Abstract
Objective.
To examine the association between ovarian conservation and oncologic outcome in surgically-treated young women with early-stage, low-grade endometrial cancer.
Methods.
This multicenter retrospective study examined women aged <50 with stage I grade 1–2 endometrioid endometrial cancer who underwent primary surgery with hysterectomy from 2000 to 2014 (US cohort n = 1196, and Japan cohort n = 495). Recurrence patterns, survival, and the presence of a metachronous secondary malignancy were assessed based on ovarian conservation versus oophorectomy.
Results.
During the study period, the ovarian conservation rate significantly increased in the US cohort from 5.4% to 16.4% (P = 0.020) whereas the rate was unchanged in the Japan cohort (6.3–8.7%, P = 0.787). In the US cohort, ovarian conservation was not associated with disease-free survival (hazard ratio [HR] 0.829, 95% confidence interval [CI] 0.188–3.663, P = 0.805), overall survival (HR not estimated, P = 0.981), or metachronous secondary malignancy (HR 1.787, 95% CI 0.603–5.295, P = 0.295). In the Japan cohort, ovarian conservation was associated with decreased disease-free survival (HR 5.214, 95% CI 1.557–17.464, P = 0.007) and an increased risk of a metachronous secondary malignancy, particularly ovarian cancer (HR 7.119, 95% CI 1.349–37.554, P = 0.021), but was not associated with overall survival (HR not estimated, P = 0.987). Ovarian recurrence or metachronous secondary ovarian cancer occurred after a median time of 5.9 years, and all cases were salvaged.
Conclusion.
Our study suggests that adoption of ovarian conservation in young women with early-stage low-grade endometrial cancer varies by population. Ovarian conservation for young women with early-stage, low-grade endometrial cancer may be potentially associated with increased risks of ovarian recurrence or metachronous secondary ovarian cancer in certain populations; nevertheless, ovarian conservation did not negatively impact overall survival.
Keywords: Endometrial cancer, Ovarian conservation, Recurrence, Survival, Secondary primary cancer
1. Introduction
Endometrial cancer is the sixth most common female malignancy worldwide, with 319,600 new estimated diagnoses in 2012. The incidence of endometrial cancer has increased globally, including in North America and Asia [1]. The vast majority of women with endometrial cancer present with early-stage disease that carries a good prognosis with surgical therapy alone [2,3]. The standard surgical treatment for early-stage disease is total hysterectomy, salpingo-oophorectomy, with lymphadenectomy reserved for high-risk patients [4]. The proposed rationale for oophorectomy is that the ovary can be a site of metastasis or synchronous/metachronous ovarian cancers, and is a source of estrogen that can be eliminated [5,6].
As the result of oophorectomy, young women with endometrial cancer will suffer from surgical menopause. The diagnosis of endometrial cancer at a young age is not uncommon, and approximately one in eight women with this disease are estimated to be premenopausal at diagnosis, and this number has increased by nearly 2% annually from the mid-1970s to the mid-2000s (Supplemental Fig. S1) [2]. Surgical menopause has major health implications in both the short- and long-term [7], and multiple studies have demonstrated that surgical menopause is associated with cardiovascular morbidity and mortality in young women [8-13]. Thus, ovarian conservation has been considered in young women with early-stage low-grade endometrial cancer to prevent surgical menopause.
The safety and oncologic outcomes related to ovarian conservation have previously been examined in early-stage endometrial cancer [14-23]. Although the number of past studies is limited and utilization of ovarian conservation remains low, these available studies suggest that ovarian conservation may not be associated with decreased overall survival compared to oophorectomy. However, recurrence patterns and secondary malignancy following ovarian conservation have not been completely examined. The objective of the study was to examine recurrence, survival, and secondary malignancy rates among women with early-stage low-grade endometrial cancer who underwent ovarian conservation at hysterectomy.
2. Patients and methods
2.1. Eligibility
This is a multicenter retrospective study conducted at 23 institutions: 13 institutions in the United States and 10 institutions in Japan. Institutional Review Board approval was obtained at each participating institution. An institutional database for endometrial cancer was queried at each participating site, and women aged <50 years who had hysterectomy-based treatment for stage I low-grade endometrial cancer between 2000 and 2014 were eligible for the study. This starting time point of the study period was chosen because ovarian conservation is a relatively new practice. Exclusion criteria included age ≥50 years, non-endometrial cancer, high-grade or unknown grade endometrial cancer, endometrial hyperplasia, stage II–IV disease, absence of hysterectomy, neoadjuvant chemotherapy or radiotherapy, and synchronous malignancy at the time of hysterectomy.
2.2. Clinical information
Patient demographics, tumor characteristics, treatment types, and survival were abstracted from archived medical records. Patient demographics at diagnosis included age, year, race/ethnicity, body mass index (BMI, kg/m2), parity (0,1, and ≥2), medical comorbidity (hypertension, diabetes mellitus, and hypercholesterolemia), medication type (metformin, statins, aspirin, and beta-blocker), cigarette use, personal history of malignancy (breast, ovarian, colorectal, and others), and personal history of adnexal surgery.
Tumor characteristics included pretreatment CA-125 level (<35 versus ≥35 IU/L), histologic type, tumor differentiation (well- versus moderately-differentiated), depth of myometrial tumor invasion (<50% versus ≥50%), cervical mucosal invasion, lympho-vascular space invasion, tumor size (≤2 versus >2 cm), peritoneal cytology results (no malignancy, atypical cells, or malignant cells), and hormonal receptor status (estrogen and progesterone receptors).
Treatment types included hysterectomy route (total abdominal hysterectomy, minimally-invasive surgery [MIS], and others), adnexal surgery (oophorectomy versus ovarian conservation), performance of lymphadenectomy (pelvic and/or para-aortic) and number of sampled nodes, adjuvant therapy (radiotherapy and chemotherapy), hormonal therapy prior to hysterectomy, and estrogen replacement therapy (ERT) after surgery. Survival information included follow-up time, disease status, and vital status. Among recurrent cases, anatomical site and salvage therapy were abstracted. Information for secondary primary cancer (SPC) was also collected during the follow-up after surgical treatment.
2.3. Study definition
The age cutoff of <50 was chosen per a prior study [19]. Obesity classification was per the CDC criteria (normal/underweight, overweight, and class I, II, and III obesity) [24]. Stage I disease was defined as T1a-b/N0-x/M0-x per the 2009 FIGO system [25]. Histology type was based on the final surgical specimen, and low-grade endometrial cancer was defined as well-/moderately-differentiated endometrioid endometrial cancer as previously described [19,26]. Ovarian conservation was defined as the retention of at least one ovary at the time of hysterectomy for endometrial cancer and was based on the past history of adnexal surgery and intraoperative findings at hysterectomy. Absence of any ovary was grouped as oophorectomy.
Disease-free survival (DFS) was defined as the time interval between hysterectomy and the first disease recurrence or death due to endometrial cancer. Overall survival (OS) was defined as the time interval between hysterectomy and death from any cause. Women without a survival event were censored at the last follow-up. SPC diagnosed >2 months after endometrial cancer was regarded as metachronous SPC whereas SPC diagnosed at the same time or within 2 months of endometrial cancer were regarded synchronous SPC [27].
In this study, we introduced a composite endpoint for outcome analysis. In this approach, oncologic outcome from endometrial cancer, all-cause mortality, and metachronous secondary malignancy were assessed together, and the time interval from hysterectomy to any of these events, whichever came first, was assessed. Additionally, the risk of ovarian recurrence of endometrial cancer or metachronous secondary ovarian cancer, termed as an ovarian adverse event, was assessed among the ovarian conservation group. The rationale for this approach is that distinguishing between endometrial cancer recurrence at the ovary and a metachronous secondary ovarian is difficult, and both are considered as adverse events related to ovarian conservation [20].
2.4. Statistical considerations
The US and Japan cohorts were analyzed separately. Differences in continuous and categorical variable were assessed with Student t or chi-square test, as appropriate. Binary logistic regression models were fitted to identify the independent factors for ovarian conservation. All preoperative factors with P < 0.05 on univariable analysis were entered in the initial model, and a conditional backward method was used to retain only the factors with P < 0.05 in the final model. Magnitude of statistical significance was expressed with odds ratio (OR) and 95% confidence interval (CI).
Joinpoint Trend Software (version 4.4.0.0, National Cancer Institute, Bethesda, MD, USA) was used to assess the temporal trends of ovarian conservation [28]. Time increments were grouped by every three calendar years to provide percent frequencies with CIs. The linear segmented regression test was used for the analysis, and log-transformation was performed to determine annual percent change of the slope with 95% CI [29]. A classification-tree model with a recursive partitioning analysis was constructed to assess the utilization patterns of ovarian conservation [30]. All independent preoperative factors of ovarian conservation were fitted in the analysis, and a chi-square automatic interaction detector method was used to construct the model.
The Kaplan-Meier method was used to construct the survival or cumulative risk curves, and differences in the curves were assessed with log-rank tests. A Cox proportional hazard regression model was used to estimate hazard ratio (HR) with 95% CI for outcome analysis. Multivariable analysis was not pre-planned as we assumed that the prognosis of young women with early-stage low-grade endometrial cancer is generally good and there would not be adequate survival events or metachronous SPC cases to perform multivariable analysis in our study [19,20]. Instead, a parsimonious adjustment by age alone was fitted as age is the strongest factor for use of ovarian conservation and survival in young women with endometrial cancer [19].
For sensitivity analysis, a group of women who received ERT in the oophorectomy group was compared to the ovarian conservation group. This group was chosen because ERT has historically been an option for post-oophorectomy hormone replacement therapy in young women but its effect has not been completely addressed in the endometrial cancer population [31]. Inter-group comparison for characteristics and outcome between the US and Japan cohorts was also examined as these two populations possess distinct difference in body habitus, a major epidemiological factor for endometrial cancer (obesity rates, 38.2% in the US versus 3.7% in Japan) [32]. In addition, association of ovarian conservation and survival outcome was adjusted for the presence of lympho-vascular space invasion and depth of myometrial tumor invasion, tumor factors that are known to impact prognosis.
All analyses were based on two-tailed hypotheses, and a P < 0.05 was considered statistically significant. Statistical Package for Social Sciences (SPSS, version 24.0, IBM Corp, Armonk, NY, USA) was used for the statistical analysis. The STROBE guidelines were consulted to outline the retrospective observational cohort study [33].
3. Results
3.1. Study cohorts
The selection schema is shown in Supplemental Fig. S2. The US cohort included 1196 women and there were 113 (9.4%, 95% CI 7.8–11.1) women who had ovarian conservation at hysterectomy. The Japan cohort consisted of 495 women including 43 (8.7%, 95% CI 6.21–1.1) women in the ovarian conservation group. Overall ovarian conservation rates were similar between the two groups (P = 0.623).
3.2. Trends of ovarian conservation
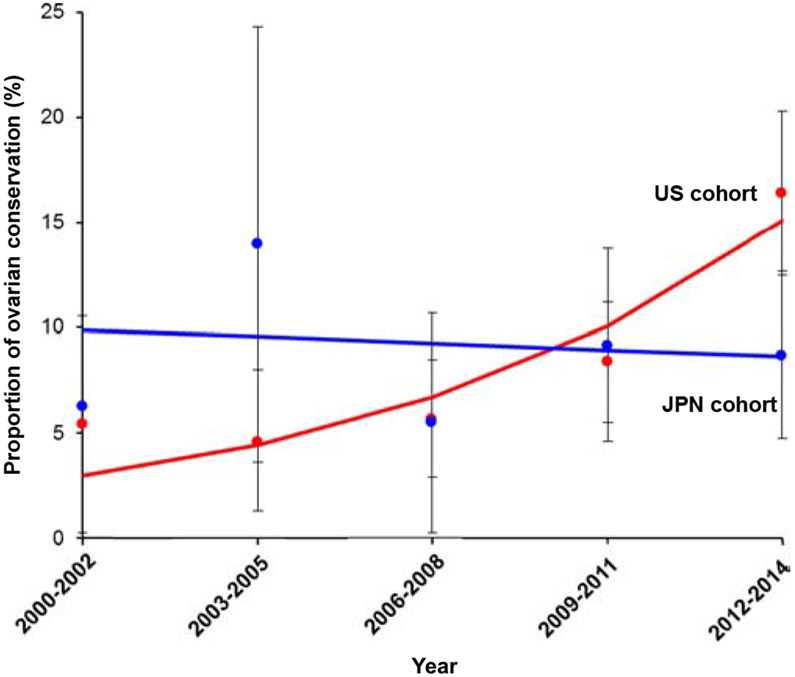
There was a significant increase in the ovarian conservation rate in the US cohort from 5.4% to 16.4% during the study period (3-fold increase, P = 0.020; Fig. 1). From 2012 to 2014 approximately one in 6 women had ovarian conservation at the time of hysterectomy. In the Japan cohort, the rate of ovarian conservation remained largely unchanged during the study period (from 6.3% to 8.7%, P = 0.787; Fig. 1).
Fig. 1.
Trends of ovarian conservation at hysterectomy. Proportion of women who had ovarian conservation at hysterectomy is shown over time for the US cohort (red color, P = 0.020) and the Japan cohort (blue color, P = 0.787). Solid lines represent the modeled value. Dots represent the observed values. Bars represent 95% confidence interval. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3. Baseline characteristics
In the US cohort (Tables 1-2), women in the ovarian conservation group were more likely to be younger, Hispanic/Asian, nulliparous, have received hormonal therapy prior to hysterectomy, have undergone MIS, and to have had small tumors as compared to those in the oophorectomy group (all, P < 0.05). They were less likely to have a comorbidity or to have undergone lymphadenectomy (P < 0.05). Younger age, recent year of diagnosis, prior hormonal therapy, and MIS remained independent preoperative factors associated with ovarian conservation in a multivariable analysis (Supplemental Table S1).
Table 1.
Patient demographics.
| Characteristic | US cohort |
Japan cohort |
||||
|---|---|---|---|---|---|---|
| |
Oophorectomy |
Ovarian conservation |
P-value | Oophorectomy |
Ovarian conservation |
P-value |
| Number | n = 1083 | n = 113 | n = 452 | n = 43 | ||
| Age (years) | 42.6 (±5.6) | 37.9 (±6.4) | <0.001 | 43.0 (±5.6) | 36.3 (±6.4) | <0.001 |
| <40 | 289 (26.7%) | 66 (58.4%) | 107 (23.7%) | 31 (72.1%) | ||
| ≥40 | 794 (73.3%) | 47 (41.6%) | 345 (76.3%) | 12 (27.9%) | ||
| Year | <0.001 | 0.602 | ||||
| 2000–2002 | 70 (6.5%) | 4 (3.5%) | 30 (6.6%) | 2 (4.7%) | ||
| 2003–2005 | 144 (13.3%) | 7 (6.2%) | 37 (8.2%) | 6 (14.0%) | ||
| 2006–2008 | 249 (23.0%) | 15 (13.3%) | 69 (15.3%) | 4 (9.3%) | ||
| 2009–2011 | 329 (30.4%) | 30 (26.5%) | 138 (30.5%) | 14 (32.6%) | ||
| 2012–2014 | 291 (26.9%) | 57 (50.4%) | 178 (39.4%) | 17 (39.5%) | ||
| Race/ethnicity | <0.001 | n/a | ||||
| White | 645 (59.6%) | 49 (43.4%) | ||||
| Black | 70 (6.5%) | 5 (4.4%) | ||||
| Hispanic | 202 (18.7%) | 41 (36.3%) | ||||
| Asian | 46 (4.2%) | 12 (10.6%) | 452 (100%) | 43 (100%) | ||
| Others | 20 (1.8%) | 3 (2.7%) | ||||
| Unknown | 100 (9.2%) | 3 (2.7%) | ||||
| Obesity | 38.4 (±11.6) | 37.9 (±10.9) | 0.927 | 25.9 (±7.1) | 24.1 (±7.0) | 0.378 |
| Normal/under weight | 130 (12.0%) | 13 (11.5%) | 247 (54.6%) | 28 (65.1%) | ||
| Overweight | 142 (13.1%) | 15 (13.3%) | 83 (18.4%) | 6 (14.0%) | ||
| Class I | 168 (15.5%) | 17 (15.0%) | 47 (10.4%) | 4 (9.3%) | ||
| Class II | 158 (14.6%) | 18 (15.9%) | 34 (7.5%) | 0 | ||
| Class III | 444 (41.0%) | 48 (42.5%) | 23 (5.1%) | 2 (4.7%) | ||
| Unknown | 41 (3.8%) | 2 (1.8%) | 18 (4.0%) | 3 (7.0%) | ||
| Parity | 0.004 | 0.005 | ||||
| 0 | 519 (47.9%) | 71 (62.8%) | 205 (45.4%) | 26 (60.5%) | ||
| 1 | 170 (15.7%) | 19 (16.8%) | 58 (12.8%) | 4 (9.3%) | ||
| ≥2 | 344 (31.8%) | 18 (15.9%) | 121 (26.8%) | 2 (4.7%) | ||
| Unknown | 50 (4.6%) | 5 (4.4%) | 68 (15.0%) | 11 (25.6%) | ||
| Diabetes mellitus | 0.068 | 0.999 | ||||
| No | 849 (78.4%) | 98 (86.7%) | 411 (90.9%) | 40 (93.0%) | ||
| Yes | 187 (17.3%) | 14 (12.4%) | 41 (9.1%) | 3 (7.0%) | ||
| Unknown | 47 (4.3%) | 1 (0.9%) | 0 | 0 | ||
| Hypertension | 0.001 | 0.207 | ||||
| No | 730 (67.4%) | 95 (84.1%) | 398 (88.1%) | 41 (95.3%) | ||
| Yes | 306 (28.3%) | 17 (15.0%) | 54 (11.9%) | 2 (4.7%) | ||
| Unknown | 47 (4.3%) | 1 (0.9%) | 0 | 0 | ||
| Hypercholesterolemia | 0.027 | 0.501 | ||||
| No | 893 (82.5%) | 104 (92.0%) | 423 (93.6%) | 42 (97.7%) | ||
| Yes | 143 (13.2%) | 8 (7.1%) | 29 (6.4%) | 1 (2.3%) | ||
| Unknown | 47 (4.3%) | 1 (0.9%) | 0 | 0 | ||
| Cigarette use | 0.647 | 0.272 | ||||
| No | 843 (77.8%) | 90 (79.6%) | 267 (59.1%) | 24 (55.8%) | ||
| Yes | 204 (18.8%) | 18 (15.9%) | 82 (18.1%) | 5 (11.6%) | ||
| Unknown | 36 (3.3%) | 5 (4.4%) | 103 (22.8%) | 14 (32.6%) | ||
| Metformin | 0.947 | 0.686 | ||||
| No | 917 (84.7%) | 97 (85.8%) | 441 (97.6%) | 42 (97.7%) | ||
| Yes | 145 (13.4%) | 14 (12.4%) | 6 (1.3%) | 1 (2.3%) | ||
| Unknown | 21 (1.9%) | 2 (1.8%) | 5 (1.1%) | 0 | ||
| Statin | 0.385 | 0.317 | ||||
| No | 963 (88.9%) | 105 (92.9%) | 429 (94.9%) | 43 (100%) | ||
| Yes | 99 (9.1%) | 6 (5.3%) | 16 (3.5%) | 0 | ||
| Unknown | 21 (1.9%) | 2 (1.8%) | 7 (1.5%) | 0 | ||
| Aspirin | 0.224 | 0.557 | ||||
| No | 1002 (92.5%) | 109 (96.5%) | 440 (97.3%) | 43 (8.8%) | ||
| Yes | 60 (5.5%) | 2 (1.8%) | 7 (1.5%) | 0 | ||
| Unknown | 21 (1.9%) | 2 (1.8%) | 5 (1.1%) | 0 | ||
| Beta-blocker | 0.805 | 0.615 | ||||
| No | 966 (89.2%) | 103 (91.2%) | 442 (97.8%) | 43 (100%) | ||
| Yes | 96 (8.9%) | 8 (7.1%) | 2 (0.4%) | 0 | ||
| Unknown | 21 (1.9%) | 2 (1.8%) | 8 (1.8%) | 0 | ||
| Prior malignancy | 0.455 | 0.682 | ||||
| None | 1025 (94.6%) | 111 (98.2%) | 429 (94.9%) | 43 (100%) | ||
| Breast | 18 (1.7%) | 0 | 12 (2.7%) | 0 | ||
| Ovarian | 5 (0.5%) | 0 | 1 (0.2%) | 0 | ||
| Colorectal | 9 (0.8%) | 0 | 3 (0.7%) | 0 | ||
| Others/unknown | 26 (2.4%) | 2 (1.8%) | 7 (1.5%) | 0 | ||
| CA-125 (IU/L) | 0.363 | 0.215 | ||||
| <35 | 258 (23.8%) | 33 (29.2%) | 340 (75.2%) | 36 (83.7%) | ||
| ≥35 | 33 (3.0%) | 2 (1.8%) | 64 (14.2%) | 2 (4.7%) | ||
| Unknown | 792 (73.1%) | 78 (69.0%) | 48 (10.6%) | 5 (11.6%) | ||
| Prior hormone therapy | <0.001 | <0.001 | ||||
| No | 887 (81.9%) | 80 (70.8%) | 423 (93.6%) | 31 (72.1%) | ||
| Yes | 133 (12.3%) | 31 (27.4%) | 16 (3.5%) | 11 (25.6%) | ||
| Unknown | 63 (5.8%) | 2 (1.8%) | 13 (2.9%) | 1 (2.3%) | ||
Mean (±standard deviation) or number (percent per column) is shown. P-values for univariable analysis. Significant P-values are emboldened.
Table 2.
Treatment type and pathological characteristics.
| Characteristic | US cohort |
Japan cohort |
||||
|---|---|---|---|---|---|---|
| |
Oophorectomy |
Ovarian conservation |
P-value | Oophorectomy |
Ovarian conservation |
P-value |
| Number | n = 1083 | n = 113 | n =452 | n = 43 | ||
| Hysterectomy type | <0.001 | 0.349 | ||||
| TAH | 642 (59.3%) | 33 (29.2%) | 256 (56.6%) | 25 (58.1%) | ||
| MIS | 375 (34.6%) | 67 (59.3%) | 55 (12.2%) | 8 (18.6%) | ||
| Others/unknown | 66 (0.3%) | 13 (1.8%) | 141 (31.2%) | 10 (23.3%) | ||
| Pelvic lymphadenectomy | <0.001 | <0.001 | ||||
| No | 533 (49.2%) | 84 (74.3%) | 196 (43.4%) | 34 (79.1%) | ||
| Yes | 550 (50.8%) | 29 (25.7%) | 245 (54.2%) | 8 (18.8%) | ||
| Unknown | 0 | 0 | 11 (2.4%) | 1 (2.3%) | ||
| Para-aortic lymphadenectomy | <0.001 | 0.237 | ||||
| No | 816 (75.3%) | 104 (92.0%) | 406 (89.8%) | 42 (97.7%) | ||
| Yes | 267 (24.7%) | 9 (8.0%) | 37 (8.2%) | 1 (2.3%) | ||
| Unknown | 0 | 0 | 9 (2.0%) | 0 | ||
| Sampled number | ||||||
| Pelvic | 11 (IQR 6–18) | 9.5 (IQR4–15) | 0.093 | 24 (IQR 15–36) | 15 (IQR 3–29) | 0.053 |
| Para-aortic | 4 (IQR 2–7) | 2 (IQR 1–4.5) | 0.076 | 35 (IQR 9–54.5) | n/a | n/a |
| Postop radiotherapy | 0.335 | 0.999 | ||||
| No | 1018 (94.0%) | 110 (97.3%) | 451 (99.8%) | 43 (100%) | ||
| Yes | 63 (5.8%) | 3 (2.7%) | 1 (0.2%) | 0 | ||
| Unknown | 2 (0.2%) | 0 | 0 | 0 | ||
| Postop chemotherapy | 0.478 | 0.666 | ||||
| No | 1069 (98.7%) | 113 (100%) | 414 (91.6%) | 41 (95.3%) | ||
| Yes | 11 (1.0%) | 0 | 36 (8.0%) | 2 (4.7%) | ||
| Unknown | 3 (0.3%) | 0 | 2 (0.4%) | 0 | ||
| Estrogen replacement therapy | 0.003 | 0.293 | ||||
| No | 875 (80.8%) | 104 (92.0%) | 396 (87.6%) | 41 (95.3%) | ||
| Yes | 107 (9.9%) | 1 (0.9%) | 40 (8.8%) | 1 (2.3%) | ||
| Unknown | 101 (9.3%) | 8 (7.1%) | 16 (3.5%) | 1 (2.3%) | ||
| Tumor differentiation | 0.073 | 0.176 | ||||
| Well | 837 (77.3%) | 96 (85.0%) | 382 (84.5%) | 40 (93.0%) | ||
| Moderate | 246 (22.7%) | 17 (15.0%) | 70 (15.5%) | 3 (7.0%) | ||
| Myometrial invasion | 0.095a | 0.999 | ||||
| <50% | 1009 (93.2%) | 110 (97.3%) | 416 (92.0%) | 40 (93.0%) | ||
| ≥50% | 72 (6.6%) | 3 (2.7%) | 36 (8.0%) | 3 (7.0%) | ||
| Unknown | 2 (0.2%) | 0 | 0 | 0 | ||
| Cervical mucosa involvement | 0.734a | 0.612 | ||||
| No | 1067 (98.5%) | 110 (97.3%) | 440 (97.3%) | 43 (100%) | ||
| Yes | 15 (1.4%) | 2 (1.8%) | 12 (2.7%) | 0 | ||
| Unknown | 1 (0.1%) | 1 (0.9%) | 0 | 0 | ||
| LVSI | 0.996a | 0.669a | ||||
| No | 1026 (94.7%) | 104 (92.0%) | 392 (86.7%) | 40 (93.0%) | ||
| Yes | 48 (4.4%) | 5 (4.4%) | 27 (6.0%) | 2 (4.7%) | ||
| Unknown | 9 (0.8%) | 4 (3.5%) | 33 (7.3%) | 1 (2.3%) | ||
| Tumor size (cm) | 0.024a | 0.270a | ||||
| ≤2 | 473 (43.7%) | 61 (54.0%) | 127 (28.1%) | 13 (30.2%) | ||
| >2 | 435 (43.5%) | 34 (30.1%) | 226 (50.0%) | 15 (34.9%) | ||
| Unknown | 175 (16.2%) | 18 (15.9%) | 99 (21.9%) | 15 (34.9%) | ||
| Peritoneal cytology | 0.361a | 0.106a | ||||
| No malignancy | 825 (76.2%) | 69 (61.1%) | 381 (84.3%) | 36 (83.7%) | ||
| Malignant/atypical cells | 63 (5.8%) | 10 (8.8%) | 48 (10.6%) | 1 (2.3%) | ||
| Not examined | 195 (18.0%) | 34 (30.1%) | 23 (5.1%) | 6 (14.0%) | ||
| Estrogen receptor | 0.471a | 0.559a | ||||
| Not expressed | 4 (0.4%) | 0 | 5 (1.1%) | 0 | ||
| Expressed | 154 (14.2%) | 20 (17.7%) | 29 (6.4%) | 2 (4.7%) | ||
| Not examined | 925 (85.4%) | 93 (82.3%) | 418 (92.5%) | 41 (95.3%) | ||
| Progesterone receptor | 0.933a | 0.559a | ||||
| Not expressed | 7 (0.6%) | 1 (0.9%) | 5 (1.1%) | 0 | ||
| Expressed | 148 (13.7%) | 19 (16.8%) | 29 (6.4%) | 2 (4.7%) | ||
| Not examined | 928 (85.7%) | 93 (82.3%) | 418 (92.5%) | 41 (95.3%) | ||
Median (IQR) or number (percent per column) is shown. P-values for univariable analysis. Significant P-values are emboldened. Abbreviations: TAH, total abdominal hysterectomy; MIS, minimally invasive surgery; IQR, interquartile range; and LVSI, lympho-vascular space invasion.
Among known results.
In the Japan cohort (Tables 1-2), women who had ovarian conservation were more likely to be younger, nulliparous, and have received prehysterectomy hormonal therapy but were less likely to have undergone pelvic lymphadenectomy compared to those who underwent oophorectomy (all, P < 0.05). Age and prior hormone therapy remained independent preoperative factors for ovarian conservation on multivariable analysis (Supplemental Table S2).
3.4. Pattern of ovarian conservation
In the US cohort (Supplemental Fig. S3), age was the strongest factor associated with ovarian conservation followed by hysterectomy type, and year of surgery. Women aged ≤36 who had MIS hysterectomy had the highest ovarian conservation rate (33.3%), and none of the women aged ≥48 who had hysterectomy in 2012 or prior had ovarian conservation (P < 0.05). In the Japan cohort, age was the only factor associated with ovarian conservation in the model. Women aged ≤41 years had a higher rate of ovarian conservation (19.3%) than those aged ≥42 (2.3%, P < 0.001).
3.5. Oncologic outcome and overall survival
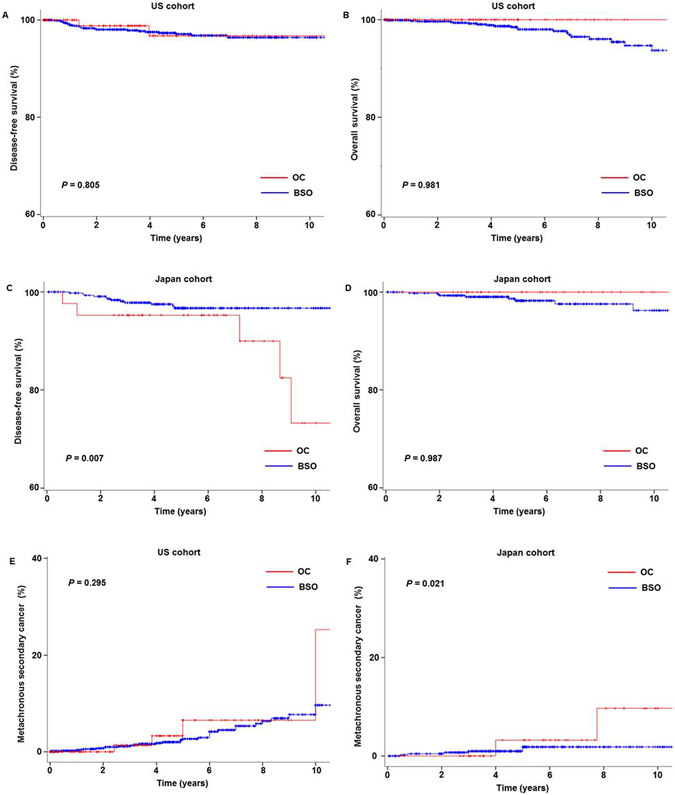
In the US cohort, the median follow-up was 4.1 (IQR 1.5–9.5) years, and there were 25 recurrences and 20 deaths noted. After controlling for age (Table 3), the ovarian conservation group had similar DFS compared to the oophorectomy group (adjusted-HR 0.829, 95% CI 0.188–3.663, P = 0.805; Fig. 2A). No deaths were noted in the ovarian conservation group, and 5-year OS rates were similar between the groups (100% versus 98.5%, P = 0.981; Fig. 2B).
Table 3.
Risk of recurrence, secondary malignancy, and mortality (age-adjusted model).
| Outcome | US cohort |
Japan cohort |
||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Primary endpoint | ||||
| DFS | 0.829 (0.188–3.663) | 0.805 | 5.211 (1.554–17.473) | 0.007 |
| OS | Not estimateda | 0.981 | Not estimateda | 0.987 |
| Recurrence site | ||||
| Local | 1.328 (0.291–6.055) | 0.714 | 8.913 (1.616–49.170) | 0.012 |
| Distant | Not estimateda | 0.988 | 3.104 (0.528–18.249) | 0.21 |
| Metachronous SPC after EMCA | ||||
| Any | 1.787 (0.603–5.295) | 0.295 | 7.119 (1.349–37.554) | 0.021 |
| Breast cancer | 3.687 (0.904–15.041) | 0.069 | Not estimateda | 0.997 |
| Colorectal cancer | Not estimateda | 0.987 | Not estimateda | 0.995 |
| Ovarian adverse eventb | ||||
| Any (recurrence/metachronous OVCA) | 1/113 | 5/43 | 0.061d | |
| Metachronous OVCA | 0/113 | 3/43 | 0.080d | |
| Ovarian recurrence | 1/113 | 2/43 | 0.381d | |
| Composite endpointc | ||||
| EMCA recurrence, death, metachronous (any) | 1.192 (0.500–2.840) | 0.692 | 4.592 (1.722–12.244) | 0.002 |
| EMCA recurrence, death, metachronous (OVCA) | 0.676 (0.157–2.918) | 0.600 | 5.228 (1.848–14.790) | 0.001 |
| EMCA recurrence, death, metachronous (breast) | 1.346 (0.511–3.546) | 0.548 | 3.592 (1.043–12.371) | 0.043 |
| EMCA recurrence, death, metachronous (breast/ovarian) | 1.346 (0.511–3.546) | 0.548 | 5.109 (1.824–14.307) | 0.002 |
| EMCA recurrence, death, metachronous (breast/ovarian/CRC) | 1.170 (0.451–3.038) | 0.747 | 4.850 (1.751–13.435) | 0.002 |
Cox proportional hazard regression model for analysis (ovarian conservation versus oophorectomy). Significant P-values are emboldened. Abbreviations: HR, hazard ration; CI, confidence interval; DFS, disease-free survival; OS, overall survival; SPC, secondary primary cancer; EMCA, endometrial cancer; OVCA, ovarian cancer; and CRC, colo-rectal cancer.
Not estimated due to no event in the ovarian conservation group.
Examined for the ovarian conservation cases.
Composite endpoint included endometrial cancer recurrence, death, and secondary primary cancer.
Comparison between the Japan and US cohort (log-rank test).
Fig. 2.
Survival outcome and metachronous secondary malignancy. Survival outcome is shown based on adnexal surgery status (ovarian conservation versus oophorectomy): (A) DFS and (B) OS in the US cohort, (C) DFS and (D) OS in the Japan cohort, and metachronous secondary malignancy in (E) the US cohort and (F) the Japan cohort. X-axis is truncated at 10 years, and Y-axis truncated to 60–100% or 0–40%. Abbreviations: DFS, disease-free survival; OS, overall survival; OC, ovarian conservation; and BSO, oophorectomy.
In the Japan cohort, the median follow-up was 5.1 (IQR 3.5–7.7) years, with 17 recurrences and 8 deaths noted. After controlling for age (Table 3), ovarian conservation was significantly associated with decreased DFS (HR 5.211, 95% CI 1.554–17.473, P = 0.007; Fig. 2C) compared to oophorectomy. When stratified by anatomical location, the ovarian conservation group had more local-recurrences compared to the oophorectomy group (P = 0.012). However, no deaths were noted in the ovarian conservation group, and 5-year OS rates were similar between the two group (100% versus 98.3%, P = 0.987; Fig. 2D). Similar results were observed after controlling for tumor factors (supplemental Table S3).
3.6. Secondary malignancy after treatment
There were 36 and 9 metachronous SPC's in the USA and Japan cohort, respectively. Ovarian conservation was not associated with any metachronous secondary malignancy in the US cohort (adjusted-HR 1.787, 95% CI 0.603–5.295, P = 0.295; Fig. 2E). Conversely, women in the Japan cohort with ovarian conservation had a higher risk for developing a metachronous secondary malignancy (adjusted-HR 7.119, 95% CI 1.349–37.554, P = 0.021; Fig. 2F) with ovarian cancer being the most common malignancy type (3 out of 9).
3.7. Composite endpoint outcome
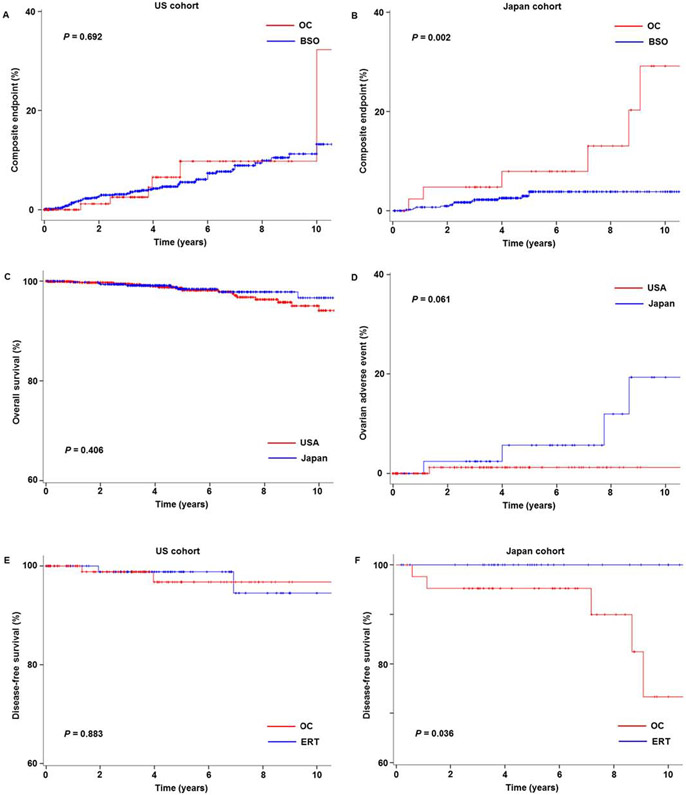
Cumulative risks of endometrial cancer recurrence, death, or secondary malignancy were assessed (Table 3). In the US cohort, ovarian conservation was not associated with the composite endpoint (adjusted-HR 1.192, 95% CI 0.500–2.840, P = 0.692; Fig. 3A). In the Japan cohort, women in the ovarian conservation group had increased risks of composite endpoint outcomes compared to those who had oophorectomy (adjusted-HR 4.592, 95% CI 1.722–12.244, P = 0.002; Fig. 3B).
Fig. 3.
Composite endpoint, ovarian adverse event, and sensitivity analysis. Composite endpoint (recurrence, death, or metachronous secondary malignancy) for (A) the US cohort and (B) the Japan cohort is shown per adnexal surgery type (ovarian conservation versus oophorectomy). (C) Overall survival and (D) cumulative risk of ovarian adnexal event (ovarian recurrence or metachronous secondary ovarian cancer) are shown based on the study cohort. Disease-free survival is shown comparing ovarian conservation and ERT for (E) the US cohort and (F) the Japan cohort. Abbreviations: OC, ovarian conservation; and ERT, estrogen replacement therapy.
3.8. US versus Japan cohort
Age was similar between the cohorts (P = 0.216). When compared to the US cohort, women in the Japan cohort had a lower BMI (mean, 25.7 versus 38.3) with more than half having a normal or underweight body habitus (55.6% versus 12.0%); were more likely to have well-differentiated (85.3% versus 78.0%), large tumors (>2 cm, 48.7% versus 39.2%), and have abnormal peritoneal cytology (9.9% versus 6.1%) (all, P < 0.05; Supplemental Tables S4-5). Compared to the Japan cohort, women in the US cohort were more likely to have class III obesity (41.1% versus 5.1%) and a medical comorbidity. Tumors in the US cohort were more likely to express estrogen (97.8% versus 86.1%) and progesterone (95.4% versus 86.1%) receptors among those tested (all, P < 0.05). The US cohort had higher risk of metachronous secondary malignancy compared to the Japan cohort (HR 2.231, 95% CI 1.074–4.636, P = 0.031), but OS was similar between the two cohorts (HR 0.707, 95% CI 0.311–1.607, P = 0.406; Fig. 3C).
3.9. Ovarian adverse event after ovarian conservation
The risk of an ovarian adverse event was examined among those who had ovarian conservation at the time of hysterectomy (n = 156). There was 1 event (isolated ovarian recurrence) among 113 women in the US cohort whereas there were 5 events (2 isolated ovarian recurrences, and 3 secondary ovarian cancers) among 43 women in the Japanese cohort with a median time to event of 5.9 years. Women in the Japan cohort had a non-statistically significant increased risk of an ovarian adverse event compared to the US cohort (HR 6.362, 95% CI 0.718–56.4, P = 0.061; Fig. 3D). All women with an ovarian adverse event underwent salvage therapy and were alive at the last follow-up.
3.10. ERT versus ovarian conservation
In the US cohort, 107 women who received ERT in the oophorectomy group were compared to 113 women who had ovarian conservation (Fig. 3E), and the ERT group had DFS similar to the ovarian conservation group (HR 0.863, 95% CI 0.121–6.142, P = 0.883). In the Japan cohort, 40 women who received ERT in the oophorectomy group were compared to 43 women who had ovarian conservation (Fig. 3F), and ovarian conservation was associated with decreased DFS compared to ERT (HR not estimated, P = 0.036). No deaths noted in either group. ERT rates among the oophorectomy groups were similar between the two cohorts, and did not change during the study period (both, P > 0.05).
4. Discussion
Key findings of our study are that adoption of ovarian conservation and outcomes of young women with early-stage low-grade endometrial cancer differed across the two study populations. Specifically, in the US cohort where there was an increase in the ovarian conservation rate, ovarian conservation was not associated with adverse oncologic outcome. Conversely, in the Japan cohort where the ovarian conservation rate remained low, ovarian conservation was associated with increased ovarian adverse events. Nevertheless, ovarian conservation did not negatively impact overall survival.
No previous studies have examined the composite outcome of recurrence, death, or metachronous secondary malignancy following ovarian conservation for young women with early-stage endometrial cancer [14-23]. Some have reported survival only, or together with recurrence. One study examined only metachronous secondary ovarian cancer after ovarian conservation but there was no control group for comparison [20]. Because (i) ovarian recurrence and metachronous secondary ovarian cancer are both ovarian adverse events and (ii) metachronous secondary ovarian cancer after ovarian conservation for endometrial cancer is difficult to distinguish from ovarian recurrence of endometrial cancer [20], assessing these two outcomes together is of benefit when examining the safety and outcome of ovarian conservation.
Increasing utilization of ovarian conservation in the US cohort is consistent with what was observed in a recent US tumor registry study [19]. It is speculated that the absence of adverse events and recognition of the long-term benefits of ovarian hormones may be the cause of this trend [8-13]. Yet, as shown in our study and others, ovarian conservation remains under-utilized and no clear guidelines exist to identify the best candidates for this approach [19,23]. Moreover, our study does not have information regarding institution-specific algorithms for ovarian conservation. Understanding the safety and efficacy for ovarian conservation in young women with early-stage low-grade endometrial cancer is an essential step toward developing a needed universal clinical practice guideline.
In the Japan cohort, women who had ovarian conservation had an increased risk of an ovarian adverse event. While it is unknown whether these findings apply to the entire Japanese population, our study suggests that different patient and tumor characteristics between the two cohorts may have resulted in the different outcomes. For instance, women in the US cohort exhibited the typical clinical characteristics of endometrial cancer (class III obesity, 41.1%) whereas those in the Japan cohort were more likely to be non-obese (normal/underweight, 55.6%). Similarly, tumors in the US cohort represent obesity-driven estrogen-expressing biology whereas endometrial cancer in the Japan cohort had more malignant/atypical cells on peritoneal cytology. This suggests that endometrial cancer in Japanese women may have a different mechanism of tumorigenesis.
Endometriosis may be one factor that is associated with non-obese endometrial cancer in Japanese women. While controversial, there seems to be a potential epidemiological link between endometriosis and an increased risk of endometrial cancer in the Asian population [34,35]. Because endometriosis is also associated with an increased risk of ovarian cancer via sustained or deep-infiltrating endometriotic lesions [36,37], it may be possible that endometrial and ovarian cancers in the Japan cohort were directly and indirectly triggered by endometriosis. As our study did not have information regarding the presence of endometriosis, further study is warranted to support this hypothesis.
Our results showed that nearly half of the study population had lymphadenectomy at hysterectomy (48.4% for the US cohort and 51.1% for the Japanese cohort). Recent population-based studies in the United States showed an increasing utilization of lymphadenectomy until the late-2000 and the subsequent downtrending for early-stage low-grade endometrial cancer [38,39]. Thus, it is speculated that relatively high utilization of lymphadenectomy in our study population may be due to the inclusion of older cases.
Strengths of our study include a patient sample which is among the largest in the literature. Rigorous eligibility criteria, inclusion of >30 covariates, and comprehensive outcome analysis enrich the quality of the data analysis. As ovarian conservation is infrequently performed and patients with this disease have a good prognosis, a multicenter retrospective observational study is the best approach to examine outcomes in young women with early-stage low-grade endometrial cancer.
We also acknowledge several limitations in the study. First, inherent to the nature of retrospective studies, unmeasured factors that may confound outcomes were not assessable. This includes the preoperative and intraoperative decision to perform ovarian conservation, menopausal status, family history of ovarian cancer, genetic assessment such as Lynch syndrome, and patient/surgeon's perspectives on ovarian conservation, all of which impact the decision-making process of ovarian conservation. This study does not have information regarding opportunistic salpingectomy at the time of ovarian conservation, and it is unknown if metachronous secondary ovarian cancer among conserved cases was via the STIC pathway [40]. A prior study showed that metachronous secondary ovarian cancer following ovarian conservation for endometrial cancer is more likely to be endometrioid histology, implying non-STIC pathway [20].
A relatively short follow-up time is another weakness of the study. This may be particularly applicable when analyzing overall survival. As our study population was young (median age, 42) and from a favorable disease group (stage I low-grade endometrial cancer), a lack of prolonged follow-up may have resulted in lead-time bias. Indeed, previous studies which have examined young women have shown that the median time to ischemic heart disease after early oophorectomy was 4.3 years and that overall survival differences between the ovarian conservation and oophorectomy groups started appearing several years after surgery [8,19]. This relatively short follow-up in our study mighty be why overall survival between the two groups was similar. Moreover, a standard follow-up protocol after surgery was not available, and it is unknown whether or not routine periodical systemic imaging as opposed to symptom-based follow-up was used in this study which can result in a lead-time bias.
Another limitation is that we did not have outcome information related to surgical menopause, including quality-of-life, cardiovascular/osseous biomarkers and events, and patient satisfaction/regret after early oophorectomy. A recent prospective study among high-risk women for hereditary ovarian cancer showed that those who had early oophorectomy were more likely to have menopausal symptoms, weight gain, lower physical function, and decision regret compared to those who had delayed oophorectomy [41]. In addition, a population-based study demonstrated increased cardiovascular events and mortality after early oophorectomy [8]. Thus, a lack of assessment of the benefits of ovarian conservation weakens the study implications.
While we have examined a relatively large sample size compared to past studies, multivariable analysis was not feasible due to the infrequency of outcome events reflecting the favorable prognosis in young women with early-stage low-grade endometrial cancer as above. Thus, complete risk and outcome assessment remain missing. Further study with a larger sample size would be necessary to examine risks and benefits of this treatment approach. This is particularly applicable to the Japan cohort. Despite a statistically increased risk of ovarian adverse events in this cohort, there were only 43 cases of ovarian conservation making the results of the analysis difficult to extrapolate to broad populations.
An additional study limitation is the lack of central pathology review. This is specifically relevant for the evaluation of ovarian adverse events to distinguish ovarian recurrence from a new primary ovarian cancer. Lastly, there may be variability in pathological testing or interpretation between the two countries, like tumor differentiation or lympho-vascular space invasion that could potentially affect the results of this study.
In summary, our study found that ovarian conservation is not negatively associated with overall survival in young women with early-stage low-grade endometrial cancer. Ovarian conservation can harbor certain risks for ovarian adverse event such as micro-metastasis (0.8% in apparently normal ovary) [42], synchronous ovarian cancer (1.7%) [6], and metachronous ovarian cancer (1.2%) (combined, 3.7%) [20]. Benefits of ovarian conservation include a reduction in ischemic heart disease by 15% and all-cause mortality by 27% particularly from heart disease by 50–60% [19]. Therefore, detailed informed consent and careful preoperative and intraoperative assessment is necessary to properly select candidates for ovarian conservation. As late events are known to occur, patient compliance with follow-up should be considered given the need for prolonged surveillance after surgery.
Supplementary Material
HIGHLIGHTS.
Outcomes following ovarian conservation (OC) for young women with early-stage low-grade endometrial cancer
OC rate: increased in US cohort but not in Japan cohort
Recurrence after OC: increased in the Japan cohort but not increased in the US cohort
Secondary primary cancer after OC: increased in the Japan cohort (ovarian cancer), but not increased in the US cohort
Overall survival after OC: similar to oophorectomy in both cohorts
Acknowledgements
We thank Drs. Yongmei Huang and Brendan H. Grubbs for their scientific inputs for this study.
Funding source
Ensign Endowment for Gynecologic Cancer Research (K.M.).
Footnotes
Declaration of competing interest
Research grant, Pfizer, Yakult Honsha, OncoThreapy Science, honoraria, Chugai, Daiichi-Sankyo, Ono Pharmaceutical, Eisai, Kyowa Hakko Kirin, and Bayer Yakuhin, advisory board, Merck Sharp and Dohme (K.H.); honorariums, Chugai and Astra Zeneca (T.E.); consultant, Tesaro and Clovis Oncology, research funding, Merck (J.D.W.); honorarium, Chugai, textbook editorial, Springer, and investigator meeting attendance expense, VBL therapeutics (K.M.); consultant, Quantgene (L.D.R.); research grant, Merck and Tesaro, advisory board/consultant, Tesaro, Stryker, Astra Zeneca, Celsion, Abbvie, Iovance, Clovis, Genentech, and speaker bureau, Astra Zeneca, Tesaro, and Merck (P.H.T.).
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ygyno.2019.08.007.
References
- [1].Torre LA, Islami F, Siegel RL, Ward EM, Jemal A, Global cancer in women: burden and trends, Cancer Epidemiol. Biomark. Prev 26 (2017) 444–457.Torre LA, Islami F, Siegel RL, Ward EM, Jemal A. Global Cancer in Women: Burden and Trends. Cancer Epidemiol Biomarkers Prev 2017;26:444-457. [DOI] [PubMed] [Google Scholar]
- [2].Cancer Stat Facts: Uterine Cancer. National Cancer Institute Surveillance, Epidemiology, and End Results Program. <https://seer.cancer.gov/statfacts/html/corp.html> (accessed 4/17/2019).
- [3].Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E, Endometrial cancer, Lancet 387 (2015) 1094–1108.Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet 2015;387:1094-1108.26354523 [Google Scholar]
- [4].Uterine Neoplasms. NCCN Clinical Practice Guidelines in Oncology. National Comprehensive Cancer Network. <https://www.nccn.org/professionals/physician_gls/> (accessed 4/17/2019). [Google Scholar]
- [5].Wright JD, Take 'em or leave 'em: management of the ovaries in young women with endometrial cancer, Gynecol. Oncol 131 (2013) 287–288.Wright JD. Take 'em or leave 'em: management of the ovaries in young women with endometrial cancer. Gynecol Oncol 2013;131:287-8. [DOI] [PubMed] [Google Scholar]
- [6].Matsuo K, Machida H, Blake EA, L. Holman L, Rimel BJ, Roman LD, Wright JD, Trends and outcomes of women with synchronous endometrial and ovarian cancer, Oncotarget 9 (2018) 28757–28771.Matsuo K, Machida H, Blake EA, Holman LL, Rimel BJ, Roman LD, Wright JD. Trends and outcomes of women with synchronous endometrial and ovarian cancer. Oncotarget 2018;9:28757-28771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Shoupe D, Parker WH, Broder MS, Liu Z, Farquhar C, Berek JS, Elective oophorectomy for benign gynecological disorders, Menopause 14 (2007) 580–585.Shoupe D, Parker WH, Broder MS, Liu Z, Farquhar C, Berek JS. Elective oophorectomy for benign gynecological disorders. Menopause 2007;14:580-5. [DOI] [PubMed] [Google Scholar]
- [8].Mytton J, Evison F, Chilton PJ, Lilford RJ, Removal of all ovarian tissue versus conserving ovarian tissue at time of hysterectomy in premenopausal patients with benign disease: study using routine data and data linkage, BMJ 356 (2017) j372.Mytton J, Evison F, Chilton PJ, Lilford RJ. Removal of all ovarian tissue versus conserving ovarian tissue at time of hysterectomy in premenopausal patients with benign disease: study using routine data and data linkage. BMJ 2017;356:j372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Parker WH, Feskanich D, Broder MS, Chang E, Shoupe D, Farquhar CM, Berek JS, Manson JE, Long-term mortality associated with oophorectomy compared with ovarian conservation in the nurses' health study, Obstet. Gynecol 121 (2013) 709–716.Parker WH, Feskanich D, Broder MS, Chang E, Shoupe D, Farquhar CM, Berek JS, Manson JE. Long-term mortality associated with oophorectomy compared with ovarian conservation in the nurses' health study. Obstet Gynecol 2013;121:709-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Parker WH, Broder MS, Chang E, Feskanich D, Farquhar C, Liu Z, Shoupe D, Berek JS, Hankinson S, Manson JE, Ovarian conservation at the time of hysterectomy and long-term health outcomes in the nurses' health study, Obstet. Gynecol 113 (2009) 1027–1037.Parker WH, Broder MS, Chang E, Feskanich D, Farquhar C, Liu Z, Shoupe D, Berek JS, Hankinson S, Manson JE. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the nurses' health study. Obstet Gynecol 2009;113:1027-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rivera CM, Grossardt BR, Rhodes DJ, Brown RD Jr., Roger VL, Melton LJ 3rd, Rocca WA, Increased cardiovascular mortality after early bilateral oophorectomy, Menopause 16 (2009) 15–23.Rivera CM, Grossardt BR, Rhodes DJ, Brown RD, Jr., Roger VL, Melton LJ, 3rd, Rocca WA. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause 2009;16:15-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rocca WA, Grossardt BR, de Andrade M, Malkasian GD, Melton LJ 3rd, Survival patterns after oophorectomy in premenopausal women: a population-based cohort study, Lancet Oncol. 7 (2006) 821–828.Rocca WA, Grossardt BR, de Andrade M, Malkasian GD, Melton LJ, 3rd. Survival patterns after oophorectomy in premenopausal women: a population-based cohort study. Lancet Oncol 2006;7:821-8. [DOI] [PubMed] [Google Scholar]
- [13].Rosenberg L, Hennekens CH, Rosner B, Belanger C, Rothman KJ, Speizer FE, Early menopause and the risk of myocardial infarction, Am. J. Obstet. Gynecol 139 (1981) 47–51.Rosenberg L, Hennekens CH, Rosner B, Belanger C, Rothman KJ, Speizer FE. Early menopause and the risk of myocardial infarction. Am J Obstet Gynecol 1981;139:47-51. [DOI] [PubMed] [Google Scholar]
- [14].Wright JD, Buck AM, Shah M, Burke WM, Schiff PB, Herzog TJ, Safety of ovarian preservation in premenopausal women with endometrial cancer, J. Clin. Oncol 27 (2009) 1214–1219.Wright JD, Buck AM, Shah M, Burke WM, Schiff PB, Herzog TJ. Safety of ovarian preservation in premenopausal women with endometrial cancer. J Clin Oncol 2009;27:1214-9. [DOI] [PubMed] [Google Scholar]
- [15].Wright JD, Jorge S, Tergas AI, Hou JY, Burke WM, Huang Y, Hu JC, Ananth CV, Neugut AI, Hershman DL, Utilization and outcomes of ovarian conservation in premenopausal women with endometrial cancer, Obstet. Gynecol 127 (2016) 101–108.Wright JD, Jorge S, Tergas AI, Hou JY, Burke WM, Huang Y, Hu JC, Ananth CV, Neugut AI, Hershman DL. Utilization and Outcomes of Ovarian Conservation in Premenopausal Women With Endometrial Cancer. Obstet Gynecol 2016;127:101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sun C, Chen G, Yang Z, Jiang J, Yang X, Li N, Zhou B, Zhu T, Wei J, Weng D, Ma D, Wang C, Kong B, Safety of ovarian preservation in young patients with early-stage endometrial cancer: a retrospective study and meta-analysis, Fertil. Steril 100 (2013) 782–787.Sun C, Chen G, Yang Z, Jiang J, Yang X, Li N, Zhou B, Zhu T, Wei J, Weng D, Ma D, Wang C, Kong B. Safety of ovarian preservation in young patients with early-stage endometrial cancer: a retrospective study and meta-analysis. Fertil Steril 2013;100:782-7. [DOI] [PubMed] [Google Scholar]
- [17].Richter CE, Qian B, Martel M, Yu H, Azodi M, Rutherford TJ, Schwartz PE, Ovarian preservation and staging in reproductive-age endometrial cancer patients, Gynecol. Oncol 114 (2009) 99–104.Richter CE, Qian B, Martel M, Yu H, Azodi M, Rutherford TJ, Schwartz PE. Ovarian preservation and staging in reproductive-age endometrial cancer patients. Gynecol Oncol 2009;114:99-104. [DOI] [PubMed] [Google Scholar]
- [18].Gonthier C, Trefoux-Bourdet A, Koskas M, Impact of conservative managements in young women with grade 2 or 3 endometrial adenocarcinoma confined to the endometrium, Int. J. Gynecol. Cancer 27 (2017) 493–499.Gonthier C, Trefoux-Bourdet A, Koskas M. Impact of Conservative Managements in Young Women With Grade 2 or 3 Endometrial Adenocarcinoma Confined to the Endometrium. Int J Gynecol Cancer 2017;27:493-499. [DOI] [PubMed] [Google Scholar]
- [19].Matsuo K, Machida H, Shoupe D, Melamed A, Muderspach LI, Roman LD, Wright JD, Ovarian conservation and overall survival in young women with early-stage low-grade endometrial cancer, Obstet. Gynecol 128 (2016) 761–770.Matsuo K, Machida H, Shoupe D, Melamed A, Muderspach LI, Roman LD, Wright JD. Ovarian Conservation and Overall Survival in Young Women With Early-Stage Low-Grade Endometrial Cancer. Obstet Gynecol 2016;128:761-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Matsuo K, Machida H, Stone RL, Soliman PT, Thaker PH, Roman LD, Wright JD, Risk of subsequent ovarian cancer after ovarian conservation in young women with stage I endometrioid endometrial cancer, Obstet. Gynecol 130 (2017) 403–410.Matsuo K, Machida H, Stone RL, Soliman PT, Thaker PH, Roman LD, Wright JD. Risk of Subsequent Ovarian Cancer After Ovarian Conservation in Young Women With Stage I Endometrioid Endometrial Cancer. Obstet Gynecol 2017;130:403-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jia P, Zhang Y, Ovarian preservation improves overall survival in young patients with early-stage endometrial cancer, Oncotarget 8 (2017) 59940–59949.Jia P, Zhang Y. Ovarian preservation improves overall survival in young patients with early-stage endometrial cancer. Oncotarget 2017;8:59940-59949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gu H, Li J, Gu Y, Tu H, Zhou Y, Liu J, Survival impact of ovarian preservation on women with early-stage endometrial cancer: a systematic review and meta-analysis, Int. J. Gynecol. Cancer 27 (2017) 77–84.Gu H, Li J, Gu Y, Tu H, Zhou Y, Liu J. Survival Impact of Ovarian Preservation on Women With Early-Stage Endometrial Cancer: A Systematic Review and Meta-analysis. Int J Gynecol Cancer 2017;27:77-84. [DOI] [PubMed] [Google Scholar]
- [23].Mandelbaum RS, Chen L, Shoupe D, Paulson RJ, Roman LD, Wright JD, Matsuo K, Patterns of utilization and outcome of ovarian conservation for young women with minimal-risk endometrial cancer, Gynecol. Oncol 154 (2019) 45–52. Mandelbaum RS, Chen L, Shoupe D, Paulson RJ, Roman LD, Wright JD, Matsuo K. Patterns of utilization and outcome of ovarian conservation for young women with minimal-risk endometrial cancer. Gynecol Oncol 2019;154:45-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Defining Adult Overwight and Obesity. Center for Disease Control and Prevention. <https://www.cdc.gov/obesity/adult/defining.html> (accessed 4/15/19). [Google Scholar]
- [25].Pecorelli S, Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium, Int J. Gynaecol. Obstet 105 (2009) 103–104.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet 2009;105:103-4. [DOI] [PubMed] [Google Scholar]
- [26].Matsuo K, Opper NR, Ciccone MA, Garcia J, Tierney KE, Baba T, Muderspach LI, Roman LD, Time interval between endometrial biopsy and surgical staging for type I endometrial cancer: association between tumor characteristics and survival outcome, Obstet. Gynecol 125 (2015) 424–433.Matsuo K, Opper NR, Ciccone MA, Garcia J, Tierney KE, Baba T, Muderspach LI, Roman LD. Time interval between endometrial biopsy and surgical staging for type I endometrial cancer: association between tumor characteristics and survival outcome. Obstet Gynecol 2015;125:424-33. [DOI] [PubMed] [Google Scholar]
- [27].Italian cancer figures, report 2013: multiple tumours, Epidemiol. Prev 37 (2013) 1–152.Italian cancer figures, report 2013: Multiple tumours. Epidemiol Prev 2013;37:1-152. [PubMed] [Google Scholar]
- [28].National Cancer Institute Joinpoint Trend Analysis Software, http://surveillance.cancer.gov/joinpoint%3e, Accessed date: 16 April 2019National Cancer Instititute Joinpoint Trend Analysis Software. <<<http://surveillance.cancer.gov/joinpoint%3e>> (accessed4/16/2019). [Google Scholar]
- [29].Kim HJ, Fay MP, Feuer EJ, Midthune DN, Permutation tests for joinpoint regression with applications to cancer rates, Stat Med. 19 (2000) 335–351.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000;19:335-51. [DOI] [PubMed] [Google Scholar]
- [30].Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, McKenna WG, Byhardt R, Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials, Int. J. Radiat. Oncol. Biol. Phys 37 (1997) 745–751.Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, McKenna WG, Byhardt R. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 1997;37:745-51. [DOI] [PubMed] [Google Scholar]
- [31].Barakat RR, Bundy BN, Spirtos NM, Bell J, Mannel RS, Randomized double-blind trial of estrogen replacement therapy versus placebo in stage I or II endometrial cancer: a Gynecologic Oncology Group Study, J. Clin. Oncol 24 (2006) 587–592.Barakat RR, Bundy BN, Spirtos NM, Bell J, Mannel RS. Randomized double-blind trial of estrogen replacement therapy versus placebo in stage I or II endometrial cancer: a Gynecologic Oncology Group Study. J Clin Oncol 2006;24:587-92. [DOI] [PubMed] [Google Scholar]
- [32].Obesity Update, OECD, 2017https://www.oecd.org/els/health-systems/Obesity-Update-2017.pdf, Accessed date: 17 April 2019Obesity Update 2017. OECD. <http://www.oecd.org/els/health-systems/Obesity-Update-2017.pdf> (accessed 4/17/2019). [Google Scholar]
- [33].von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies, BMJ 335 (2007) 806–808.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806-8.17947786 [Google Scholar]
- [34].Nagai K, Hayashi K, Yasui T, Katanoda K, Iso H, Kiyohara Y, Wakatsuki A, Kubota T, Mizunuma H, Disease history and risk of comorbidity in women's life course: a comprehensive analysis of the Japan Nurses' Health Study baseline survey, BMJ Open 5 (2015), e006360.Nagai K, Hayashi K, Yasui T, Katanoda K, Iso H, Kiyohara Y, Wakatsuki A, Kubota T, Mizunuma H. Disease history and risk of comorbidity in women's life course: a comprehensive analysis of the Japan Nurses' Health Study baseline survey. BMJ Open 2015;5:e006360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kok VC, Tsai HJ, Su CF, Lee CK, The risks for ovarian, endometrial, breast, colorectal, and other cancers in women with newly diagnosed endometriosis or adenomyosis: a population-based study, Int. J. Gynecol. Cancer 25 (2015) 968–976.Kok VC, Tsai HJ, Su CF, Lee CK. The Risks for Ovarian, Endometrial, Breast, Colorectal, and Other Cancers in Women With Newly Diagnosed Endometriosis or Adenomyosis: A Population-Based Study. Int J Gynecol Cancer 2015;25:968-76. [DOI] [PubMed] [Google Scholar]
- [36].Kobayashi H, Sumimoto K, Moniwa N, Imai M, Takakura K, Kuromaki T, Morioka E, Arisawa K, Terao T, Risk of developing ovarian cancer among women with ovarian endometrioma: a cohort study in Shizuoka, Japan, Int. J. Gynecol. Cancer 17 (2007) 37–43.Kobayashi H, Sumimoto K, Moniwa N, Imai M, Takakura K, Kuromaki T, Morioka E, Arisawa K, Terao T. Risk of developing ovarian cancer among women with ovarian endometrioma: a cohort study in Shizuoka, Japan. Int J Gynecol Cancer 2007;17:37-43. [DOI] [PubMed] [Google Scholar]
- [37].Anglesio MS, Papadopoulos N, Ayhan A, Nazeran TM, Noe M, Horlings HM, Lum A, Jones S, Senz J, Seckin T, Ho J, Wu RC, Lac V, Ogawa H, Tessier-Cloutier B, Alhassan R, Wang A, Wang Y, Cohen JD, Wong F, Hasanovic A, Orr N, Zhang M, Popoli M, McMahon W, Wood LD, Mattox A, Allaire C, Segars J, Williams C, Tomasetti C, Boyd N, Kinzler KW, Gilks CB, Diaz L, Wang TL, Vogelstein B, Yong PJ, Huntsman DG, Shih IM, Cancer-associated mutations in endometriosis without cancer, N. Engl. J. Med 376 (2017) 1835–1848.Anglesio MS, Papadopoulos N, Ayhan A, Nazeran TM, Noe M, Horlings HM, Lum A, Jones S, Senz J, Seckin T, Ho J, Wu RC, Lac V, Ogawa H, Tessier-Cloutier B, Alhassan R, Wang A, Wang Y, Cohen JD, Wong F, Hasanovic A, Orr N, Zhang M, Popoli M, McMahon W, Wood LD, Mattox A, Allaire C, Segars J, Williams C, Tomasetti C, Boyd N, Kinzler KW, Gilks CB, Diaz L, Wang TL, Vogelstein B, Yong PJ, Huntsman DG, Shih IM. Cancer-Associated Mutations in Endometriosis without Cancer. N Engl J Med 2017;376:1835-1848.28489996 [Google Scholar]
- [38].Melamed A, Rauh-Hain JA, Clemmer JT, Diver EJ, Hall TR, Clark RM, Uppal S, Goodman A, Boruta DM 2nd, Changing trends in lymphadenectomy for endometrioid adenocarcinoma of the endometrium, Obstet. Gynecol 126 (2015) 815–822.Melamed A, Rauh-Hain JA, Clemmer JT, Diver EJ, Hall TR, Clark RM, Uppal S, Goodman A, Boruta DM, 2nd. Changing Trends in Lymphadenectomy for Endometrioid Adenocarcinoma of the Endometrium. Obstet Gynecol 2015;126:815-22. [DOI] [PubMed] [Google Scholar]
- [39].Matsuo K, Machida H, Ragab OM, Takiuchi T, Pham HQ, Roman LD, Extent of pelvic lymphadenectomy and use of adjuvant vaginal brachytherapy for early-stage endometrial cancer, Gynecol. Oncol 144 (2017) 515–523.Matsuo K, Machida H, Ragab OM, Takiuchi T, Pham HQ, Roman LD. Extent of pelvic lymphadenectomy and use of adjuvant vaginal brachytherapy for early-stage endometrial cancer. Gynecol Oncol 2017;144:515-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kurman RJ, Shih Ie M., The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory, Am. J. Surg. Pathol 34 (2010) 433–443.Kurman RJ, Shih Ie M. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol 2010;34:433-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lu KH, Nebgen DR, Norquist B, Bowen DJ, Bakkum-Gamez JN, Romero I, Long-Roche K, Levine DA, Soletsky B, Carter J, Hickey M, Crase J, Gavin K, Polinsky D, Swisher EM, WISP: A prospective, multicenter trial of salpingectomy with delayed oophorectomy versus risk reducing salpingo-oophorectomy in women at increased risk for hereditary ovarian cancer, 50th Annual Meeting on Women's Cancer, Honolulu, HI, March 16–19, 2019.Lu KH, Nebgen DR, Norquist B, Bowen DJ, Bakkum-Gamez JN, Romero I, Long-Roche K, Levine DA, Soletsky B, Carter J, Hickey M, Crase J, Gavin K, Polinsky D, Swisher EM. WISP: A prospective, multicenter trial of salpingectomy with delayed oophorectomy versus risk reducing salpingo-oophorectomy in women at increased risk for hereditary ovarian cancer. 50th Annual meeting on Women'’s Cancer, Honolulu, HI, March 16-19, 2019. [Google Scholar]
- [42].Lin KY, Miller DS, Bailey AA, Andrews SJ, Kehoe SM, Richardson DL, Lea JS, Ovarian involvement in endometrioid adenocarcinoma of uterus, Gynecol. Oncol 138 (2015) 532–535.Lin KY, Miller DS, Bailey AA, Andrews SJ, Kehoe SM, Richardson DL, Lea JS. Ovarian involvement in endometrioid adenocarcinoma of uterus. Gynecol Oncol 2015;138: 532-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.