Conrad et al. provide further evidence that interictal spikes help localize the seizure onset zone, but show that spike spatial distribution also fluctuates in response to sleep and seizures. An average of 12 hours of intracranial recordings are therefore required to capture this variability in the interictal network.
Keywords: EEG, epilepsy, interictal spikes, intracranial EEG, epilepsy surgery
Abstract
The location of interictal spikes is used to aid surgical planning in patients with medically refractory epilepsy; however, their spatial and temporal dynamics are poorly understood. In this study, we analysed the spatial distribution of interictal spikes over time in 20 adult and paediatric patients (12 females, mean age = 34.5 years, range = 5–58) who underwent intracranial EEG evaluation for epilepsy surgery. Interictal spikes were detected in the 24 h surrounding each seizure and spikes were clustered based on spatial location. The temporal dynamics of spike spatial distribution were calculated for each patient and the effects of sleep and seizures on these dynamics were evaluated. Finally, spike location was assessed in relation to seizure onset location. We found that spike spatial distribution fluctuated significantly over time in 14/20 patients (with a significant aggregate effect across patients, Fisher’s method: P < 0.001). A median of 12 sequential hours were required to capture 80% of the variability in spike spatial distribution. Sleep and postictal state affected the spike spatial distribution in 8/20 and 4/20 patients, respectively, with a significant aggregate effect (Fisher’s method: P < 0.001 for each). There was no evidence of pre-ictal change in the spike spatial distribution for any patient or in aggregate (Fisher’s method: P = 0.99). The electrode with the highest spike frequency and the electrode with the largest area of downstream spike propagation both localized the seizure onset zone better than predicted by chance (Wilcoxon signed-rank test: P = 0.005 and P = 0.002, respectively). In conclusion, spikes localize seizure onset. However, temporal fluctuations in spike spatial distribution, particularly in relation to sleep and post-ictal state, can confound localization. An adequate duration of intracranial recording—ideally at least 12 sequential hours—capturing both sleep and wakefulness should be obtained to sufficiently sample the interictal network.
Introduction
Epilepsy affects 1% of the population and is a major cause of disability worldwide (Hauser and Hesdorffer, 1990). Among patients with epilepsy, one-third have seizures that cannot be controlled by medication (Wiebe et al., 1999; Kwan et al., 2011). Many of these patients may benefit from surgery or devices (Wiebe et al., 2001; Noe et al., 2013; Heck et al., 2014; Vonck and Boon, 2015; Englot et al., 2017). However, outcomes from these therapies remain imperfect, in part because the optimal location for surgery and device placement remains unknown.
Interictal spikes are brief electrographic discharges seen in epileptic patients during seizure-free intervals and are an important biomarker of epilepsy (Rosati et al., 2003; Staley et al., 2005; Staley and Dudek, 2006). Spike location is used clinically to map epileptic networks for epilepsy surgery, and resecting regions with frequent spikes correlates with improved surgical outcomes (Kim et al., 2010; Dworetzky and Reinsberger, 2011; Lee et al., 2014). However, spikes often arise outside regions of seizure onset, complicating the presumed relationship between spike and seizure generation (Alarcon et al., 1994; Marsh et al., 2010). An additional challenge is that multiple spatially distinct spike populations often co-exist in the same patient, and the proportion of spikes in each population can vary over time (Janca et al., 2018). An improved understanding of the spatial dynamics of interictal spikes will help elucidate mechanisms underlying the epileptic network and will inform their use in surgical planning.
To address these fundamental gaps in knowledge, we investigated the spatial dynamics of interictal spikes on intracranial EEG to answer three questions: (i) whether and how the spatial distribution of spikes changes over time; (ii) what mechanisms underlie temporal fluctuations in spike spatial distribution; and (iii) whether spike spatial dynamics localize the seizure onset zone.
Materials and methods
Patient selection and intracranial EEG recording
This retrospective study was approved by the Hospital of the University of Pennsylvania (HUP) Institutional Review Board. Twenty-eight patients with medically refractory, localization-related epilepsy who underwent intracranial EEG recording during presurgical evaluation at HUP or the Mayo Clinic Rochester, were included. All examined patients had at least 24 h of available intracranial EEG data, at least one seizure, and post-implant head CT demonstrating implanted electrode locations. All clinical decisions were made without influence from this study. Patient outcomes were determined at 24 months by the International League Against Epilepsy (ILAE) scale (Class 1: completely seizure-free; Class 6: >100% increase over baseline seizure days). Study procedures were conducted in accordance with the Declaration of Helsinki.
Intracranial EEG recording and electrode localization
Electrode configurations (Ad Tech Medical Instruments) consisted of linear cortical strips and 2 D cortical grid arrays (2.3 mm diameter with 10 mm intercontact spacing), and linear depth electrodes (1.1 mm diameter with 10 mm intercontact spacing). Recordings were acquired at 512 Hz at HUP and 500 Hz at the Mayo Clinic and referenced to an electrode distant from the suspected epileptogenic zone. A notch filter removed 60 Hz noise (order 2 Butterworth filter, bandstop with high-pass 61 Hz and low-pass 59 Hz; parameters were chosen based on visual detection of a subset of data with a high amount of 60 Hz noise) (Supplementary Fig. 1). Pre-electrode implantation MPRAGE T1-weighted MRI studies were obtained for each patient on a 3 T Siemens Magnetom Trio scanner (Siemens). Spiral CT images (Siemens) were obtained post-electrode implantation. Electrode coordinates were identified by cross-referencing the CT images with a surgical cartoon map. The pre-implant MRI was registered using the Advanced Normalization Toolkit (ANTs) to post-resection imaging as well as the post-implant CT in order to segment the resection zone (Avants et al., 2011). Resection zones were estimated semi-automatically with the use of a random forest classifier feature as part of the ITK-SNAP toolkit (Yushkevich et al., 2006), from which we identified the electrodes overlying resected cortex.
Marking seizure times and the seizure onset zone
Seizures were identified by a clinician reviewing the full duration of the EEG record for clinical purposes, and confirmed in a clinical case conference discussion. A board-certified epileptologist (F.M., A.K., K.D., B.L.) then reviewed the seizures and remarked the earliest electrographic change and end of each seizure, as well as the location of seizure onset. After running our automatic spike detector (Brown et al., 2007), we discovered two additional subclinical electrographic seizures (one for Patient HUP111A and one for Patient Study019). We analysed these times as interictal data given that they were not noted in the clinical record. These represented 0.85% of all seizures observed.
Interictal spike detection
We restricted our analysis to 12 h before and 12 h after each seizure for each patient. If a patient’s first and last seizure were <200 h apart, then we analysed the entire duration from 12 h before the first seizure to 12 h after the last seizure in order to obtain spike detections over the complete dataset. The data were segmented into minute-long segments. Each segment underwent automated spike detection using a previously validated detector, which is described in the original paper (Brown et al., 2007). Briefly, the segment was high-pass (>10 Hz) and low-pass (<40 Hz) filtered. Peaks in this high frequency signal were identified and subjected to the following criteria: (i) an absolute amplitude threshold; (ii) an amplitude threshold relative to the surrounding baseline; (iii) maximum (220 ms) and minimum (10 ms) duration thresholds; and (iv) the requirement of an after-going slow wave. Absolute and relative amplitude thresholds were manually adjusted for each patient according to the Brown et al. (2007) protocol. The algorithm was modified such that the relative amplitude threshold for detection was chosen relative to the surrounding minute of EEG data, rather than the entire record, as this produced more accurate spike detections by visual analysis. Minute-long segments were excluded from analysis if the sum of the absolute value of the amplitude was <1 μV (consistent with electrode disconnection) or if the square root of the sum of the square difference in amplitude between adjacent time points was >50 000 μV (consistent with amplifier saturation or EEG noise). Spikes detected across >80% of all electrodes within 400 ms were discarded, as these were also more likely to be artefactual on visual analysis. Spikes occurring within 1 min of the earliest electrographic change of each seizure were discarded to avoid defining early and difficult-to-identify ictal activity as interictal (Davis et al., 2018). For each spike detection, the spike peak was defined as the maximum of the absolute deviation of the high frequency signal relative to the baseline (which would occasionally result in spike peaks during the downward deflection of the spike).
Spike sequence detection
After detecting individual spikes, we next identified spike sequences in which interictal spikes occurred across multiple electrodes in close temporal proximity. We calculated these sequences using a previously described algorithm (Tomlinson et al., 2016, 2019). Briefly, the first spike was defined as the leader of a candidate spike sequence. Spikes occurring within 50 ms of the leader or within 15 ms of the previous spike were appended to the sequence. Sequences with fewer than five spikes were discarded. We required each spike in a sequence to be close enough in space to the preceding spike so as to not exceed a propagation velocity of 10 m/s (Hirsch and Gilbert, 1991; González-Burgos et al., 2000). To allow for the possibility of rapid connections, connections exceeding this velocity were preserved if these connections accounted for >5% of all electrode-to-electrode spike connections for the electrode. Sequences were discarded if ≥50% of the spikes in the sequence occurred within 2 ms of each other, as these were more likely to be artefactual on visual inspection. The spike sequencing step preceded all subsequent analysis so that we could investigate features of spike propagation, and because spikes occurring in multi-electrode sequences were less likely to be artefact by visual analysis.
Clustering by spike location
For each subject, a k-means algorithm clustered interictal spikes into regions according to the x, y, and z coordinates of their corresponding electrode locations (Fig. 1A). The number of clusters was chosen for each patient by visual identification of the inflection point in a plot of the sum of square error as a function of cluster number (Soni Madhulatha, 2012; Bholowalia and Kumar, 2014). Supplementary Fig. 2 shows the sum of square error as a function of cluster number for each patient and the optimal cluster number chosen for each patient. The clustering algorithm was performed 30 times for each patient and the result that produced the lowest sum of the distances between each spike location and its cluster centroid was chosen. We then selected 50 random spike sequences from each cluster for visual analysis. Clusters were discarded if >50% of the analysed spike sequences appeared to be artefact in order to balance the need for high detector specificity without discarding a large number of true spikes. If all of a patient’s clusters were discarded by this method, then the patient was discarded from further analysis. Supplementary Fig. 3 shows an example of this process and Supplementary Table 1 shows the percentage of artefactual sequences in each cluster for all patients. In subsequent analyses, we used cluster distribution (i.e. the proportion of spikes in each cluster) as a surrogate for spike spatial distribution.
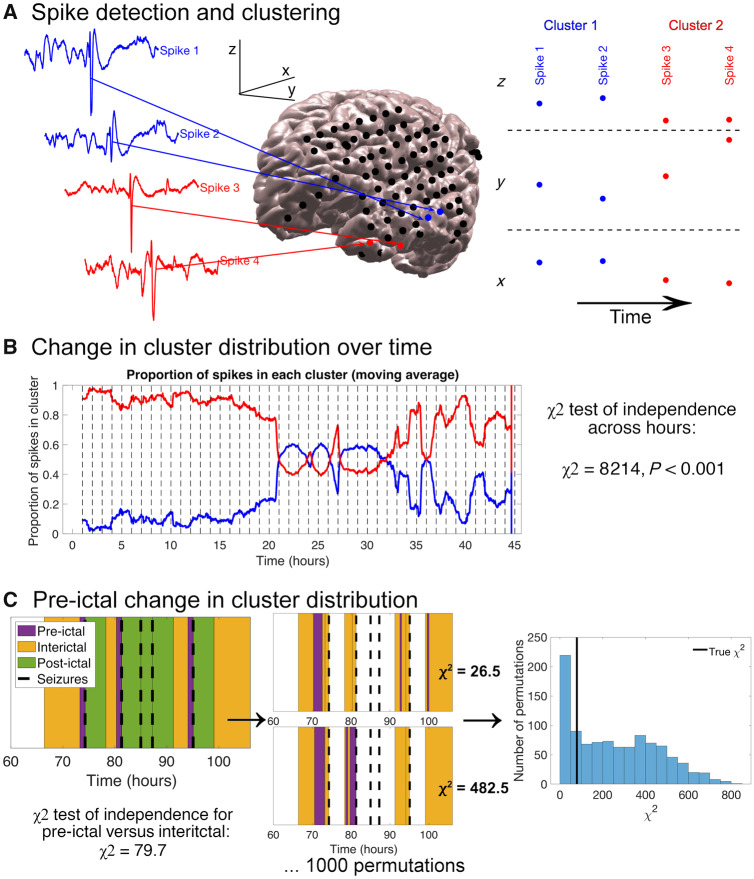
Figure 1.
Methods for spike detection and spatial clustering, determining change in cluster distribution over time, and determining pre-ictal change in cluster distribution. All data shown are for Patient HUP078. (A) Examples of four spike detections, the electrodes where they were detected, and electrode x, y and z coordinates. Two clusters (labelled red and blue) were identified. (B) Algorithm for determining the change in spike cluster distribution over time. The EEG recording was divided into hour-long bins (vertical dotted lines in the plot). The number of spikes in each cluster was compared across hour-long bins using a χ2 test for independence. (C) Algorithm for determining the pre-ictal change in cluster distribution. The number of spikes in each cluster was compared between the pre-ictal and interictal time periods with a χ2 independence test. Seizures occurring during the ‘postictal’ period—within 4 h—of a preceding seizure were defined to have no ‘pre-ictal’ period, as was the case for the third and fourth seizure shown above. We then computed 1000 random permutations of pre-ictal start times. The pre-ictal period durations varied across permutations so as to keep the number of spikes constant. We obtained a new pre-ictal versus interictal chi-squared statistic for each permutation. The true χ2 statistic was compared to the distribution of χ2 statistics from the permutations to determine there was a significant difference between the pre-ictal and interictal cluster distributions. In the example above, 706/1000 random permutations had larger χ2 statistics than the true χ2 statistic, yielding a P-value of 0.71.
Change in spike spatial distribution over time
To test whether spike spatial distribution changed over time, the EEG record was divided into hour-long segments. The number of spikes in each cluster was compared across each hour-long segment using a χ2 test of independence on the contingency table of cluster number and segment number (Fig. 1B). The null hypothesis of this test is that the relative proportions of spikes in each cluster are identical across different hour-long segments, and so a significant χ2 statistic implies temporal variability in the spike spatial distribution. An alpha of 0.0025 (post-Bonferroni correction of an original alpha of 0.05 given that we tested 20 patients) was used to determine significance for each patient. Fisher’s combined probability test was used to combine the individual patient P-values and obtain an aggregate P-value (Fisher, 1934). Fisher’s method combines the P-values for multiple independent tests bearing on the same hypothesis. In our case, we hypothesized that the spike spatial distribution changes over time, without predicting a consistent pattern of change across patients. We compared the ILAE scores between patients with a significant change in spike spatial distribution over time and those without using a Wilcoxon rank sum test. We used χ2 independence tests to compare the number of patients with a change in spike spatial distribution over time between those with temporal lobe and extra-temporal lobe-onset seizures, between those with mesial temporal lobe sclerosis and those with other pathologies, and between those with all grid and strip electrodes and those with grid, strip, and depth electrodes.
We next estimated the recording duration required to accurately capture the variability in the spike spatial distribution. Only patients with more than one spike cluster were analysed (17 of 20 patients). We first identified the predominant spike region for each patient, defined as the cluster with the highest proportion of spikes across the entire record. We divided the EEG record into hour-long segments, and calculated the proportion of spikes in that cluster in each hour-long segment. We identified the 5th and 95th percentiles of proportions across hours and defined the difference between them as the variability in spike spatial distribution. We then examined subsets ranging in duration from 1 h up to the entire duration of the EEG record. For each duration, we considered all possible subsets of consecutive hours with that total duration. For each subset, we again obtained the 5th and 95th percentile proportions of spikes in the predetermined predominant cluster, defining the difference between these percentiles as the variability in spike spatial distribution for that duration. We then identified the shortest duration for which the average spatial distribution variability was >80% of the spatial distribution variability observed in the full dataset. Eighty per cent was chosen arbitrarily as an adequate percentage of total variability. We correlated this duration with both spike frequency and ILAE surgical outcome score using Spearman rank correlation. We also compared this duration between patients with temporal lobe onset seizures and those with extra-temporal lobe onset seizures using a Wilcoxon rank sum test.
Change in spike spatial distribution with sleep
To study the effect of sleep/wake state on spike spatial distribution, we used the alpha/delta ratio (Jobert et al., 1994; Agarwal and Gotman, 2001; Šušmáková and Krakovská, 2007; Lajnef et al., 2015). A lower alpha/delta ratio implies relatively higher delta power, suggesting that the patient is in a sleep state. We calculated the absolute power ratio between alpha (8–13 Hz) and delta (1–4 Hz) frequencies across all channels, averaging over 2000-s bins (this duration was chosen to optimize computational speed). We again identified the predominant spike cluster, and determined the proportion of spikes in this cluster in each time window (studying only patients with at least two spike clusters). We used the proportion of spikes in the predominant cluster as a surrogate for the spike spatial distribution, as a change in the proportion of spikes in the predominant cluster implies a change in the relative distribution of spikes throughout the clusters. To determine if spike spatial distribution varies with alpha/delta ratio, we developed a binomial regression model (logit link function) with autoregressive errors with the proportion of spikes in the predominant cluster as the response and the alpha/delta ratio as the predictor. We used the GLARMA package in R to perform this analysis (R Core Team, 2014; Dunsmuir et al., 2015). We chose an order of AR(2) as this captured the significant values for the partial correlations of the residuals of the original binomial regression model for the majority of patients. An alpha of 0.0025 (post-Bonferroni correction) was used to determine significance for each patient. We aggregated P-values using Fisher’s method to test for aggregate changes in the spike spatial distribution with the alpha/delta ratio. To test whether alpha/delta ratio was associated with an overall increase or decrease in the proportion of spikes in the predominant cluster (and thus a trend towards narrower or broader spike spatial distribution), we aggregated the model z-scores across patients and used a one-sample two-sided t-test to determine if the combined z-scores were significantly different from zero.
We also tested for sleep-related changes in spike rates, distance from the seizure onset zone, and extent of spike propagation. For spike rates, we determined the number of spikes in each 2000-s bin. We developed a Poisson regression model with autoregressive errors [AR(2) chosen based on the partial correlation of the residuals of the original Poisson model] with the spike count as the response and the alpha/delta ratio as the predictor. For distance from the seizure onset zone, we averaged the distance between each spike and its nearest seizure onset zone electrode for all spikes in each 2000-s bin. We defined this distance as the response and the alpha/delta ratio the predictor of a linear regression model with AR(2) errors. For spike propagation, we measured the average sequence length, defined as the number of electrodes in a spike sequence, in each 2000-s bin. A longer sequence length suggests more extensive spike propagation. We defined this length as the response and the alpha/delta ratio the predictor of a linear regression model with AR(2) errors. To test for group-level correlation between these measures and the alpha/delta ratio, we aggregated the model t-statistics across patients using a one-sample two-sided t-test.
Given the possibility that spikes may affect the calculation of the alpha/delta ratio and subsequently confound this analysis, we performed an additional analysis to test the effect of spikes on the alpha/delta ratio. To do this, we identified all spikes, including those thought to belong to artefactual clusters (so as to increase the sensitivity of spike detections). We took the time period from 0.5 s before the spike peak to 1 s after the spike peak (the longer post-peak duration chosen to be enough time to include the after-going slow wave). We recalculated the alpha/delta power ratio for each electrode, removing from calculation the times of the spikes detected on that electrode. We then averaged the alpha/delta ratio over all electrodes and over each 2000-s bin. We compared the vector of alpha/delta ratios across 2000-s bins obtained from this new analysis with the vector obtained from the original analysis using the Pearson correlation coefficient.
Peri-ictal change in spike spatial distribution
We compared the spike spatial distribution between different time periods surrounding seizures. We defined the postictal period to be the 4 h after each seizure, chosen based on evidence for a postictal energy increase that can persist 4–6 h after the seizure, and with the goal of minimizing overlapping seizure periods (Litt et al., 2001). The pre-ictal period was defined as the time from 60 min up to 1 min before the seizure (the 1-min choice is discussed in the subsection on spike detection), excluding any times in the postictal period of a preceding seizure (this would sometimes exclude a seizure’s entire pre-ictal period from analysis). The 1-h pre-ictal period was chosen given evidence for a pre-ictal energy increase that may occur 50 min prior to seizures (Litt et al., 2001), and for synchronization changes that may occur hours before seizures (Le Van Quyen et al., 2005). This time period was also chosen to balance the need for including a sufficient number of pre-ictal spikes while remaining as close as possible to the seizure to capture any pre-ictal change that exists. All remaining spikes were classified as interictal. We compared the cluster distributions between the pre-ictal and interictal periods, and then between the postictal period and all other times using a χ2 statistic. To test if the difference between spike spatial distributions in different peri-ictal periods was larger than expected between randomly chosen periods, we used a permutation test (n = 1000) in which we chose random start times from which we obtained a set of consecutive spikes equal in number to the spikes in the true peri-ictal period (Fig. 1C). We compared the χ2 test statistic for the true peri-ictal cluster distributions to the array of 1000 χ2 statistics obtained from our permutation tests to obtain a P-value (alpha = 0.0025). We combined P-values across patients using Fisher’s method to test whether there is a significant group-level change in spike spatial distribution in the pre-ictal or postictal periods. Finally, we repeated our analyses after (i) redefining the postictal period to be 1 h after each seizure; and (ii) redefining the pre-ictal period to be 4 h before each seizure in order to determine the sensitivity of the results to our choices of pre- and postictal duration.
We also tested for group-level peri-ictal changes in spike rates, distance from the seizure onset zone, and spike propagation (defined as spike sequence length as described in the section on sleep). We compared these measures in the pre-ictal, postictal, and interictal periods for all patients using a Friedman test (Friedman, 1937).
Seizure onset zone localization
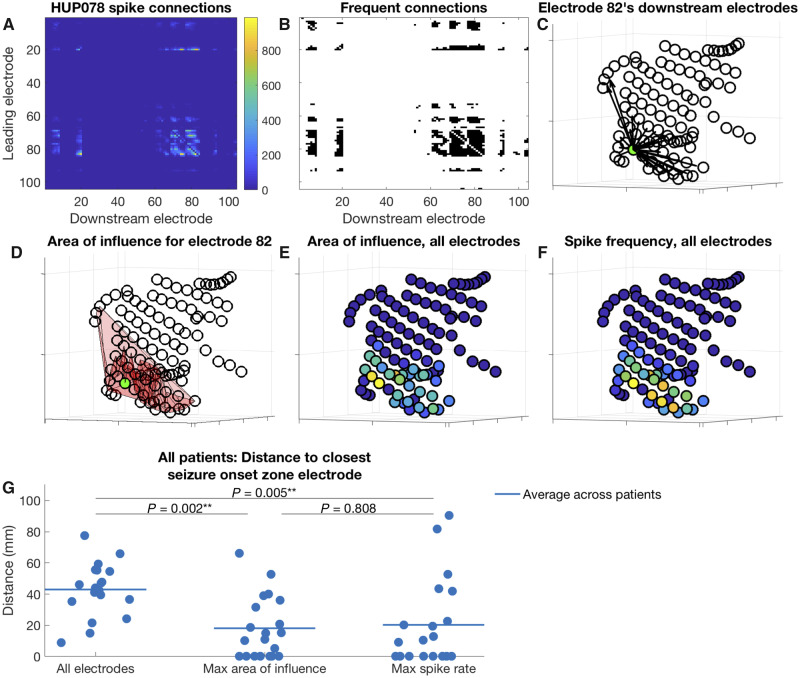
We examined whether features of spike topology localize the seizure onset zone. First, we identified the electrode with maximum spike frequency for each patient. Next, to determine if spike propagation added information to localizing the seizure onset zone, we examined all spike sequences, where spike sequences were calculated as defined in the ‘Spike sequence detection’ section. We constructed a matrix of ‘leader-downstream’ electrode connections—where the ‘leader’ is the first electrode in the spike sequence and the ‘downstream’ electrodes are those that appear later—encoding the number of times that each electrode appeared downstream in spike sequences led by each other electrode. We identified the elements of the matrix with significantly more connections than a threshold value defined by a test in which we generated 1000 connection matrices, each time randomly distributing the total number of true connections across elements in the matrix. With this analysis, the null distribution connection matrix is one in which all pairs of leader-downstream electrode connections are equally likely. The threshold was defined as the 95th percentile number of connections across the randomly generated matrices. For each electrode, this resulted in an array of other electrodes that appeared downstream in its spike sequences more often than expected by chance. We then calculated the surface area connecting each electrode and its set of downstream electrodes, defining the electrode’s area of influence. Intuitively, an electrode’s area of influence describes how far spikes originating in that electrode tend to propagate. We then calculated the distance between the electrode with the largest area of influence and its nearest seizure onset zone electrode, as well as the distance between the electrode with the highest spike rate and its nearest seizure onset zone. We aggregated these distances across all patients. We used Wilcoxon signed-rank tests to compare these distances with the average distance between every electrode and its nearest seizure onset zone to determine if the most frequently spiking electrode and the electrode with the largest area of influence are closer to the seizure onset zone than predicted by chance (Fig. 5).
Figure 5.
Electrode area of influence and distance between electrodes of interest and nearest seizure onset zone. (A–F) An example for Patient HUP078, and (G) aggregate data for all patients. (A) Matrix of downstream connections. For each electrode represented by a row i in the matrix, the colour in each column j represents the number of times the electrode j appears downstream in spike sequences led by the electrode i. (B) Connections from the matrix shown in A occurring more frequently than expected by chance (shown in black). (C) Locations of the electrodes that appear downstream in spike sequences led by the green electrode (electrode 82, chosen for display arbitrarily) more often than expected by chance. Each arrow connects the green electrode to one of its downstream electrodes. (D) The area of influence for the green electrode (electrode 82), which is the area connecting the green electrode with its downstream electrodes (the pink shaded area). (E and F) Area of influence and spike frequency for all electrodes, respectively. Yellow colours indicate higher values. (G) Distance from various electrodes of interest to their nearest seizure onset zone electrodes. Left: Cluster of points demonstrates, for each patient, the average distance from every electrode to its nearest seizure onset zone electrode. Middle: Cluster demonstrates the distance for each patient from the electrode with the highest area of influence to its nearest seizure onset zone electrode. Right: Cluster demonstrates the distance for each patient from the electrode with the maximum sequence frequency to its nearest seizure onset zone electrode. Horizontal lines show the average for each cluster across patients. The P-values are the result of Wilcoxon signed-rank tests comparing the distances for each group.
We also identified whether the electrode with the maximum spike frequency and the electrode with maximum area of influence were amongst the electrodes overlying resected cortex for that patient. We compared the ILAE outcomes between patients who had these regions resected and those that did not with Wilcoxon rank-sum tests. We next determined whether the predominant (most frequently spiking) cluster better localized the seizure onset zone than non-predominant spike clusters. For each patient with multiple spike clusters (17 patients), we calculated the distance between each cluster’s centroid and the nearest seizure onset zone electrode. We determined the number of patients for whom this distance was smallest for the predominant cluster. We compared this number to the expected number and variance assuming a Bernoulli distribution for the probability that a patient’s predominant cluster was closest to the seizure onset zone, using a one-sided z-test (alpha = 0.05).
Finally, to test whether the area of influence measure localized the seizure onset zone independent of spike rate, we repeated the above analysis, changing the test used to identify frequent leader-downstream electrode connections so that we permuted either the rows (representing the leader electrodes) or the columns (representing downstream electrodes). The former test identified, for each downstream electrode, the leader electrodes that led the downstream electrodes in spike sequences significantly more than other leader electrodes. The latter test identified, for each leader electrode, the downstream electrodes that followed the leader electrode in spike sequences significantly more than other downstream electrodes.
Statistical analysis
All statistical analyses were performed using MATLAB R2018 (Natick, MA), with the exception of the generalized linear models with autoregressive errors designed for the alpha/delta ratio analysis, which were fit in R using the GLARMA package (R Core Team, 2014; Dunsmuir et al., 2015). We also include on GitHub MATLAB code to determine the relationship between the spike spatial distribution and the alpha/delta ratio using a generalized linear model without autoregressive errors so that the reader can perform the analysis entirely in MATLAB. The individual statistical tests used for each analysis are described in the relevant sections.
Data availability
All data can be made available on reasonable request. Analysis scripts are freely available on GitHub (https://github.com/erinconrad/spike-propagation). All EEG records are hosted by The International Epilepsy Electrophysiology Portal (https://www.ieeg.org/) under the Virtual Cortical Resection dataset (free account required) (Wagenaar et al., 2013; Kini et al., 2016). An example dataset containing 500 s of data for a single patient (Study029) as well as a dataset containing the electrode locations, seizure times, and de-identified clinical information are available as MATLAB structures in the GitHub repository. The ReadMe in the GitHub repository explains how to run an example script demonstrating the code using the example dataset.
Results
Clinical characteristics and spike detection
Eight of the 28 patients included in the study were excluded because >50% of sample sequences appeared to be artefactual for each of the patient’s clusters, indicating poor performance of the spike detector for that patient. Supplementary Table 1 shows the percentage of sample sequences in each cluster that appeared to be artefactual. Supplementary Fig. 3 shows example sequences for Patient Study028, who had three clusters, one of which was discarded due to artefactual-appearing sequences. The final study therefore included 20 patients (12 females, mean age = 34.5 years, range = 5–58). A variety of clinical histories, pathologies, and electrode coverages were represented (Table 1). There were on average 83.3 electrodes (range = 48–120) implanted per patient. We recorded 6.2 seizures per patient (range 1–36). Table 2 shows the sample duration, spike rates, and detector accuracy for each patient. A mean of 75.5 h (range 24.0–141.7) was evaluated for each patient (note that this number is lower than 24 h × the number of seizures because seizures often occurred within 24 h of each other). The mean interictal spike frequency was 39.8 spikes/electrode/h averaged over all electrodes per patient (range 4.3–115.5). Individual electrode rates ranged from a minimum of 0.09 spikes/min to a maximum of 6.74 spikes/min, averaged across patients. The median ILAE score was 2 (range ILAE 1–ILAE 5). Supplementary Table 2 shows clinical data for the eight patients excluded due to poor spike detector performance. There was no significant difference between the included and excluded patients for any clinical measure.
Table 1.
Subject demographic and clinical information
| Subject ID | Sex | Age at onset, years | Age at surgery, years | Seizure onset | Pathology | ILAE outcome | Total grid electrode contacts, n | Total strip electrode contacts, n | Total depth electrode contacts, n |
|---|---|---|---|---|---|---|---|---|---|
| HUP064 | M | 3 | 22 | LFL | MCD | 1 | 64 | 22 | 0 |
| HUP070 | M | 12 | 33 | LFPL | MCD | 2 | 64 | 14 | 0 |
| HUP074 | F | 5 | 25 | LTL | Cortical tuber + FCD + gliosis | 1 | 64 | 32 | 24 |
| HUP075 | F | 35 | 57 | LTL | HS/MTS | 5 | 64 | 34 | 16 |
| HUP078 | M | 2 mo | 54 | LTL | HS/MTS | 4 | 64 | 24 | 16 |
| HUP080 | F | 35 | 41 | LTL | Gliosis | 2 | 64 | 22 | 16 |
| HUP086 | F | 18 | 25 | LTL | Gliosis | 1 | 64 | 44 | 8 |
| HUP088 | F | 13 mo | 24 | LFL | HS/MTS | 1 | 0 | 46 | 16 |
| HUP094 | F | 20 | 48 | RTL | HS/MTS | 2 | 0 | 64 | 22 |
| HUP105 | M | 27 | 39 | RTL | HS/MTS + tumour | 1 | 0 | 44 | 16 |
| HUP106 | F | 24 | 45 | LTL | HS/MTS | 2 | 64 | 36 | 16 |
| HUP107 | M | 5 | 36 | RTL | HS/MTS | 1 | 64 | 38 | 16 |
| HUP111A | F | 29 | 40 | RTL | HS/MTS | 1 | 0 | 32 | 16 |
| HUP116 | F | 51 | 58 | RTL | NR | 1 | 0 | 0 | 50 |
| Study016 | F | 5 | 36 | RFTL | Gliosis | 4 | 48 | 16 | 0 |
| Study019 | M | 31 | 33 | LTL | Gliosis | 5 | 60 | 28 | 8 |
| Study020 | M | 5 | 10 | RFL | Gliosis | 4 | 40 | 16 | 0 |
| Study022 | F | 21–30 | 21–30 | LTL | Gliosis | 5 | 44 | 12 | 0 |
| Study028 | M | 4 | 5 | LFPL | Gliosis | 4 | 64 | 8 | 0 |
| Study029 | F | Unknown | Unknown | RTL | Gliosis | 5 | 24 | 30 | 8 |
Two-year post-surgical outcomes were based on the ILAE classification system (class 1–6).
F = female; FCD = focal cortical dysplasia; HS/MTS = hippocampal sclerosis/mesial temporal sclerosis; HUP = Hospital of the University of Pennsylvania; LFL = left frontal lobe; LFPL = left frontoparietal lobe; LTL = left temporal lobe; M = male; MCD = malformation of cortical development; mo = months; NR = no resection; RFL = right frontal lobe; RFTL = right frontotemporal lobe; RTL = right temporal lobe.
Table 2.
EEG and spike detector information
| Patient ID | Duration, h | Number of seizures | Raw spike rate (spikes/channel/h) | Post clustering spike rate (spikes/channel/h) | Total spike count | Accuracy rate, % |
|---|---|---|---|---|---|---|
| HUP064 | 24.0 | 1 | 4.3 | 0.3 | 597 | 61.0 |
| HUP070 | 97.7 | 8 | 70.2 | 7.1 | 53 857 | 86.0 |
| HUP074 | 101.0 | 5 | 29.0 | 2.0 | 24 313 | 100.0 |
| HUP075 | 24.2 | 1 | 30.1 | 1.9 | 5 189 | 80.0 |
| HUP078 | 44.8 | 5 | 60.0 | 18.5 | 86 410 | 98.0 |
| HUP080 | 80.1 | 4 | 12.4 | 1.4 | 11 176 | 100.0 |
| HUP086 | 33.6 | 2 | 37.6 | 7.8 | 30 482 | 66.7 |
| HUP088 | 35.5 | 3 | 115.5 | 2.6 | 5 702 | 82.0 |
| HUP094 | 72.0 | 3 | 55.9 | 1.8 | 11 191 | 76.0 |
| HUP105 | 130.6 | 2 | 23.4 | 1.3 | 10 437 | 84.0 |
| HUP106 | 116.4 | 4 | 15.5 | 3.9 | 52 122 | 100.0 |
| HUP107 | 123.2 | 13 | 12.1 | 3.8 | 55 402 | 99.0 |
| HUP111A | 54.6 | 5 | 58.3 | 15.9 | 41 597 | 89.0 |
| HUP116 | 40.9 | 3 | 24.6 | 2.7 | 5 476 | 100.0 |
| Study016 | 141.7 | 6 | 29.4 | 4.1 | 37 057 | 88.7 |
| Study019 | 130.9 | 36 | 30.6 | 5.9 | 73 573 | 97.3 |
| Study020 | 91.7 | 7 | 15.9 | 2.5 | 12 910 | 97.5 |
| Study022 | 83.5 | 7 | 68.0 | 15.5 | 72 306 | 92.5 |
| Study028 | 35.7 | 5 | 21.3 | 5.7 | 14 580 | 96.0 |
| Study029 | 48.6 | 3 | 82.1 | 39.3 | 118 488 | 85.3 |
The accuracy rate is the per cent of sample spike sequences that were determined to be real spike sequences on visual analysis after removal of artefactual clusters.
Clustering by spike location
On average, 0.7 artefactual clusters were removed per patient (range 0–3), with an average of 2.3 distinct spike location clusters remaining (range 1–4). The mean distance between cluster centroids was 5.5 cm (range 1.8–18.1 cm). After removing spikes belonging to artefact-heavy clusters, the mean spike density was 7.2 spikes/electrode/h (range 0.3–39.3), and the percentage of sample spike sequences determined to be true spike sequences by visual inspection was 89.0% (range 61–100% across patients), similar to that reported for other spike detectors (Ung et al., 2017b).
Change in spike spatial distribution over time
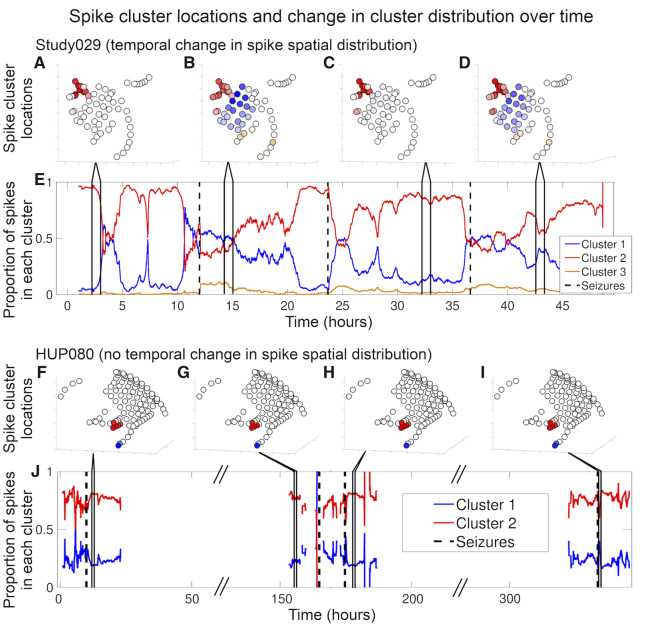
Figure 2 shows an example of spike spatial distribution over time for two patients. Seventeen of 20 patients had more than one spike cluster after removing artefactual clusters. Of these 17 patients, 14 had a significant change in cluster distribution across hour-long bins by χ2 test of independence, implying temporal variability in spike spatial distribution. Supplementary Table 3 shows individual patient P-values and χ2 statistics. The combined P-value for a change in cluster distribution over time across all 20 patients was P < 0.001 (Fisher’s method). There was no significant difference in clinical outcome between patients with a significant temporal change in spike spatial distribution (median ILAE 3) and those without (median ILAE 1) (Wilcoxon rank sum test: P = 0.18, U = 26). There was no difference in the number of patients with a significant change in spike spatial distribution between those with temporal lobe versus non-temporal lobe seizure origin [χ2 independence test: P = 0.57, χ2(1) = 0.32], between those with mesial temporal sclerosis versus other pathology [χ2 independence test: P = 0.92, χ2(1) = 0.01], or between those with only grid and strip electrodes versus those with grid, strips, and depths [χ2 independence test: P = 0.39, χ2(1) = 0.73].
Figure 2.
Spike location and cluster distribution over time. Patient Study029 (A–E) demonstrated a significant change in cluster distribution over time and Patient HUP080 (F–J) did not. (A–D and F–I) Diagrams of each patient’s electrodes and the average spike frequency in each electrode for four arbitrarily chosen hour-long segments as indicated by the lines connecting to subplots E and J, respectively. The colour shows which cluster the spikes in the corresponding electrodes were assigned to, based on their location. The brightness represents spike frequency, where brighter colours indicate higher spike rates and whiter colours indicate lower spike rates. (E and J) One hour moving average (overlap of 1 h minus 1 s, showing the average of the hour leading up to the indicated time) of the proportion of spikes in a given cluster. Black dashed vertical lines denote seizures. The diagonal hash marks in the x-axis of J represent times in which we spliced together different periods in Patient HUP080’s record to minimize white space in the plot, as these times were prolonged interictal periods that were not subjected to analysis. Supplementary Table 3 shows the statistics determining the change in spike spatial distribution over time for all patients.
Number of hours needed to capture variability in spike spatial distribution
Figure 3 shows an example, for one patient, of the variability in spike spatial distribution as a function of the subsample duration, and a summary graph showing the number of hours needed to capture 80% of the variability in spike spatial distribution for each patient. The median number of sequential hours needed to capture 80% of the total variability in spike spatial distribution was 12 h (range 6–85 h across patients, interquartile range = 11.8 h), and the median percentage duration was 16.3% of the total recording duration (range 7.4–64.9%). There was no correlation between this duration and ILAE score [Spearman rank correlation, r(15) = 0.03, P = 0.91] or between this duration and spike frequency [Spearman rank correlation, r(15) = 0.29, P = 0.26]. There was no difference in this duration between patients with temporal lobe onset seizures (13 patients, mean 17.0 h) and non-temporal lobe onset seizures (four patients, mean 11.5 h) (Wilcoxon rank sum: P = 0.34, U = 17).
Figure 3.
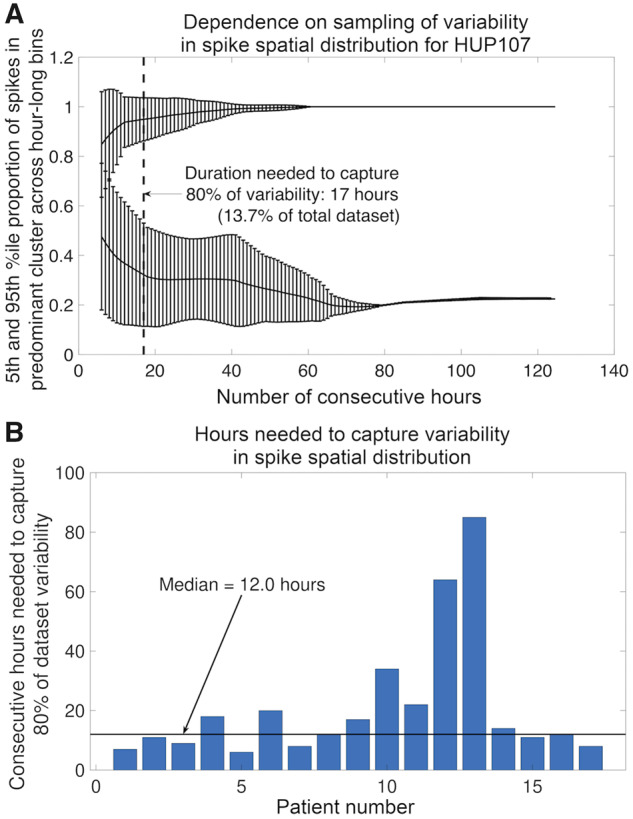
Hours needed to capture variability in spike spatial distribution. (A) Variability in spike spatial distribution as a function of sampling time for Patient HUP107. The x-axis shows the number of consecutive hours sampled, ranging from 1 h to the total number of hours in the dataset. The top line is the 95th percentile proportion of spikes in the predominant cluster (where the predominant cluster was selected from the entire dataset) across hour-long bins, averaged over all possible subsets with a duration of sequential hours indicated by the x-axis. The bottom line is the 5th percentile proportion of spikes in the predominant cluster across hour-long bins, averaged over all subsets. Error bars indicate the standard deviation of the proportion over all subsets of the given duration. The dotted line and arrow indicate the sample duration for which the difference between the 95th and 5th percentile proportions of spikes in the predominant cluster is equal to 80% of the difference across the full dataset duration. (B) The number of hours for each patient needed to capture 80% of the variability in spike spatial distribution. The horizontal black line shows the median number of hours across all patients.
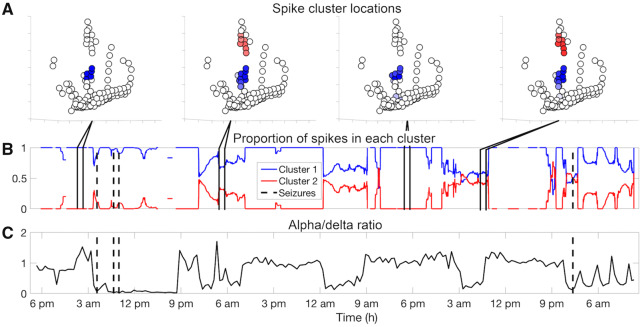
Correlation between sleep and spike spatial distribution
Figure 4 shows a single patient example of the relationship between spike spatial distribution and alpha/delta power ratio. There was a significant relationship between the proportion of spikes in the predominant cluster and alpha/delta ratio for eight of 20 patients (Patients HUP074, HUP078, HUP106, HUP107, Study016, Study019, Study022, and Study029), suggesting that for these eight patients, the spike location distribution was related to sleep versus wakefulness. Supplementary Table 4 shows the model coefficients, z-scores, and P-values for all patients. The aggregate P-value was P < 0.001 (Fisher’s method). There was no consistent relationship across patients between the proportion of spikes in the predominant cluster and the alpha/delta ratio [one-sample two-sided t-test: P = 0.16, t(16) = −1.5]. When we examined only the eight patients with a significant relationship between the proportion of spikes in the predominant cluster and the alpha delta ratio, there was again no consistent direction to this relationship [one-sample two-sided t-test: P = 0.27, t(7) = −1.2].
Figure 4.
The relationship between sleep and spike spatial distribution. Patient HUP106 is shown. (A) Diagrams of Patient HUP106’s electrodes and the average spike frequency in each electrode for four arbitrarily chosen hour-long segments as indicated by the lines connecting to B. The colour shows which cluster the spikes in the corresponding electrodes were assigned to, based on their location. The brightness represents spike frequency, where brighter colours indicate higher spike rates and whiter colours indicate lower spike rates. (B) Moving average of 1 h of the proportion of spikes in a given cluster. In this patient, the spikes in the red cluster occur predominantly at night. The black dashed vertical lines denote seizures (four seizures are shown). (C) Alpha-delta power ratio over time to estimate sleep epochs. Each time point is the alpha/delta power ratio averaged over a 2000-s bin and averaged over all electrodes. A low alpha/delta ratio indicates relatively higher delta power, suggesting sleep. In this patient, spikes occurring in the red cluster appear predominantly during periods of low alpha/delta power. Supplementary Tables 4 and 5 show the statistics determining the postictal and pre-ictal change in spike spatial distribution for all patients.
There was no aggregate correlation between the alpha-delta ratio and the distance from the nearest seizure onset zone electrode [one-sample two-sided t-test: P = 0.50, t(19) = 0.69]. We observed a non-significant trend of higher sequence length with lower alpha/delta ratio, suggesting more extensive spike propagation with sleep [one-sample two-sided t-test: P = 0.061, t(19) = −2.0]. Eighteen of the 20 patients (all except Patients HUP116 and Study028) exhibited a significant correlation between spike rate and alpha/delta ratio. For all but four of these patients (Patients HUP070, HUP074, HUP075 and HUP078) this was a negative correlation, implying higher spike rate during sleep. In aggregate, spike rate was negatively correlated with alpha/delta ratio [one-sample two-sided t-test: P = 0.003, t(19) = −3.4]. The Pearson correlation coefficient comparing alpha/delta ratios when spikes are included and when spikes are excluded was >0.99 for every patient, suggesting that spikes do not largely affect the calculation of the alpha/delta ratio.
Peri-ictal change in spike spatial distribution
Four patients (Patients HUP107, Study019, Study022, and Study029) exhibited a significant change in cluster distribution during the postictal period compared to all other periods. The group P-value for postictal change in spike spatial distribution was <0.001 (Fisher’s method). When the postictal period was defined as 1 h rather than 4 h after seizures to test the sensitivity of our result to the duration of the postictal changes, three of the 20 patients (Patients Study019, Study022, and Study029) continued to demonstrate a significant postictal change in spike spatial distribution (the P-value for Patient HUP107 increased from <0.001 to 0.006). The group P-value remained <0.001 (Fisher’s method). When we defined the preictal period to be 4 h rather than 1 h before the seizure, the same four patients (Patients HUP107, Study019, Study022, and Study029) continued to demonstrate a significant postictal change in spike spatial distribution, and the group P-value remained <0.001 (Fisher’s method). Together, these findings suggest that there is a postictal change in spike spatial distribution for a minority of patients, and that this result is robust to the choice of definition of postictal and pre-ictal period.
No patient had a significant difference in cluster distribution between the pre-ictal and interictal period. The group P-value was 0.99 (Fisher’s method) (this high P-value largely stems from our conservative choice to set the individual P-values for patients with only one cluster to 1). When we changed the definition of postictal duration to 1 h, 2 of 20 patients (Patients Study019 and Study028) now had a significant difference between the pre-ictal and interictal cluster distribution; however, the group P-value remained non-significant at 0.16. When we changed the definition of preictal duration to 4 h, one patient (Patient HUP064) now had a significant pre-ictal change in spike spatial distribution, and the group P-value became significant at P = 0.026. Although this result is statistically significant, the fact that the significant group P-value is largely driven by Patient HUP064, with the lowest number of spikes and the lowest spike detector accuracy (Table 2), renders the result less robust. Altogether these analyses suggest that there is likely no pre-ictal change in spike spatial distribution in our patient dataset. Supplementary Tables 5 and 6 show the χ2 statistics and P-values for the results of the permutation tests for all patients for the postictal and pre-ictal analyses, respectively.
There was no difference in the proportion of spikes in the predominant cluster between the pre-ictal, postictal, and interictal periods when aggregated across patients [Friedman test: P = 0.19, χ2(2) = 3.3]. There was no difference in spike rates, distance from the seizure onset zone, or spike propagation between the pre-ictal, postictal, and interictal time periods [Friedman test, spike rate: P = 0.95, χ2(2) = 0.1; seizure onset zone distance: P = 0.71, χ2(2) = 0.7; spike propagation: P = 0.70, χ2(2) = 0.7].
Seizure onset localization
Figure 5 shows comparisons in distances between various electrodes of interest and the seizure onset zone. The electrode with the largest area of influence was a different electrode from the electrode with the maximum spike frequency in 19 of 20 patients. The average distance between these two electrodes was 2.8 ± 2.0 cm (range 0–8.0 cm). The average Spearman’s rank correlation coefficient between electrode spike frequency and area of influence was 0.70 (range 0.49–0.84). The largest area of influence was on average 47.7 cm2 (range 2.3–180.4 cm2). The electrode with the largest area of influence was on average 1.8 ± 2.0 cm from its nearest seizure onset zone electrode, compared to an average distance of 2.0 ± 2.8 cm for the electrode with maximum spike frequency, and 4.3 ± 1.7 cm for all electrodes. Both the electrode with maximum spike frequency and the electrode with maximum area of influence were significantly closer to the seizure onset zone than expected by chance (Wilcoxon signed-rank test, max frequency versus average: P = 0.005, W = −150; max area of influence versus average: P = 0.002, W = −170). There was no significant difference in the distance to the nearest seizure onset zone between the electrode with the maximal spike frequency and the electrode with the largest area of influence (Wilcoxon signed-rank test: P = 0.81, W = 9). Eight of 20 patients underwent resection of the cortex underlying the electrode with maximum spike frequency. There was no significant difference in ILAE scores of patients for whom this electrode was resected (median ILAE 2.0) than for whom it was not (3.0) (Wilcoxon rank sum: P = 0.44, U = 38). Ten patients underwent resection of the cortex underlying the electrode with maximum area of influence. There was no significant difference in ILAE score for patients for whom this electrode was resected (1.5) and those for whom it was not (2.0) (Wilcoxon rank sum: P = 0.66, U = 44). The predominant spike cluster was also the nearest spike cluster to a seizure onset zone electrode in 10/17 patients, which was not more frequent than predicted by chance (z-test, P = 0.078).
Finally, we permuted either the rows or columns of the connection matrix to define the electrodes forming the area of influence (in order to generate a spike rate-agnostic area of influence). The electrode with the highest area of influence was not closer to the nearest seizure onset zone than predicted by chance (permute rows: average distance: 3.6 cm ± 4.8 cm, Wilcoxon signed-rank test: P = 0.14, W = −80; permute columns: average distance: 3.9 cm ± 3.5 cm, Wilcoxon signed-rank test: P = 0.53, W = −34). This suggests that the area of influence measure, when calculated independent of spike rate, does not significantly localize the seizure onset zone.
Discussion
The presumed relationship between ictal and interictal networks motivates the use of interictal spike location to guide epilepsy surgical planning. However, spike location dynamics are poorly understood. Our overall findings suggest that the spatial distribution of spikes changes over time and that this change is related in part to sleep and in part to seizures.
The spatial distribution of spikes fluctuates over time
Most patients had multiple spatially distinct regions of spikes, agreeing with previous studies (Alarcon et al., 1997; Bourien et al., 2005; Oishi et al., 2006; Pittau et al., 2014; Janca et al., 2018). The majority of the patients also demonstrated hour-to-hour shifts in the proportion of spikes in each region. A median of 12 sequential hours were required to capture 80% of the variability in spike spatial distribution, although there was substantial variability across patients, with some requiring fewer hours and some requiring many more to capture this variability. Marsh et al. (2010) and Janca et al. (2018) have previously reported temporal variability in spike location in paediatric patients. To our knowledge, this is the first study to quantify the recording duration needed to assess the temporal variability of spike spatial distribution, and the first to examine the contribution of seizures to this variability.
The change in spatial distribution may reflect changes in spike activity in static spatial regions or actual migration of the regions themselves. Our analysis is unable to distinguish between these two possibilities. Performing spatial clustering analysis across many time periods could answer this question. However, because of the need to remove artefactual clusters, this process would require either the development of an unsupervised algorithm for removing artefactual clusters or extensive visual analysis by humans.
Temporal variability in the spatial distribution of spikes should be considered when mapping interictal spikes for surgical planning. Therefore, clinical decisions incorporating interictal spike location should include an adequate duration of interictal EEG data—ideally at least 12 consecutive hours, although some patients may require many more—to avoid undersampling the variability in spike location.
Effect of sleep on the frequency and spatial distribution of spikes
We observed an overall increase in spike rates during periods of low alpha/delta ratio, although the opposite was true for a minority of patients. This suggests an overall increase in spike rates during sleep, which has been observed by other groups (Foldvary-Schaefer and Grigg-Damberger, 2006; Derry and Duncan, 2013; Karoly et al., 2016). We also found a significant relationship between spike spatial distribution and alpha/delta ratio, suggesting a change in spike spatial distribution with sleep. This finding is consistent with previous studies (Rocamora et al., 2013; Del Felice et al., 2015; Janca et al., 2018; Lambert et al., 2018), although this effect was driven by a minority of patients (8/20) in our study. There are multiple potential mechanisms for this effect: first, sleep may preferentially increase spikes in certain regions of the brain (Janca et al., 2018). This is seen in Fig. 4, where periods of low alpha/delta power ratio were associated with a disproportionate increase in spikes in the red cluster, leading to both a change in spike spatial distribution and an increase in overall spike rates. Alternatively, sleep may produce changes on a whole-brain level, facilitating a broader distribution of spikes (Del Felice et al., 2015). We found a trend towards more extensive spike propagation during sleep, although this was non-significant (P = 0.06).
Peri-ictal changes in spike frequency and spatial distribution
We found no peri-ictal change in spike rates. Previous reports on peri-ictal spike rates have been inconsistent (Gotman and Marciani, 1985; Kaibara and Blume, 1988; Gotman and Koffler, 1989; Spencer et al., 2008; So and Blume, 2010; Krishnan et al., 2014; Karoly et al., 2016). We also found no clear pre-ictal change in spike spatial distribution. We observed a postictal change in spike spatial distribution for a minority (4 of 20) of the patients studied, with a significant aggregate effect. Several hypothesized mechanisms for postictal changes in spike rates may also bear on our finding of the postictal change in spike spatial distribution, including postictal hyperpolarization, the administration of anti-seizure drugs, and a tendency for patients to fall asleep or to have increased delta activity postictally (Kaibara and Blume, 1988; So and Blume, 2010). The latter hypothesis raises the possibility that the postictal change in spike spatial distribution may be driven by the alpha/delta ratio-related change in spatial distribution (Aminoff, 1980; Sharbrough, 2005). Our low number of seizures per patient (median 4.5) limited our ability to detect small changes in peri-ictal spike location distribution on an individual patient level. Long-term recording may reveal peri-ictal trends (Baud et al., 2018).
Other factors that may influence spike spatial distribution
Given our small number of patients and their heterogeneous exposure to anti-seizure drugs, we were unable to analyse the effect of specific anti-seizure drugs on spike spatial distribution (Spencer et al., 2008). We also could not investigate a post-electrode implantation effect because we only analysed the 24 h surrounding seizures, which on average began 74 h after recording initiation (Sun et al., 2018). Finally, all of our patients were thought to have localization-related epilepsy. Patients with generalized epilepsy may exhibit different temporal patterns in spike topology.
Localizing the seizure onset zone
Electrodes with the most frequent spikes were closer to the seizure onset zone than predicted by chance, agreeing with previous studies (Hufnagel et al., 2000; Asano et al., 2003; Marsh et al., 2010; Mégevand et al., 2014; Liu et al., 2016). However, the predominant spike cluster did not outperform other spike clusters at localizing the seizure onset zone. Furthermore, we found no correlation between sleep versus wakefulness or peri-ictal state with localization of spikes to the seizure onset zone. Therefore, although spikes help to localize the seizure onset zone, it remains unclear which spikes localize best.
The area of influence measure tested whether spike propagation adds additional information to localizing the seizure onset zone, as observed by other groups (Alarcon et al., 1997; Hufnagel et al., 2000). Our specific hypothesis was that the region responsible for the largest area of downstream spike propagation would preferentially localize to the seizure onset zone. While the electrode with the largest area of influence was closer to the seizure onset zone than expected by chance, it was not significantly closer than the electrode with the most frequent spikes (nor was it significantly further away). Furthermore, the largest area of influence electrode did not significantly localize the seizure onset zone when we calculated area of influence using a method controlling for spike rate. Thus, spike propagation does not clearly add localizing value over spike rate alone to mapping the seizure onset zone. Interestingly, the electrode with the largest area of influence was usually distinct from the electrode with the most frequent spikes. The fact that this electrode is distinct from the most frequently spiking electrode but localizes the seizure onset zone equally well suggests that this electrode may add additional useful information about seizure dynamics. For instance, perhaps upon reaching the region with the largest area of influence, seizures are able to propagate broadly throughout the brain. This ‘gatekeeper’ hypothesis could be tested by comparing the involvement of this region in seizures that remain focal and seizures that secondarily generalize.
Limitations
Given our limited electrode coverage as well as the imperfect sensitivity of the spike detector, there may be additional populations of spikes that we failed to record. Also, variability in spike location may occur over longer time scales than those studied here, perhaps in part related to the trauma of brain implantation or medication manipulation. The alpha/delta power ratio is an imperfect surrogate for sleep, which limits our understanding of its effect on spike spatial distribution. In particular, prior work showing a post-ictal increase in delta power suggests that the presence of seizures may confound the relationship between sleep and spike spatial distribution (Aminoff, 1980; Sharbrough, 2005). Furthermore, the interrupted sleep patterns typical of epilepsy monitoring units also limit this analysis. Future studies with more rigorous methods of staging sleep are needed to verify our findings of sleep-related changes in spike spatial distribution. Chronic implanted devices could also help probe both long-term trends and sleep-related changes in spike spatial distribution (Ung et al., 2016, 2017a; Baud et al., 2018; Nurse et al., 2018). Also, the effect of changing anti-seizure drugs on the alpha-delta ratio is unknown and so potentially confounds this analysis. Studies of larger numbers of patients exposed to similar anti-seizure drugs might elucidate this effect. The spatial distribution of high frequency oscillations, not studied here, is also important for understanding network dynamics (Worrell et al., 2004; Gliske et al., 2018; Guragain et al., 2018).
This study was intended to be hypothesis-generating, which resulted in a large number of tests being performed. This process, by nature, increases the probability of type I errors. Correcting for 20 independent tests did not change the significance of the major results in our study (the localization of the most frequently spiking electrode to the seizure onset zone would become non-significant with an alpha of 0.0025, but the localization of the highest area of influence electrode to the seizure onset zone would remain significant). But this does not change the primary fact that we are performing this analysis to generate testable hypothesis in a prospective-blinded fashion in the future. In these future studies, complete correction for multiple comparisons will be performed.
Conclusion
Spikes can be used to localize the seizure onset zone, but the spatial dynamics of spikes are not well described. Understanding the temporal dynamics of spike spatial distribution may elucidate interictal networks and inform surgical planning. Many patients have multiple spatially distinct populations of interictal spikes and the spatial distribution of spikes changes over time. Both sleep and postictal network changes may contribute to spike spatial dynamics. Attempts to identify the location of interictal spikes for surgical planning purposes should analyse a minimum of 12 consecutive hours of interictal data, ideally including both sleep and wakefulness, in order to capture the temporal variability in spike spatial distribution.
Supplementary Material
Acknowledgements
We thank Dr Ammar Kheder and Dr Fadi Mikhail for their contributions to determining seizure onset times and locations. We thank Dr Steve Schmitt and Dr Abdhi Sarkar for their discussions related to statistical approaches.
Funding
E.C. received funding from NIH/NINDS (R25 NS065745). S.T. was supported by the University of Rochester CTSA award number TL1 TR002000 from the National Center for Advancing Translational Sciences of the National Institutes of Health. K.O. and J.W. have no funding to report. B.L., E.M., and K.D. received funding from NIH/NINDS (R01 NS099348). E.M. additionally received funding from NIH/NICHD IDDRC (U54 HD086984). K.D. additionally received funding from NIH/NINDS (K23 NS073801) and the Thornton Foundation. B.L. additionally received funding from The Mirowski Family Foundation, and Neil and Barbara Smit. R.S. received funding from the NIH (R01MH112845 and R01NS060910), the National Multiple Sclerosis Society, and the Race to Erase MS.
Competing interests
The authors report no competing interests.
Glossary
Abbreviation
- ILAE =
International League Against Epilepsy
References
- Agarwal R, Gotman J. Computer-assisted sleep staging. IEEE Trans Biomed Eng 2001; 48: 1412–23. [DOI] [PubMed] [Google Scholar]
- Alarcon G, Garcia Seoane JJ, Binnie CD, Martin Miguel MC, Juler J, Polkey CE, et al. Origin and propagation of interictal discharges in the acute electrocorticogram. Implications for pathophysiology and surgical treatment of temporal lobe epilepsy. Brain 1997; 120: 2259–82. [DOI] [PubMed] [Google Scholar]
- Alarcon G, Guy CN, Binnie CD, Walker SR, Elwes RD, Polkey CE. Intracerebral propagation of interictal activity in partial epilepsy: implications for source localisation. J Neurol Neurosurg Psychiatry 1994; 57: 435–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminoff MJ. EEG findings in patients with neurologic disorders In: Electrodiagnosis in Clinical Neurology. New York: Churchill Livingstone; 1980. p. 41–66. [Google Scholar]
- Asano E, Muzik O, Shah A, Juhász C, Chugani DC, Sood S, et al. Quantitative interictal subdural EEG analyses in children with neocortical epilepsy. Epilepsia 2003; 44: 425–34. [DOI] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 2011; 54: 2033–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud MO, Kleen JK, Mirro EA, Andrechak JC, King-Stephens D, Chang EF, et al. Multi-day rhythms modulate seizure risk in epilepsy. Nat Commun 2018; 9: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bholowalia P, Kumar A. EBK-means: a clustering technique based on elbow method and k-means in WSN. Int J Comput Appl Technol 2014; 105: 17–24. [Google Scholar]
- Bourien J, Bartolomei F, Bellanger JJ, Gavaret M, Chauvel P, Wendling F. A method to identify reproducible subsets of co-activated structures during interictal spikes. Application to intracerebral EEG in temporal lobe epilepsy. Clin Neurophysiol 2005; 116: 443–55. [DOI] [PubMed] [Google Scholar]
- Brown MW 3rd, Porter BE, Dlugos DJ, Keating J, Gardner AB, Storm PB Jr, et al. Comparison of novel computer detectors and human performance for spike detection in intracranial EEG. Clin Neurophysiol 2007; 118: 1744–52. [DOI] [PubMed] [Google Scholar]
- Davis KA, Devries SP, Krieger A, Mihaylova T, Minecan D, Litt B, et al. The effect of increased intracranial EEG sampling rates in clinical practice. Clin Neurophysiol 2018; 129: 360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Felice A, Storti SF, Manganotti P. Sleep affects cortical source modularity in temporal lobe epilepsy: a high-density EEG study. Clin Neurophysiol 2015; 126: 1677–83. [DOI] [PubMed] [Google Scholar]
- Derry CP, Duncan S. Sleep and epilepsy. Epilepsy Behav 2013; 26: 394–404. [DOI] [PubMed] [Google Scholar]
- Dunsmuir WTM, Scott DJ, Others. The glarma package for observation-driven time series regression of counts. J Stat Softw 2015; 67: 1–36. [Google Scholar]
- Dworetzky BA, Reinsberger C. The role of the interictal EEG in selecting candidates for resective epilepsy surgery. Epilepsy Behav 2011; 20: 167–71. [DOI] [PubMed] [Google Scholar]
- Englot DJ, Birk H, Chang EF. Seizure outcomes in nonresective epilepsy surgery: an update. Neurosurg Rev 2017; 40: 181–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RA. Statistical Methods for Research Workers. 4th Ed. Edinburgh: Oliver & Boyd; 1934. [Google Scholar]
- Foldvary-Schaefer N, Grigg-Damberger M. Sleep and epilepsy: what we know, don’t know, and need to know. J Clin Neurophysiol 2006; 23: 4–20. [DOI] [PubMed] [Google Scholar]
- Friedman M. The use of ranks to avoid the assumption of normality implicit in the analysis of variance. J Am Stat Assoc 1937; 32: 675–701. [Google Scholar]
- Gliske SV, Irwin ZT, Chestek C, Hegeman GL, Brinkmann B, Sagher O, et al. Variability in the location of high frequency oscillations during prolonged intracranial EEG recordings. Nat Commun 2018; 9: 2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Burgos G, Barrionuevo G, Lewis DA. Horizontal synaptic connections in monkey prefrontal cortex: an in vitro electrophysiological study. Cereb Cortex 2000; 10: 82–92. [DOI] [PubMed] [Google Scholar]
- Gotman J, Koffler DJ. Interictal spiking increases after seizures but does not after decrease in medication. Electroencephalogr Clin Neurophysiol 1989; 72: 7–15. [DOI] [PubMed] [Google Scholar]
- Gotman J, Marciani MG. Electroencephalographic spiking activity, drug levels, and seizure occurrence in epileptic patients. Ann Neurol 1985; 17: 597–603. [DOI] [PubMed] [Google Scholar]
- Guragain H, Cimbalnik J, Stead M, Groppe DM, Berry BM, Kremen V, et al. Spatial variation in high-frequency oscillation rates and amplitudes in intracranial EEG. Neurology 2018; 90: e639–e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser WA, Hesdorffer DC. Epilepsy Foundation of America. Epilepsy: Frequency, Causes and Consequences. New York, NY: Demos Medical Pub; 1990. [Google Scholar]
- Heck CN, King-Stephens D, Massey AD, Nair DR, Jobst BC, Barkley GL, et al. Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: final results of the RNS System Pivotal trial. Epilepsia 2014; 55: 432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch JA, Gilbert CD. Synaptic physiology of horizontal connections in the cat’s visual cortex. J Neurosci 1991; 11: 1800–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hufnagel A, Dümpelmann M, Zentner J, Schijns O, Elger CE. Clinical relevance of quantified intracranial interictal spike activity in presurgical evaluation of epilepsy. Epilepsia 2000; 41: 467–78. [DOI] [PubMed] [Google Scholar]
- Janca R, Krsek P, Jezdik P, Cmejla R, Tomasek M, Komarek V, et al. The sub-regional functional organization of neocortical irritative epileptic networks in pediatric epilepsy. Front Neurol 2018; 9: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobert M, Schulz H, Jähnig P, Tismer C, Bes F, Escola H. A computerized method for detecting episodes of wakefulness during sleep based on the alpha slow-wave index (ASI). Sleep 1994; 17: 37–46. [PubMed] [Google Scholar]
- Kaibara M, Blume WT. The postictal electroencephalogram. Electroencephalogr Clin Neurophysiol 1988; 70: 99–104. [DOI] [PubMed] [Google Scholar]
- Karoly PJ, Freestone DR, Boston R, Grayden DB, Himes D, Leyde K, et al. Interictal spikes and epileptic seizures: their relationship and underlying rhythmicity. Brain 2016; 139: 1066–78. [DOI] [PubMed] [Google Scholar]
- Kim DW, Kim HK, Lee SK, Chu K, Chung CK. Extent of neocortical resection and surgical outcome of epilepsy: intracranial EEG analysis. Epilepsia 2010; 51: 1010–7. [DOI] [PubMed] [Google Scholar]
- Kini LG, Davis KA, Wagenaar JB. Data integration: combined imaging and electrophysiology data in the cloud. Neuroimage 2016; 124: 1175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan B, Vlachos I, Faith A, Mullane S, Williams K, Alexopoulos A, et al. A novel spatiotemporal analysis of peri-ictal spiking to probe the relation of spikes and seizures in epilepsy. Ann Biomed Eng 2014; 42: 1606–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan P, Schachter SC, Brodie MJ. Drug-resistant epilepsy. N Engl J Med 2011; 365: 919–26. [DOI] [PubMed] [Google Scholar]
- Lajnef T, Chaibi S, Ruby P, Aguera P-E, Eichenlaub J-B, Samet M, et al. Learning machines and sleeping brains: automatic sleep stage classification using decision-tree multi-class support vector machines. J Neurosci Methods 2015; 250: 94–105. [DOI] [PubMed] [Google Scholar]
- Lambert I, Roehri N, Giusiano B, Carron R, Wendling F, Benar C, et al. Brain regions and epileptogenicity influence epileptic interictal spike production and propagation during NREM sleep in comparison with wakefulness. Epilepsia 2018; 59: 235–43. [DOI] [PubMed] [Google Scholar]
- Lee C, Kim JS, Jeong W, Chung CK. Usefulness of interictal spike source localization in temporal lobe epilepsy: electrocorticographic study. Epilepsy Res 2014; 108: 448–58. [DOI] [PubMed] [Google Scholar]
- Le Van Quyen M, Soss J, Navarro V, Robertson R, Chavez M, Baulac M, et al. Preictal state identification by synchronization changes in long-term intracranial EEG recordings. Clin Neurophysiol 2005; 116: 559–68. [DOI] [PubMed] [Google Scholar]
- Litt B, Esteller R, Echauz J, D’Alessandro M, Shor R, Henry T, et al. Epileptic seizures may begin hours in advance of clinical onset: a report of five patients. Neuron 2001; 30: 51–64. [DOI] [PubMed] [Google Scholar]
- Liu S, Sha Z, Abosch A, Henry T, Ince NF Identification of seizure onset zone using automatically detected spike and high-frequency oscillation in human intracranial EEG. In: 2016 24th Signal Processing and Communication Application Conference (SIU). Piscataway, NJ: IEEE; 2016. p. 2241–4.
- Marsh ED, Peltzer B, Brown MW III, Wusthoff C, Storm PB Jr, Litt B, et al. Interictal EEG spikes identify the region of electrographic seizure onset in some, but not all, pediatric epilepsy patients. Epilepsia 2010; 51: 592–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mégevand P, Spinelli L, Genetti M, Brodbeck V, Momjian S, Schaller K, et al. Electric source imaging of interictal activity accurately localises the seizure onset zone. J Neurol Neurosurg Psychiatry 2014; 85: 38–43. [DOI] [PubMed] [Google Scholar]
- Noe K, Sulc V, Wong-Kisiel L, Wirrell E, Van Gompel JJ, Wetjen N, et al. Long-term outcomes after nonlesional extratemporal lobe epilepsy surgery. JAMA Neurol 2013; 70: 1003–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse ES, John SE, Freestone DR, Oxley TJ, Ung H, Berkovic SF, et al. Consistency of long-term subdural electrocorticography in humans. IEEE Trans Biomed Eng 2018; 65: 344–52. [DOI] [PubMed] [Google Scholar]
- Oishi M, Kameyama S, Masuda H, Tohyama J, Kanazawa O, Sasagawa M, et al. Single and multiple clusters of magnetoencephalographic dipoles in neocortical epilepsy: significance in characterizing the epileptogenic zone. Epilepsia 2006; 47: 355–64. [DOI] [PubMed] [Google Scholar]
- Pittau F, Mégevand P, Sheybani L, Abela E, Grouiller F, Spinelli L, et al. Mapping epileptic activity: sources or networks for the clinicians? Front Neurol 2014; 5: 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing [Internet]. 2014. Available from: http://www.R-project.org/ (29 November 2019, date last accessed).
- Rocamora R, Andrzejak RG, Jiménez-Conde J, Elger CE. Sleep modulation of epileptic activity in mesial and neocortical temporal lobe epilepsy: a study with depth and subdural electrodes. Epilepsy Behav 2013; 28: 185–90. [DOI] [PubMed] [Google Scholar]
- Rosati A, Aghakhani Y, Bernasconi A, Olivier A, Andermann F, Gotman J, et al. Intractable temporal lobe epilepsy with rare spikes is less severe than with frequent spikes. Neurology 2003; 60: 1290–5. [DOI] [PubMed] [Google Scholar]
- Sharbrough FW. 12, Nonspecific abnormal EEG patterns. In: Niedermeyer E, Da Silva FL, editors. Electroencephalography: basic principles, clinical applications, and related fields. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. p. 235.
- Soni Madhulatha T. An overview on clustering methods. IOSR J Eng 2012; 2: 719–25. [Google Scholar]
- So NK, Blume WT. The postictal EEG. Epilepsy Behav 2010; 19: 121–6. [DOI] [PubMed] [Google Scholar]
- Spencer SS, Goncharova II, Duckrow RB, Novotny EJ, Zaveri HP. Interictal spikes on intracranial recording: behavior, physiology, and implications. Epilepsia 2008; 49: 1881–92. [DOI] [PubMed] [Google Scholar]
- Staley K, Hellier JL, Dudek FE. Do interictal spikes drive epileptogenesis? Neuroscientist 2005; 11: 272–6. [DOI] [PubMed] [Google Scholar]
- Staley KJ, Dudek FE. Interictal spikes and epileptogenesis. Epilepsy Curr 2006; 6: 199–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun FT, Arcot Desai S, Tcheng TK, Morrell MJ. Changes in the electrocorticogram after implantation of intracranial electrodes in humans: the implant effect. Clin Neurophysiol 2018; 129: 676–86. [DOI] [PubMed] [Google Scholar]
- Šušmáková K, Krakovská A. Classification of waking, sleep onset and deep sleep by single measures. Guigoz Sci Rev 2007; 7: 34–8. [Google Scholar]
- Tomlinson SB, Bermudez C, Conley C, Brown MW, Porter BE, Marsh ED. Spatiotemporal mapping of interictal spike propagation: a novel methodology applied to pediatric intracranial EEG recordings. Front Neurol 2016; 7: 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson SB, Wong JN, Conrad EC, Kennedy BC, Marsh ED. Reproducibility of interictal spike propagation in children with refractory epilepsy. Epilepsia 2019; 60: 898–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ung H, Baldassano SN, Bink H, Krieger AM, Williams S, Vitale F, et al. Intracranial EEG fluctuates over months after implanting electrodes in human brain. J Neural Eng 2017a; 14: 056011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ung H, Cazares C, Nanivadekar A, Kini L, Wagenaar J, Becker D, et al. Interictal epileptiform activity outside the seizure onset zone impacts cognition. Brain 2017b; 140: 2157–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ung H, Davis KA, Wulsin D, Wagenaar J, Fox E, McDonnell JJ, et al. Temporal behavior of seizures and interictal bursts in prolonged intracranial recordings from epileptic canines. Epilepsia 2016; 57: 1949–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonck K, Boon P. Epilepsy: closing the loop for patients with epilepsy. Nat Rev Neurol 2015; 11: 252–4. [DOI] [PubMed] [Google Scholar]
- Wagenaar JB, Brinkmann BH, Ives Z, Worrell GA, Litt B A multimodal platform for cloud-based collaborative research. In: 2013 6th International IEEE/EMBS Conference on Neural Engineering (NER). Piscataway, NJ: IEEE; 2013. p. 1386–9.
- Wiebe S, Bellhouse DR, Fallahay C, Eliasziw M. Burden of epilepsy: the Ontario Health Survey. Can J Neurol Sci 1999; 26: 263–70. [DOI] [PubMed] [Google Scholar]
- Wiebe S, Blume WT, Girvin JP, Eliasziw M; Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med 2001; 345: 311–8. [DOI] [PubMed] [Google Scholar]
- Worrell GA, Parish L, Cranstoun SD, Jonas R, Baltuch G, Litt B. High‐frequency oscillations and seizure generation in neocortical epilepsy. Brain 2004; 127: 1496–506. [DOI] [PubMed] [Google Scholar]
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 2006; 31: 1116–28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data can be made available on reasonable request. Analysis scripts are freely available on GitHub (https://github.com/erinconrad/spike-propagation). All EEG records are hosted by The International Epilepsy Electrophysiology Portal (https://www.ieeg.org/) under the Virtual Cortical Resection dataset (free account required) (Wagenaar et al., 2013; Kini et al., 2016). An example dataset containing 500 s of data for a single patient (Study029) as well as a dataset containing the electrode locations, seizure times, and de-identified clinical information are available as MATLAB structures in the GitHub repository. The ReadMe in the GitHub repository explains how to run an example script demonstrating the code using the example dataset.