-activated Ca2+ [CRAC] channel”), when the SR gets depleted. In nonmuscle cells, it takes rather long (many seconds) for SOCE to be activated (Wu et al., 2006) because STIM has to migrate by diffusion within the ER membrane to reach ER–plasma membrane junctions where it gets close enough to interact with Orai and triggers its opening. In skeletal muscle, the response of Orai1 to SR depletion is fast (less than a second; Launikonis and Ríos, 2007). It could be demonstrated, using a mechanically skinned fiber approach with a Ca2+ indicator trapped in the sealed TTs, that the reaction is rapid enough to activate SOCE (measurable as a depletion signal) after each individual action potential in a series of excitation events (Koenig et al., 2019). Probably the rapid activation is made possible by prearranged clusters of Orai1 with a specific STIM1 splice variant (STIM1L) colocalized with RyR1 in the triad (Darbellay et al., 2011).
Muscle fatigue and calcium entry units
SR Ca2+ depletion becomes a major challenge for muscle performance during strenuous exercise (Cheng et al., 2018). If electrical motor nerve stimulation via implanted electrodes is imposed for days or weeks, fast-twitch muscles undergo a transition to the more energy-efficient and fatigue-resistant slow phenotype. The fast-to-slow transition involves a remodeling of the membrane compartments of ECC as has been demonstrated by Eisenberg et al. (1984). They found that both the density of TT and SR gradually decrease during the stimulation period and recover with a similarly slow time course. Recently, an interesting discovery was made in mice that had been exposed to a 1-h period of intense treadmill running to cause fatigue. Muscles of these mice exhibited gross structural alterations of the internal membranes within <1 h following the strenuous muscle activity (Boncompagni et al., 2017; Michelucci et al., 2018, 2019); parts of the longitudinal SR formed extensive stacks of flat SR pockets, and the TT sent branches into these regions to form junctions with the new SR derivatives. In parallel with these morphological changes, a higher constitutive Ca2+ entry and a strong transient up-regulation of the SOCE rate in response to repeated tetanic stimulation was revealed by Mn2+ quenching of intracellular fura-2 fluorescence and was accompanied by improved force generation (Michelucci et al., 2019). A further enhancement of SOCE was observed when depleting the SR using SERCA blockers. These functional effects were completely absent in preparations of inducible, muscle-specific Orai1 knockout and muscle-specific dominant-negative Orai1 transgenic mice. The findings suggest that the contact regions between exercise-induced TT branches and SR stacks are sites of increased SOCE activity. They were, therefore, termed calcium entry units (CEU) and proposed to help muscle compensate for the excess loss of SR Ca2+ taking place during exercise. The results were consistent with the role of Orai1 in reducing muscle fatigue in mice during repetitive, high-frequency excitation that had previously been established (Wei-Lapierre et al., 2013). The consequences of the lack of SOCE activity for the human organism have become apparent in patients suffering from inherited disorders caused by loss of function mutations in Orai1 or STIM1; in addition to severe immunodeficiency, muscular hypotonia and atrophy are among the symptoms of these diseases (Michelucci et al., 2018).
Lack of calsequestrin boosts the development of Ca2+ entry units
The new paper by Michelucci et al. (2020) in the present issue of this journal describes the situation in fast muscles of CASQ1 knock-out mice. Eliminating its main Ca2+ buffer should drastically reduce the Ca2+ storage capacity of the SR and, as one might think, dramatically weaken these animals’ skeletal muscles. Surprisingly, they behave quite normally and show only mild signs of atrophy (Paolini et al., 2007). How do they cope with the lack of calsequestrin?
In early structural work on the CASQ1-null mouse, substantial remodeling of the Ca2+ release units was noticed (Paolini et al., 2007). The present paper highlights the resemblance of certain remodeled membrane structures in CASQ1-null muscle to the CEUs that were found to reversibly form in normal muscle during strenuous exercise (Michelucci et al., 2019). Thus, the chronic SR filling impairment resulting from the lack of CASQ1 may have caused a substantial up-regulation of CEUs during muscle cell development. In this way the SR and its rapid voltage-controlled Ca2+-release mechanism would be connected more tightly to the extracellular Ca2+ pool. This hypothesis is supported in the new study by a large array of experimental findings resulting from the combination of ultrastructural, molecular, and physiological investigations.
The morphometric analysis of electron micrographs revealed a large increase in the number of SR stacks in the I-band region and in TT branching to this location as well as in the length of common contacts between these two elements. Much higher SOCE activity than in the wild type was found as judged by the Mn2+ quenching technique. As a correlate of SOCE activity, a gradual build-up of Ca2+ release and force development from initially low levels could be recorded during repeated tetanic stimulations. This went in parallel with a progressive improvement of the SR filling state as measured with an SR-targeted Ca2+ indicator. Finally, significantly higher expression levels of Orai1, of both STIM1 isoforms (L and S) and of the SERCA pump were found by Western blotting.
The picture that emerges from these findings is the following: repetitive action potential activation initially leads to a rapid strong depletion in the terminal cisternae of CASQ1-null muscle accompanied by excessive Ca2+ loss to the TT lumen, presumably via the sodium-calcium exchanger (NCX) and the plasma membrane Ca2+ ATPase (PMCA). The SR depletion acts as the signal for STIM1 molecules to recruit Orai1 channels of prearranged CEUs in the TT branches. SERCA pumps in the membranes of adjacent SR stacks take up the external Ca2+ that enters the narrow inter-membrane gap of the CEU. The raised Ca2+ concentration in the stack compartments of the SR feeds a flux of Ca2+ to the terminal cisternae which counteracts the rapid decline in driving force for Ca2+ release. In this way, CEU-based SOCE sort of mimics the replenishment of free Ca2+ in the terminal cisternae that in wild type muscle is provided by dissociation from CASQ1. The morphological and physiological data of CASQ1-null skeletal muscle indicate a permanent state very similar to the one reached in wild type mice after strenuous exercise (Fig. 1), and they provide a plausible explanation for the lack of obvious disabilities in these mice without having to postulate compensatory up-regulation of alternative SR Ca2+ buffers.
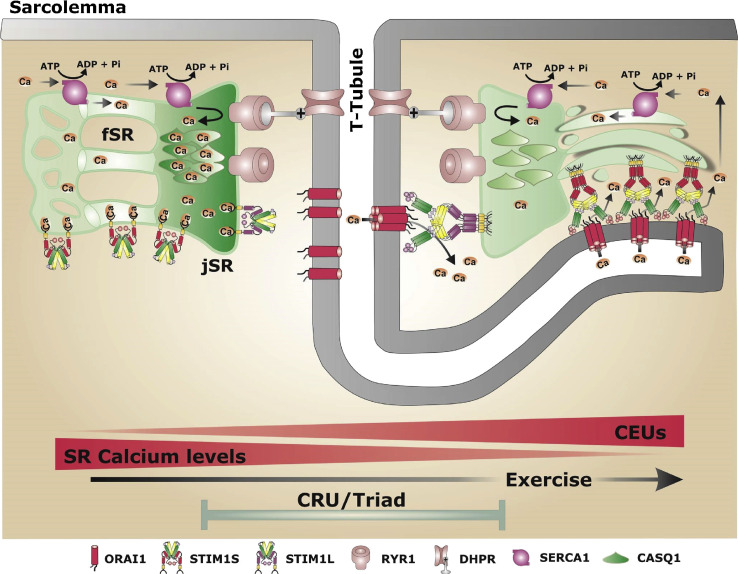
Figure 1.
Formation of calcium entry units. Structural changes from the normal state of a well-loaded SR (left) to a state of Ca2+ depletion (right). Depletion leads to the formation of CEUs characterized by longitudinal branches of the transverse tubular system (T-Tubule) and stacks of the longitudinal SR which are connected by STIM1–Orai1 bridges. The right side of the drawing shows the case of Ca2+-depleted calsequestrin (CASQ1) in the terminal cisternae. Similar CEUs are now reported as permanent structures in CASQ1-null muscle fibers as means to cope with the compromised Ca2+ storing ability. fSR, free (nonjunctional) SR that does not interact with TTs; jSR, junctional SR (terminal cisternae). Figure reproduced from Michelucci et al., 2018, with permission.
The CEUs constitutively present in CASQ1-null muscle or forming on demand in wild type cells may be part of an extensive Ca2+ nanocourse network as has recently been identified in vascular smooth muscle (Duan et al., 2019). Targeted indicators should make it possible in the future to visualize the travel routes of Ca2+, specifically to measure Ca2+ entry via the CEUs, and to search for intra-SR gradients associated with the flux from the CEU to the CRU. It would also be interesting to learn more about the specific protein content of the CEU regions. Which are the molecular components that maintain these structures and control their formation? The study indicates that Orai1 is present. Is STIM preassembled, too, or does it have to migrate there from different regions of the SR? And which of the STIM isoforms are involved? Interestingly, CASQ1 has been reported to interact with STIM1 to inhibit its redistribution (Zhang et al., 2016). Furthermore, the structural characteristics of the CEUs may bring about functional changes in addition to up-regulated SOCE. Are the new longitudinal TT derivatives excitable like their transversal counterparts? This would seem luxurious since this compartment apparently lacks voltage-activated Ca2+ release. However, the electrical capacitance increase caused by these elements might affect excitability and conduction velocity in the “regular” TTs. Electrical recordings combined with measurements using voltage-sensitive dyes may provide the answers.
The new findings by Michelucci et al. (2020) are intriguing, and we can expect that CEUs will gain increasing attention in future studies on altered or diseased states of skeletal muscle. In particular, studying the pathophysiology of CASQ1-related myopathies (Rossi et al., 2020) will profit from these results.
Acknowledgments
Eduardo Ríos served as editor.
The author declares no competing financial interests.
References
- Bannister R.A., and Beam K.G.. 2013. CaV1.1: The atypical prototypical voltage-gated Ca2+ channel. Biochim. Biophys. Acta. 1828:1587–1597. 10.1016/j.bbamem.2012.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister R.A., Pessah I.N., and Beam K.G.. 2009. The skeletal L-type Ca2+ current is a major contributor to excitation-coupled Ca2+ entry. J. Gen. Physiol. 133:79–91. 10.1085/jgp.200810105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi C.P., and Shanes A.M.. 1959. Calcium influx in skeletal muscle at rest, during activity, and during potassium contracture. J. Gen. Physiol. 42:803–815. 10.1085/jgp.42.4.803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boncompagni S., Michelucci A., Pietrangelo L., Dirksen R.T., and Protasi F.. 2017. Exercise-dependent formation of new junctions that promote STIM1-Orai1 assembly in skeletal muscle. Sci. Rep. 7:14286 10.1038/s41598-017-14134-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brum G., Fitts R., Pizarro G., and Ríos E.. 1988a Voltage sensors of the frog skeletal muscle membrane require calcium to function in excitation-contraction coupling. J. Physiol. 398:475–505. 10.1113/jphysiol.1988.sp017053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brum G., Ríos E., and Stéfani E.. 1988b Effects of extracellular calcium on calcium movements of excitation-contraction coupling in frog skeletal muscle fibres. J. Physiol. 398:441–473. 10.1113/jphysiol.1988.sp017052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A.J., Place N., and Westerblad H.. 2018. Molecular Basis for Exercise-Induced Fatigue: The Importance of Strictly Controlled Cellular Ca2+ Handling. Cold Spring Harb. Perspect. Med. 8 a029710 10.1101/cshperspect.a029710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbellay B., Arnaudeau S., Bader C.R., Konig S., and Bernheim L.. 2011. STIM1L is a new actin-binding splice variant involved in fast repetitive Ca2+ release. J. Cell Biol. 194:335–346. 10.1083/jcb.201012157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayal A., Schrötter K., Pan Y., Föhr K., Melzer W., and Grabner M.. 2017. The Ca2+ influx through the mammalian skeletal muscle dihydropyridine receptor is irrelevant for muscle performance. Nat. Commun. 8:475 10.1038/s41467-017-00629-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirksen R.T. 2009. Checking your SOCCs and feet: the molecular mechanisms of Ca2+ entry in skeletal muscle. J. Physiol. 587:3139–3147. 10.1113/jphysiol.2009.172148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan J., Navarro-Dorado J., Clark J.H., Kinnear N.P., Meinke P., Schirmer E.C., and Evans A.M.. 2019. The cell-wide web coordinates cellular processes by directing site-specific Ca2+ flux across cytoplasmic nanocourses. Nat. Commun. 10:2299 10.1038/s41467-019-10055-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg B.R., Brown J.M., and Salmons S.. 1984. Restoration of fast muscle characteristics following cessation of chronic stimulation. The ultrastructure of slow-to-fast transformation. Cell Tissue Res. 238:221–230. 10.1007/BF00217292 [DOI] [PubMed] [Google Scholar]
- Ferreira Gregorio J., Pequera G., Manno C., Ríos E., and Brum G.. 2017. The voltage sensor of excitation-contraction coupling in mammals: Inactivation and interaction with Ca2+. J. Gen. Physiol. 149:1041–1058. 10.1085/jgp.201611725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzini-Armstrong C. 1999. The sarcoplasmic reticulum and the control of muscle contraction. FASEB J. 13(9002, Suppl 2):S266–S270. 10.1096/fasebj.13.9002.S266 [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C. 2018. The relationship between form and function throughout the history of excitation-contraction coupling. J. Gen. Physiol. 150:189–210. 10.1085/jgp.201711889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Ochoa E.O., and Schneider M.F.. 2018. Voltage sensing mechanism in skeletal muscle excitation-contraction coupling: coming of age or midlife crisis? Skelet. Muscle. 8:22 10.1186/s13395-018-0167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig X., Choi R.H., Schicker K., Singh D.P., Hilber K., and Launikonis B.S.. 2019. Mechanistic insights into store-operated Ca2+ entry during excitation-contraction coupling in skeletal muscle. Biochim. Biophys. Acta Mol. Cell Res. 1866:1239–1248. 10.1016/j.bbamcr.2019.02.014 [DOI] [PubMed] [Google Scholar]
- Kurebayashi N., and Ogawa Y.. 2001. Depletion of Ca2+ in the sarcoplasmic reticulum stimulates Ca2+ entry into mouse skeletal muscle fibres. J. Physiol. 533:185–199. 10.1111/j.1469-7793.2001.0185b.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launikonis B.S., and Ríos E.. 2007. Store-operated Ca2+ entry during intracellular Ca2+ release in mammalian skeletal muscle. J. Physiol. 583:81–97. 10.1113/jphysiol.2007.135046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launikonis B.S., Stephenson D.G., and Friedrich O.. 2009. Rapid Ca2+ flux through the transverse tubular membrane, activated by individual action potentials in mammalian skeletal muscle. J. Physiol. 587:2299–2312. 10.1113/jphysiol.2009.168682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttgau H.C., and Spiecker W.. 1979. The effects of calcium deprivation upon mechanical and electrophysiological parameters in skeletal muscle fibres of the frog. J. Physiol. 296:411–429. 10.1113/jphysiol.1979.sp013013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan D.H., and Reithmeier R.A.. 1998. Ion tamers. Nat. Struct. Biol. 5:409–411. 10.1038/nsb0698-409 [DOI] [PubMed] [Google Scholar]
- MacLennan D.H., and Wong P.T.. 1971. Isolation of a calcium-sequestering protein from sarcoplasmic reticulum. Proc. Natl. Acad. Sci. USA. 68:1231–1235. 10.1073/pnas.68.6.1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno C., Figueroa L.C., Gillespie D., Fitts R., Kang C., Franzini-Armstrong C., and Rios E.. 2017. Calsequestrin depolymerizes when calcium is depleted in the sarcoplasmic reticulum of working muscle. Proc. Natl. Acad. Sci. USA. 114:E638–E647. 10.1073/pnas.1620265114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer W., Herrmann-Frank A., and Lüttgau H.C.. 1995. The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres. Biochim. Biophys. Acta. 1241:59–116. 10.1016/0304-4157(94)00014-5 [DOI] [PubMed] [Google Scholar]
- Michelucci A., García-Castañeda M., Boncompagni S., and Dirksen R.T.. 2018. Role of STIM1/ORAI1-mediated store-operated Ca2+ entry in skeletal muscle physiology and disease. Cell Calcium. 76:101–115. 10.1016/j.ceca.2018.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelucci A., Boncompagni S., Pietrangelo L., García-Castañeda M., Takano T., Malik S., Dirksen R.T., and Protasi F.. 2019. Transverse tubule remodeling enhances Orai1-dependent Ca2+ entry in skeletal muscle. eLife. 8 e47576 10.7554/eLife.47576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelucci A., Boncompagni S., Pietrangelo L., Takano T., Protasi F., and Dirksen R.T.. 2020. Pre-assembled Ca2+ entry units and constitutively active Ca2+ entry in skeletal muscle of calsequestrin-1 knockout mice. J Gen Physiol. 152 e202012617 10.1085/jgp.202012617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolini C., Quarta M., Nori A., Boncompagni S., Canato M., Volpe P., Allen P.D., Reggiani C., and Protasi F.. 2007. Reorganized stores and impaired calcium handling in skeletal muscle of mice lacking calsequestrin-1. J. Physiol. 583:767–784. 10.1113/jphysiol.2007.138024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H., Wu S., Dunker A.K., and Kang C.. 2003. Polymerization of calsequestrin. Implications for Ca2+ regulation. J. Biol. Chem. 278:16176–16182. 10.1074/jbc.M300120200 [DOI] [PubMed] [Google Scholar]
- Prakriya M., and Lewis R.S.. 2015. Store-Operated Calcium Channels. Physiol. Rev. 95:1383–1436. 10.1152/physrev.00020.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ríos E. 2018. Calcium-induced release of calcium in muscle: 50 years of work and the emerging consensus. J. Gen. Physiol. 150:521–537. 10.1085/jgp.201711959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ríos E., Figueroa L., Manno C., Kraeva N., and Riazi S.. 2015. The couplonopathies: A comparative approach to a class of diseases of skeletal and cardiac muscle. J. Gen. Physiol. 145:459–474. 10.1085/jgp.201411321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi D., Gamberucci A., Pierantozzi E., Amato C., Migliore L., and Sorrentino V.. 2020. Calsequestrin, a key protein in striated muscle health and disease. J. Muscle Res. Cell Motil. 10.1007/s10974-020-09583-6 [DOI] [PubMed] [Google Scholar]
- Royer L., and Ríos E.. 2009. Deconstructing calsequestrin. Complex buffering in the calcium store of skeletal muscle. J. Physiol. 587:3101–3111. 10.1113/jphysiol.2009.171934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolf R., Magalhães P.J., and Pozzan T.. 2006. Direct in vivo monitoring of sarcoplasmic reticulum Ca2+ and cytosolic cAMP dynamics in mouse skeletal muscle. J. Cell Biol. 173:187–193. 10.1083/jcb.200601160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishmarev D. 2020. Excitation-contraction coupling in skeletal muscle: recent progress and unanswered questions. Biophys. Rev. 12:143–153. 10.1007/s12551-020-00610-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztretye M., Yi J., Figueroa L., Zhou J., Royer L., Allen P., Brum G., and Ríos E.. 2011. Measurement of RyR permeability reveals a role of calsequestrin in termination of SR Ca2+ release in skeletal muscle. J. Gen. Physiol. 138:231–247. 10.1085/jgp.201010592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursu D., Schuhmeier R.P., and Melzer W.. 2005. Voltage-controlled Ca2+ release and entry flux in isolated adult muscle fibres of the mouse. J. Physiol. 562:347–365. 10.1113/jphysiol.2004.073882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walser M. 1961. Ion association. VI. Interactions between calcium, magnesium, inorganic phosphate, citrate and protein in normal human plasma. J. Clin. Invest. 40:723–730. 10.1172/JCI104306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei-Lapierre L., Carrell E.M., Boncompagni S., Protasi F., and Dirksen R.T.. 2013. Orai1-dependent calcium entry promotes skeletal muscle growth and limits fatigue. Nat. Commun. 4:2805 10.1038/ncomms3805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M.M., Buchanan J., Luik R.M., and Lewis R.S.. 2006. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J. Cell Biol. 174:803–813. 10.1083/jcb.200604014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Wang L., Li S., Xue J., and Luo D.. 2016. Calsequestrin-1 Regulates Store-Operated Ca2+ Entry by Inhibiting STIM1 Aggregation. Cell. Physiol. Biochem. 38:2183–2193. 10.1159/000445574 [DOI] [PubMed] [Google Scholar]