Dear editor,
We read with interest the recent article by Terpos et al. 1 They reviewed different hematologic findings and complications of COVID‐19. Especially, we are interested in lymphopenia in severe COVID‐19 patients, which is a predictor factor of severity and mortality. We aimed to report the occurrence of lymphopenia, lymphocyte subsets, and its impact on ICU mortality in critically ill patients with COVID‐19.
In this single‐center cohort, we included adult patients with confirmed COVID‐19 infection by a positive reverse‐transcriptase‐polymerase‐chain‐reaction (RT‐PCR) assay of a nasopharyngeal swab, admitted in the intensive care unit (ICU) of the Mohammed VIth university hospital of the Marrakech region (Morocco), from March 19, 2020 to May 15, 2020. We collected demographic data, comorbidities, clinical signs at the ICU admission, laboratory findings, chest CT scan if available, outcomes, time from onset of the first symptom to ICU admission, and sequential organ failure assessment (SOFA) scores. We expressed continuous variables as medians and interquartile (IQR) ranges or means (standard deviations (SD)), as appropriate, and compared using independent group Student's t test or the Mann‐Whitney U test. Categorical variables were described using percentages and compared using the χ2‐test, although Fisher's exact test was used when the data were sparse. We performed univariable to evaluate the risk factors of mortality. The analysis was processed by spss 10.0 for Windows (SPSS, Chicago, IL, USA). A P‐value of <.05 was considered statistically significant.
Of 1618, COVID‐19 patients hospitalized in our teaching center, 55 (3.4%) were admitted to the ICU. The mean age was 59 (16.5) years (Min‐Max: 21‐90); 74.5% were men. Among all the patients, 84% had chronic medical conditions. The common comorbidities were hypertension (42%) and diabetes (34%). The frequent symptoms were dyspnoea (85%) and cough (80%). The median length from the onset of symptoms to ICU admission was 7 (6‐8) days. The median SOFA score at admission was 5 (4‐17). The length from the onset of symptoms to ICU admission was an independent risk factor of lymphopenia <1000/mm3 (OR 1.5; 95% CI 1.006‐2.2; P = .04).
We noted that lymphopenia <1000/mm3 was present in 53% of our patients. We performed lymphocyte subset counts in 43.6% (24/55) of cases on admission (Table 1). CD3 + T cells (normal range 1000‐2200/mm3) decreased in 66.6% (16/24) of patients. CD4 + T cells (normal range 530‐1300/mm3) decreased in 70.8% (17/24) of patients. CD8 + T cells (normal range 330‐920/mm3) decreased in 54.2% (13/24) of patients. B cells (normal range 110‐570/mm3) decreased in 45.8% (11/24) of patients, and natural killer cells (normal range 70‐480/mm3) decreased in 29.1% (7/24) of patients. Statistically, comparing survivors to nonsurvivors, the difference was not significant in CD3+ (P = .1), CD4+ (P = .1), CD8+ (P = .3) T cells, B cells (P = .8), NK cells (P = .5), and CD4+/CD8 + ratio (P = .5).
Table 1.
lymphocytes subsets count on admission and outcomes in 24 patients and outcomes
| Patients | CD3+ | CD4+ | CD8+ | CD4+/CD8+ | B cells | NK cells | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | 246 | 119 | 124 | 0.96 | 55 | 80 | Deceased |
| 2 | 656 | 452 | 185 | 2.44 | 453 | 246 | Deceased |
| 3 | 1084 | 647 | 376 | 1.72 | 353 | 114 | Survivor |
| 4 | 209 | 74 | 93 | 0.8 | 10 | 1 | Survivor |
| 5 | 626 | 413 | 205 | 2.01 | 76 | 262 | Deceased |
| 6 | 1051 | 400 | 584 | 0.68 | 70 | 151 | Survivor |
| 7 | 1620 | 1100 | 474 | 2.32 | 213 | 97 | Survivor |
| 8 | 875 | 479 | 390 | 1.22 | 188 | 271 | Survivor |
| 9 | 158 | 101 | 36 | 2.8 | 70 | 17 | Deceased |
| 10 | 1886 | 654 | 1270 | 0.51 | 116 | 774 | Deceased |
| 11 | 582 | 238 | 323 | 0.73 | 85 | 81 | Survivor |
| 12 | 452 | 219 | 122 | 1.8 | 113 | 53 | Survivor |
| 13 | 280 | 158 | 97 | 1.62 | 77 | 61 | Deceased |
| 14 | 751 | 365 | 367 | 0.99 | 288 | 527 | Survivor |
| 15 | 2644 | 1163 | 1396 | 0.83 | 434 | 346 | Survivor |
| 16 | 500 | 323 | 135 | 2.39 | 89 | 82 | Survivor |
| 17 | 824 | 314 | 791 | 0.4 | 91 | 68 | Survivor |
| 18 | 249 | 144 | 100 | 1.44 | 91 | 261 | Deceased |
| 19 | 576 | 412 | 151 | 2.72 | 59 | 26 | Survivor |
| 20 | 708 | 438 | 246 | 1.78 | 242 | 115 | Deceased |
| 21 | 114 | 39 | 63 | 0.62 | 185 | 21 | Deceased |
| 22 | 1134 | 547 | 732 | 0.74 | 249 | 115 | Survivor |
| 23 | 1056 | 987 | 429 | 2.3 | 540 | 223 | Survivor |
| 24 | 1589 | 675 | 562 | 1.2 | 378 | 178 | Survivor |
Liu Z et al 2 found that CD4 + T cells diminished in 56.4% of patients, CD8 + T cells diminished in 71.8% of patients, B cells diminished in 69.2% of patients, and NK cells diminished in 76.9% patients. In addition, the severe patients had lower lymphocyte count (P = .0007), CD4 + T cells (P = .024), CD8 + T cells (P = .005), and B cells (P = .018), but the difference was not significant in CD4+/CD8 + ratio (P = .392) and NK cells (P = .177), compared to cases with mild severity. 3
Lymphopenia <1000/mm3 on admission was more frequent in nonsurvivors (67% vs 30%; P = .01) compared with survivors. The lymphocyte counts on day 3, day 4, day 5, and day 7 of hospitalization were predictor factors of the ICU mortality in univariable analysis (Figure 1) and the lowest count of lymphocyte was on day 2 after hospitalization in survivors. Compared to patients with lymphopenia >1000/mm3, those with lymphopenia <1000/mm3 needed more inotrope use (43% vs 12%; P = .01), with an increased ICU mortality rate (79% vs 44%; P = .01).
Figure 1.
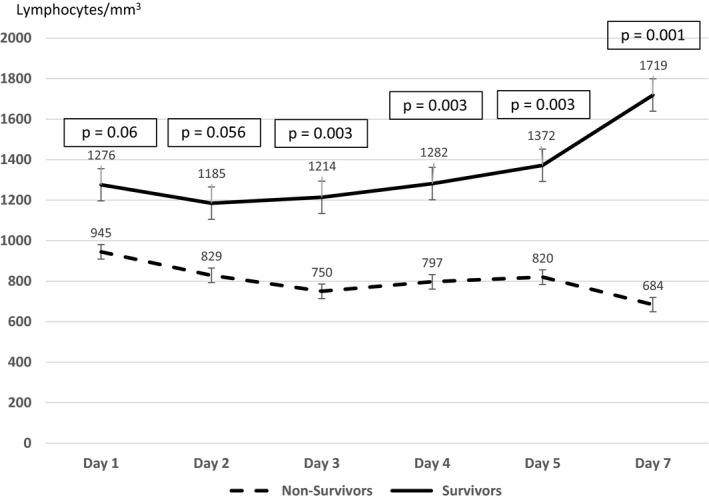
the impact of the lymphocyte counts on the ICU mortality in univariable analysis
A recent meta‐analysis proposed that lymphopenia is an important hematological signal of severe COVID‐19 and a lymphopenia <1500/mm3 could be a practical parameter to predict severe outcomes. 4 Moreover, it was a risk factor of myocardial injury 5 and acute respiratory distress syndrome (ARDS). 6 Besides, it was a risk factor for death with a nadir of lymphocytes on day 7 in survivors. 7 This nadir was on day 2 in our study as a result of the delay in the hospitalization of our patients. Additionally, Tan et al 8 showed that the kinetic of the lymphocyte percentage between two time points (10‐12 days and 17‐19 days after symptom onset) was a credible marker of the severity in COVID‐19 cases; indeed, in the death group, the lymphocyte% was more than 10% on the first time point and <5% on the second time point. As well, the neutrophil‐to‐lymphocyte ratio was an independent risk factor for the occurrence of critical events. 9
In conclusion, lymphopenia is a frequent biological disorder in patients with COVID‐19. It is a predictor factor of the severity, the myocardial injury, the occurrence of ARDS, and a risk factor of ICU mortality. Furthermore, it is a useful tool for predicting poor outcomes. Other larger sample studies are needed to validate risk factors and the lymphocyte threshold.
CONFLICT OF INTEREST
All authors declare no competing interests.
ETHICAL APPROVAL
Informed consent was waived because of the emergency of the disease. All research was conducted following the national guidelines and regulations. No patient identifiers were collected.
ACKNOWLEDGEMENTS
We thank the staff of the Laboratory of Immunology. We also greatly appreciate the efforts of healthcare workers in the department of critical care and anesthesia.
Amra Ziadi, Abdelhamid Hachimi, Brahim Admou, and Abdenasser M. Samkaoui contributed equally to this study.
REFERENCES
- 1. Terpos E, Ntanasis‐Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID‐19. Am J Hematol. 2020;95(7):834‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu Z, Long W, Tu M, et al. Lymphocyte subset (CD4+, CD8+) counts reflect the severity of infection and predict the clinical outcomes in patients with COVID‐19. J Infect. 2020;81(2):318‐356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang F, Nie J, Wang H, et al. Characteristics of peripheral lymphocyte subset alteration in COVID‐19 pneumonia. J Infect Dis. 2020;221:1762‐1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhao Q, Meng M, Kumar R, et al. Lymphopenia is associated with severe coronavirus disease 2019 (COVID‐19) infections: a systemic review and meta‐analysis. Int J Infect Dis. 2020;96:131‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients With COVID‐19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tan L, Wang Q, Zhang D, et al. Lymphopenia predicts disease severity of COVID‐19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu J, Liu Y, Xiang P, et al. Neutrophil‐to‐lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J Transl Med. 2020;18:206. [DOI] [PMC free article] [PubMed] [Google Scholar]


