Proteome-wide profiling of HPV-specific T cell immunity in oropharyngeal cancer (OPC) patients reveals broad and multiple antigen-specific reactivities directed to HPV-encoded proteins E1, E2, E4, E5 E6, E7, and L1. These observations provide novel insights for future development of cellular immunotherapies for HPV-associated OPC patients.
Abstract
Cellular immunotherapeutics targeting the human papillomavirus (HPV)–16 E6 and E7 proteins have achieved limited success in HPV-positive oropharyngeal cancer (OPC). Here we have conducted proteome-wide profiling of HPV-16–specific T cell responses in a cohort of 66 patients with HPV-associated OPC and 22 healthy individuals. Unexpectedly, HPV-specific T cell responses from OPC patients were not constrained to the E6 and E7 antigens; they also recognized E1, E2, E4, E5, and L1 proteins as dominant targets for virus-specific CD8+ and CD4+ T cells. Multivariate analysis incorporating tumor staging, treatment status, and smoking history revealed that treatment status had the most significant impact on HPV-specific CD8+ and CD4+ T cell immunity. Specifically, the breadth and overall strength of HPV-specific T cell responses were significantly higher before the commencement of curative therapy than after therapy. These data provide the first glimpse of the overall human T cell response to HPV in a clinical setting and offer groundbreaking insight into future development of cellular immunotherapies for HPV-associated OPC patients.
Graphical Abstract
Introduction
Head and neck cancers are the sixth most common type of cancer worldwide, and their incidence is growing (Kreimer et al., 2018). Human papillomavirus (HPV) can be detected in 38–56% of oropharyngeal cancer (OPC) cases in Australia, Japan, North America, and Northern and Western Europe (de Martel et al., 2020). Other estimates indicate an HPV-positive OPC incidence of 60–70% in the United States (Chaturvedi et al., 2013). Although the worldwide prevalence of head and neck squamous cell carcinoma (HNSCC) has decreased over the past few decades, the incidence of HPV-associated OPC has increased (Chaturvedi et al., 2011). Despite the recent approval of two very effective HPV vaccines that prevent primary infection, the impact of vaccination on the incidence of HPV-associated OPC is not expected to become evident until 2060 (Gillison et al., 2015). The morbidity associated with treatment for OPC and the favorable long-term survival demonstrate an ongoing need to develop better prognostic markers for this disease to allow better selection of de-escalation strategies (Gillison et al., 2019; Jones et al., 2020; Mehanna et al., 2019).
HPV serotype 16 (HPV-16) is the predominant viral type associated with OPC and other HPV-positive cancers, including cervical cancer and anogenital cancer. HPV has an 8-kb genome that encodes six early genes (E1, E2, E4, E5, E6, and E7) that are involved in replication and transcription and two late genes (L1 and L2) that encode capsid proteins. In infected malignant cells, HPV-16 is known to integrate into the host genome, leading to disruption of E2 gene expression and induction of E6 and E7, which have known oncogenic function. As a consequence, the majority of immunotherapeutic developmental work in HPV has concentrated on the E6 and E7 antigens. However, recent cancer genome sequencing in HNSCC showed that >70% of cancers contain a hybrid episomal form of virus, indicative of the potential presence of other HPV antigens in tumor tissue (Morgan et al., 2017; Nulton et al., 2017). Furthermore, previous reports have demonstrated mRNA expression and high titers of serum antibodies against E1, E2, E4, and E5, in addition to E6 and E7, in HPV-positive OPC (Anderson et al., 2015; Gleber-Netto et al., 2019; Krishna et al., 2018), which further supports the persistence of all early antigens and their immunogenicity in OPC.
Virus-specific T cells have been shown to play an important role in remission of HPV-associated cancers, and immunodeficiency correlates with poor survival in HNSCC patients (Masterson et al., 2014). However, most previous studies have concentrated solely on the association of T cell responses against E6 and E7 with the prognosis of patients with OPC (Albers et al., 2005; Masterson et al., 2016). Therefore, in the present study, we adopted a proteome-wide profiling approach to study HPV-specific T cell immunity in a cohort of 66 OPC patients. The frequency, magnitude, and antigen specificity of HPV-specific T cell responses were further correlated to patient demographics, including the impact of curative therapy, disease staging, and smoking history.
Results
HPV-specific CD8+ and CD4+ T cells from OPC patients display broad, multiantigen-specific reactivity
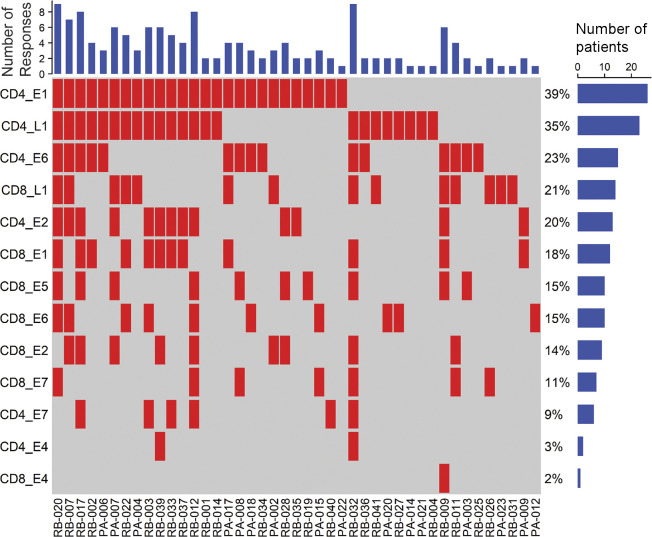
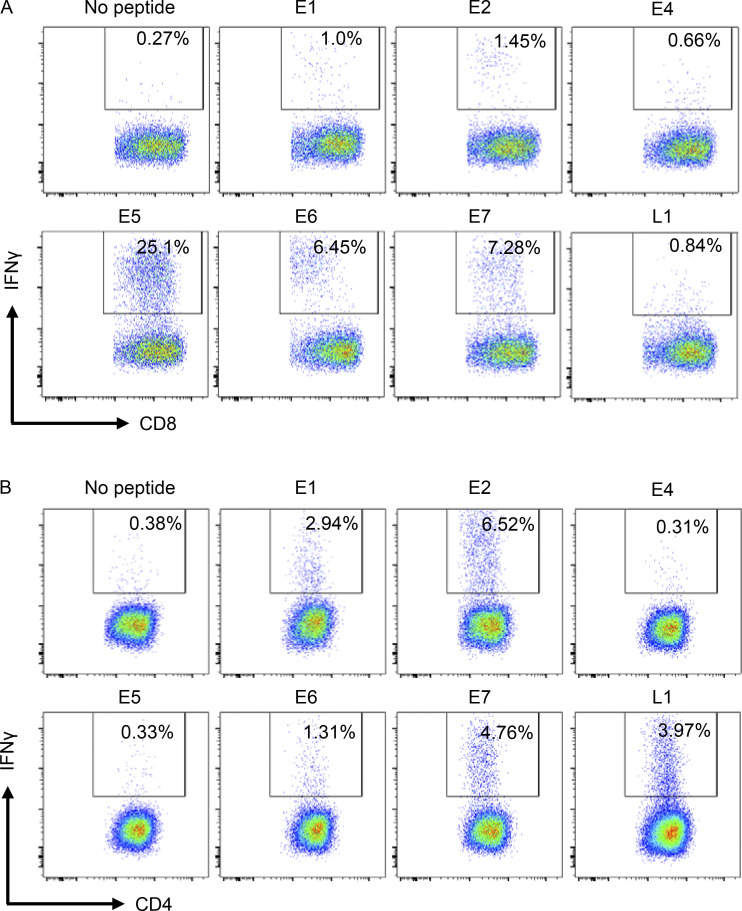
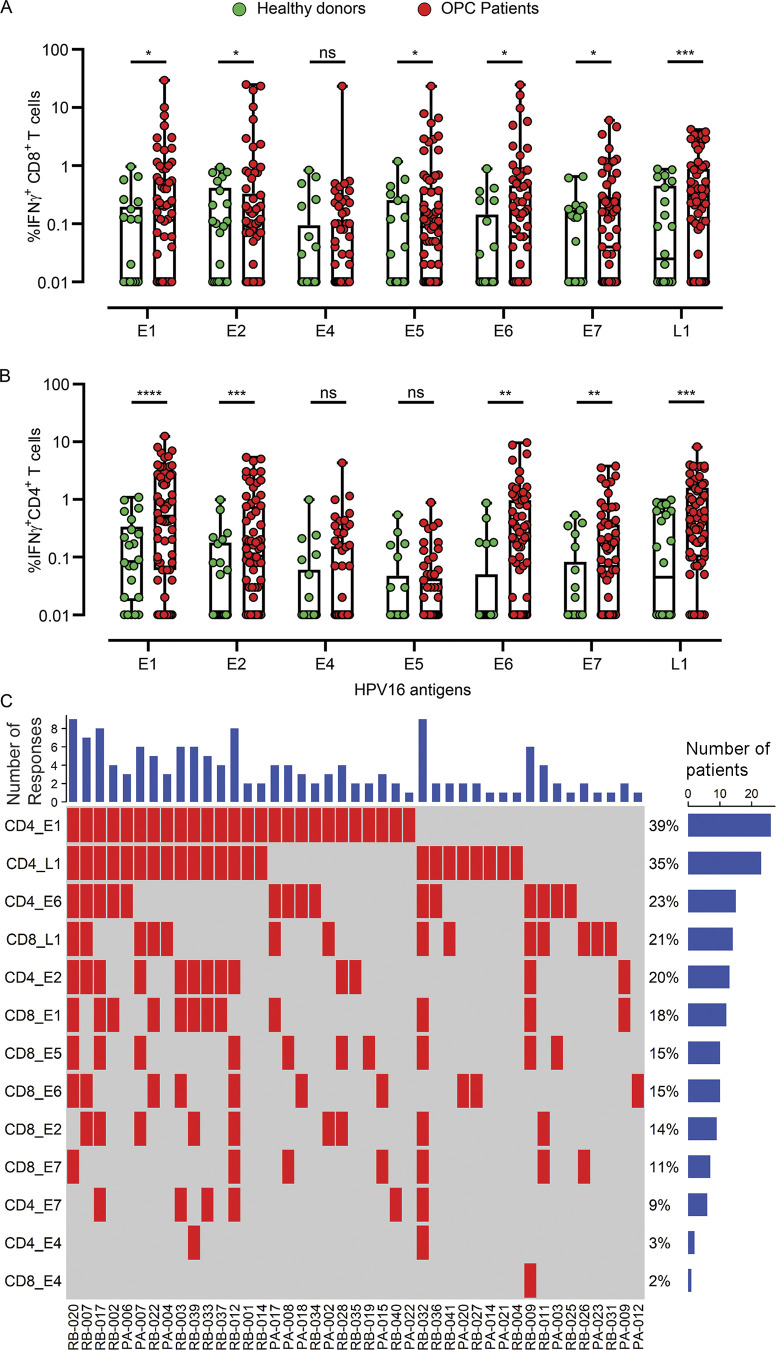
To comprehensively analyze HPV-specific T cell immunity, we recruited a cohort of 66 OPC patients and 22 healthy volunteers as control subjects (Table S1). This cohort was predominantly male (91%), with a median age of 59.06 ± 7.79 yr at diagnosis. The majority (61.19%) of these patients were diagnosed with OPC of the tonsillar region and recruited after therapy (62.68%; Table S1). Our initial studies indicated that it was difficult to detect HPV-specific T cells ex vivo in peripheral blood mononuclear cells (PBMC) from both OPC patients and healthy volunteers (data not shown). To enhance the sensitivity of detection of HPV-specific T cells, PBMC from OPC patients and healthy volunteers were stimulated with overlapping peptide pools (OPPs) from HPV-encoded antigens E1, E2, E4, E5, E6, E7, and L1 and cultured for 14 d in the presence of IL-2. HPV antigen–specific reactivity of these in vitro–expanded T cells was then assessed by intracellular cytokine staining (ICS). A representative flow cytometric analysis showing frequency of IFN-γ–positive CD8+ and CD4+ T cells after recall with either E1, E2, E4, E5, E6, E7, or L1 or left unstimulated from an OPC patient (RB-012) is shown in Fig. 1, A and B. This analysis showed that HPV-specific CD8+ and CD4+ T cell responses (≥1% above the no-peptide control) were detected in the majority of OPC patients (43 of 66) in our cohort (Fig. 2, A and B). In contrast, HPV-specific T cell responses were less prominent and detected at a lower frequency in healthy volunteers (3 of 22). T cells from OPC patients not only reacted against E6 and E7 but also consistently displayed comparable reactivity against E1, E2, E4, E5, and L1 proteins.
Figure 1.
Representative FACS plots showing HPV-specific CD8+ and CD4+ T cell reactivity in T cells expanded with HPV-16 PepMix. PBMC from an OPC patient (RB-012) stimulated with OPPs of HPV-encoded antigens E1, E2, E4, E5, E6, E7, and L1 and cultured for 14 d in the presence of recombinant IL-2. At day 14 of the culture, the cells either were restimulated with HPV-16 E1, E2, E4, E5, E6, E7, and L1 OPPs or were left unstimulated (no peptide), and 4 h later, IFN-γ expression was assessed by performing an ICS flow cytometry assay. (A and B) Each plot shows viable CD8+ (A) and CD4+ (B) T cells expressing IFN-γ following stimulation with HPV-16 antigen OPPs The number inside the box indicates the percentage of IFN-γ–positive CD8+ or CD4+ T cells.
Figure 2.
Proteome-wide profiling of HPV-16–specific T cell responses in the peripheral blood of OPC patients and healthy volunteers. PBMC from OPC patients (n = 66) and healthy volunteers (n = 22) were stimulated with OPPs covering the entire sequence of HPV-encoded antigens E1, E2, E4, E5, E6, E7, and L1; cells were cultured in the presence of IL-2. On day 14, these cells were restimulated with individual antigen OPPs, and antigen-specific reactivity was assessed by intracellular IFN-γ FACS staining. (A and B) Box-and-whisker plots show HPV-16–specific CD8+ T cell (A) and CD4+ T cell (B) reactivity in OPC patients and healthy volunteers. Each patient’s sample screening was repeated a minimum of three times independently. (C) OPC patients showing CD8+ and CD4+ T cell responses ≥1% IFN-γ+ (n = 43) were analyzed using a response matrix, which was memo sorted and displayed as response plots. Each row of the response plot shows the presence or absence of a CD4+ or CD8+ T cell response to an individual HPV antigen, and each column shows the response for an individual patient. The bar graph on top shows the number of antigen-specific responses observed for each patient. The percentage adjacent to each row shows the antigen-specific T cell response frequency among all OPC patients recruited in the study (n = 66). The bar graph on the right side shows the number of patients responding to each antigen. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; ns, not significant; two-tailed t test with Welch’s correction.
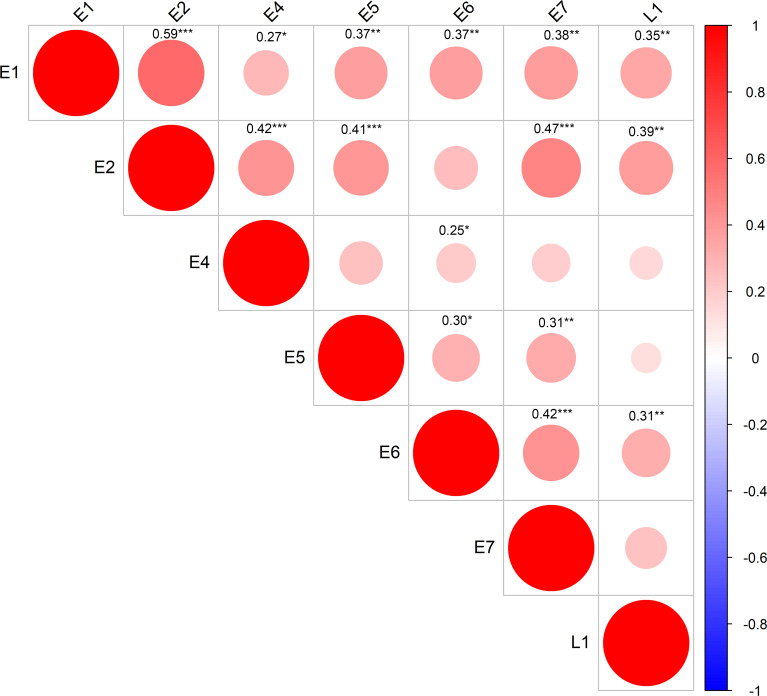
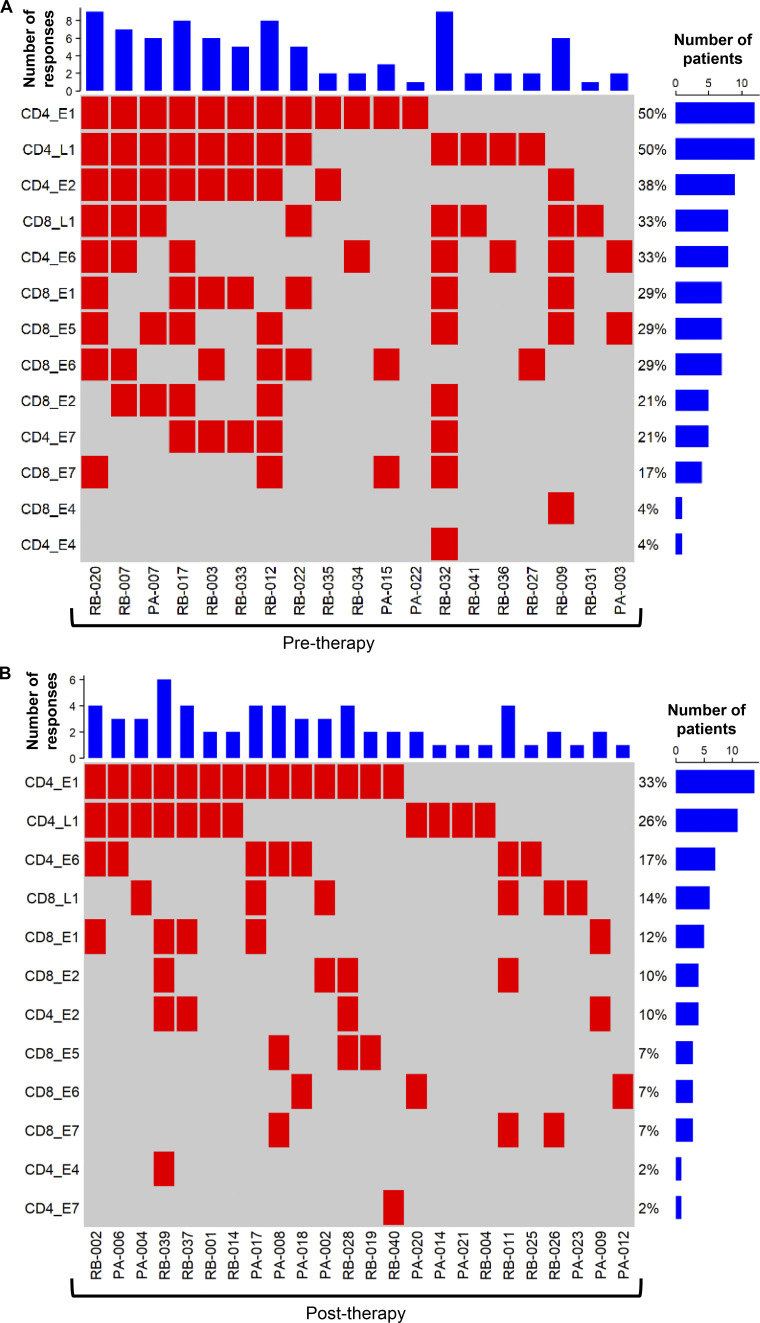
In-depth analysis of these responses emphasized the recognition of multiple antigens by patients, with 30% of patients having T cell responses against three or more HPV antigens (Fig. 2 C). Furthermore, when CD4+ and CD8+ T cell responses against each antigen were tallied, two patients (RB-020 and RB-032) had nine discrete T cell responses. Although the predominant targets were E1 (39% of patients) and L1 (35% of patients) for CD4+ T cells and L1 (21% of patients), E5 (15% of patients), and E6 (15% of patients) for CD8+ T cells, all antigens were recognized by CD4+ and CD8+ T cells from at least one patient, excluding CD4+ T cell recognition of E5 (Fig. 2 C). This demonstrates the diversity of the HPV-specific T cell response in OPC patients. Correlative analysis showed significant corecognition of antigens, including a correlation of E1 recognition with all other antigens (P < 0.05). E2-specific responses showed a positive correlation with all other antigens except E6; E6-specific responses with all other antigens except E2; and L1-specific responses with E1, E2, and E6 (Fig. 3).
Figure 3.
Correlations between individual HPV antigen–specific T cell responses across OPC patients. Patient CD8+ and CD4+ T cell responses with reactivity ≥1% IFN-γ+ (n = 43 patients) were analyzed using a Pearson correlation matrix. This analysis derived correlations between the T cell responses to different HPV antigens, with the size and color of the circles corresponding to the correlation coefficients. Only significant correlations are shown (confidence = 0.95). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
We extended our T cell reactivity analysis to map HLA class I– and class II–restricted T cell epitopes within HPV-encoded antigens using standard two-dimensional peptide matrices. Data from these assays were then used to precisely map T cell epitopes using N-terminal and C-terminal trimming. A representative analysis is shown in Fig. S1 and Fig. S2. HLA restriction of these epitopes was determined by assessing T cell reactivity against peptide-loaded, HLA-matched and -mismatched lymphoblastoid cell lines. Table 1 and Table 2 contain comprehensive lists of CD8+ and CD4+ HPV T cell epitopes and their HLA restrictions.
Figure S1.
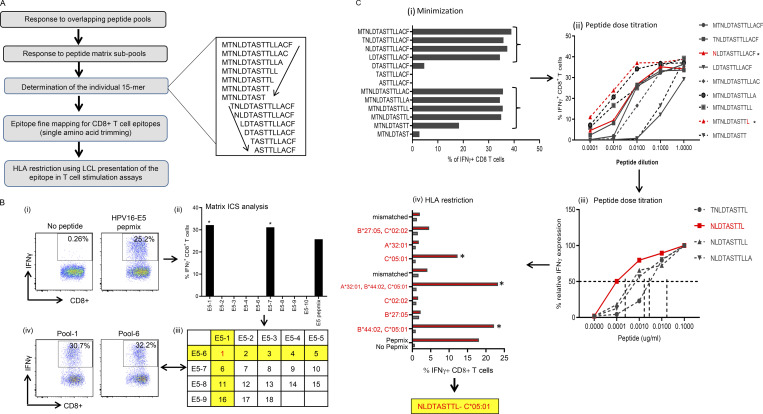
Mapping of HPV-16 epitopes recognized by CD8+ T cells from OPC patients. (A) Overview of the process for mapping HPV epitopes recognized by T cells from OPC patients. An example of the trimming of a 15–amino acid sequence to define the minimal T cell epitope is shown to the right of the flowchart. The amino acid sequence was trimmed from the C-terminal and N-terminal ends, as indicated by the arrows. (B) Representative data showing the identification of a T cell determinant. (i) T cell cultures were generated by stimulating PBMC with OPPs from HPV-16 antigens. After 14 d in culture, the T cells were restimulated with OPPs from individual antigens, and intracellular IFN-γ production was analyzed by flow cytometry. Reactive T cells were further analyzed to determine the cognate peptide. (ii) Subpools of peptides were made for each antigen (nine subpools for E5 are pictured) and used in an IFN-γ ICS assay. The T cell response against each subpool is shown in the bar graph. (iii) The responses against each subpool were overlaid on a two-dimensional matrix, which shows the common individual peptides among the pools. The positive responses against pools 1 and 6 are highlighted in yellow, showing the common peptide 1 in red (row and column highlighted in yellow; common peptides in red text). (iv) Flow cytometric plots showing the T cell responses against subpools 1 and 6, assessed by intracellular IFN-γ staining. (C) Fine epitope mapping and HLA restriction. (i) Minimization of the active epitope within the 15–amino acid sequences derived from the peptide matrix analysis. T cell cultures responding to peptide 1 from the E5 subpools were tested against a range of shorter peptides within the peptide 1 sequence. The shorter peptides were synthesized by sequentially trimming one amino acid from the N-terminus and C–terminus down to a nine–amino acid peptide. T cell cultures were assayed for the expression of IFN-γ upon stimulation with each of the trimmed peptides at a concentration of 1 µg/ml for 45 h. The figure shows the CD8+ T cell response for each trimmed peptide from E5 subpool 1 (MTNLDTASTTLLACF). The peptides that stimulated a T cell response, denoted by brackets, were further analyzed in a dose titration assay. (ii) Peptide dose titration assay. T cell cultures were stimulated with serial decimal dilutions of selected minimized peptide sequences to determine the exact epitope sequence within the longer peptides. A representative example of the titration assay is shown; in this example, NLDTASTTL is the likely minimal epitope sequence. (iii) Modified peptide sequences were tested in a dose titration assay to determine if the addition of extra amino acids at the N- or C-terminus could enhance peptide specificity. The example pictured demonstrates that NLDTASTTL is the likely minimal epitope sequence targeted by HPV-16–E5–specific CD8+ T cells from this patient. (iv) Representative HLA class I restriction analysis for epitopes mapped from HPV antigens. A panel of lymphoblastoid cell lines with one HLA allele matched to the patient were loaded with each individual peptide for 1 h before coincubation with T cells for 4 h. Responses were assessed by intracellular IFN-γ staining. The OPC patients whose T cells responded to NLDTASTTL had a class I HLA type of A*32:01, B*27:05, B*44:02, C*02:02, or C*05:01. In this example, the strongest response was elicited against antigen-presenting cells expressing HLA-C*05:01; therefore, this was determined to be the HLA restriction of NLDTASTTL. Asterisk indicates positive response.
Figure S2.
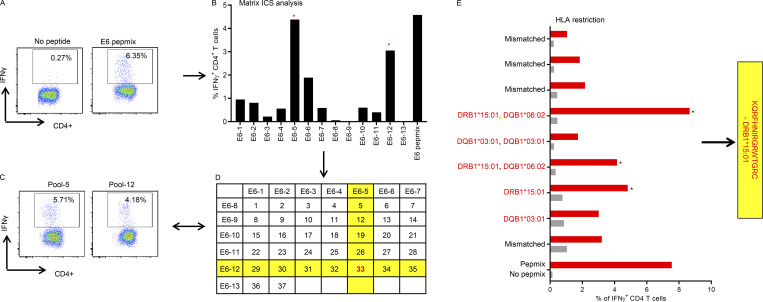
HPV-16–specific CD4+ T cell epitope mapping in OPC patients. Representative data showing the identification of a CD4+ T cell epitope. (A) T cell cultures were generated by stimulating PBMC with OPPs from HPV-16 antigens. After 14 d in culture, T cells were restimulated with OPPs from individual antigens, and intracellular IFN-γ production was analyzed by flow cytometry. Reactive T cells were further analyzed to determine the cognate peptide. (B) Subpools of peptides were made for each antigen (13 subpools for E6 are pictured) and used in an intracellular IFN-γ assay. The T cell response against each subpool is shown in the bar graph. The CD4+ T cell responses against subpools 5 and 12 were selected as a positive response. (C) Flow cytometric plots showing the CD4+ T cell responses against subpools 5 and 12, assessed by intracellular IFN-γ staining. (D) The responses against each subpool were overlaid on a two-dimensional matrix, which shows the common individual peptides among the pools. The positive responses against subpools 5 and 12 are highlighted in yellow, showing the common peptide 33 in red. This peptide, KQRFHNIRGRWTGRC, was synthesized and further characterized. (E) Representative HLA class II restriction analysis for CD4+ epitopes mapped from HPV antigens. A panel of lymphoblastoid cell lines with one HLA allele matching to the patient were loaded with each individual peptide for 1 h before coincubation with T cells for 4 h. The OPC patients whose T cells responded to KQRFHNIRGRWTGRC had a class II HLA type of DRB1*08:10, DRB1*15:01, DQB1*06:02, or DQB1*03:01. In this example, the strongest response was elicited against antigen-presenting cells expressing HLA-DRB1*15:01; therefore, this was determined to be the HLA restriction of KQRFHNIRGRWTGRC. Asterisk indicates positive response.
Table 1. Mapped HPV-16 CD8+ T cell epitopes.
| Antigen | Epitope | HLA restriction | Number of +ve patients | Number of patients with HLA |
|---|---|---|---|---|
| HPV-16-E1 | FELSQMVQW | B*18:01 | 1 | 5 |
| B*44:02 | 1 | 24 | ||
| TLLQQYCLYLa | A*02:01 | 2 | 34 | |
| SEIAYKYAQL | B*18:01 | 1 | 5 | |
| B*44:02 | 1 | 24 | ||
| RPFKSNKST | B*07:02 | 1 | 17 | |
| ALDGNLVSMDV | A*02:01 | 1 | 34 | |
| YLHNRLVVF | B*08:01 | 2 | 14 | |
| SRWPYLHNR | B*27:05 | 1 | 6 | |
| HPV-16-E2 | TLQDVSLEVYLa | A*02:01 | 3 | 34 |
| YICEEASVTVVa | A*02:01 | 1 | 34 | |
| NKVWEVHAGGQVILC | A*01:01 | 2 | 24 | |
| HRDSVDSAPI | B*27:05 | 3 | 6 | |
| YVHEGIRTYa | B*35:01 | 1 | 6 | |
| QVDYYGLYY | A*01:01 | 1 | 24 | |
| HPAATHTKAVa | B*07:02 | 2 | 17 | |
| HPV-16-E4 | WPTTPPRPI | B*07:02 | 1 | 17 |
| HPV-16-E5 | NLDTASTTL | C*05:01 | 5 | 21 |
| NLDTASTTL | C*08:02 | 2 | 3 | |
| SAFRCFIVYa | B*35:01 | 1 | 6 | |
| B*35:43 | 1 | 1 | ||
| HPV-16-E6 | HDIILECVYa | B*18:01 | 2 | 5 |
| TIHDIILECVa | A*02:01 | 1 | 34 | |
| AFRDLCIVYa | C*07:02 | 3 | 19 | |
| GRWTGRCMSCa | B*27:05 | 4 | 6 | |
| HPV-16-E7 | LEDLLMGTLGIa | C*04:01 | 2 | 9 |
| HVDIRTLEDLLMGTLa | A*02:01 | 1 | 34 |
Previously published epitope sequences.
Table 2. Mapped HPV-16 CD4+ T cell epitopes.
| Antigen | Epitope | HLA restriction | Number of patients |
|---|---|---|---|
| HPV-16-E1 | VSFSELVRPFKSNKSTCCD | DRB1*15:01 | 1 |
| FLRYQGVEFMSFLTALKRF | Not defined | 1 | |
| AAMLAKFKELYGVSFSELV | Not defined | 1 | |
| PSIADSIKTLLQQYC | Not defined | 1 | |
| GWFYVEAVVEKKTGD | Not defined | 1 | |
| CTFELSQMVQWAYDN | Not defined | 1 | |
| AKFKELYGVSFSELVRPFK | DRB1*07:01 | 1 | |
| YDNDIVDDSEIAYKYAQLA | Not defined | 1 | |
| QGIPKKNCILLYGAANTGK | Not defined | 1 | |
| LFGMSLMKFLQGSVICFVN | Not defined | 1 | |
| QGVEFMSFLTALKKRF | DRB1*09:01 | 1 | |
| LFGMSLMKFLQGSVICFVN | Not defined | 1 | |
| WKSFFSRTWSRLSLH | DRB1*04:01 | 3 | |
| FMSFLTALKRFLQGIPKKN | DRB1*11:04, DRB*04:01 | 2 | |
| VEAVVEKKTGDAISDDENE | ND | 1 | |
| HPV-16-E2 | SVDSAPILTAFNSSHa | DQA1*05:01–DQB1*03:01 | 1 |
| LRYRFKKHCTLYYTAVa | DRB1*15:01 | 2 | |
| HIDYWKHMRLECALYa | DRB1*13:02 | 2 | |
| NVCQDKILTHYENDSTDLRa | Not defined | 1 | |
| QVVPTLAVSKNKALQAIELa | Not defined | 1 | |
| VSLEVYLTAPTGCIKKHGYa | Not defined | 1 | |
| HPV-16-E6 | KQRFHNIRGRWTGRCMSCCa | DRB1*15:01/DQB1*03:01 | 3 |
| EKQRHLDKKQRFHNIRGRWa | DRB1*13:02/DQR1*13:03 | 2 | |
| VYDFAFRDLCIVYRDa | Not defined | 1 | |
| AFRDLCIVYRDGNPYAVCDa | Not defined | 2 | |
| VCDKCLKFYSKISEYRHYCa | Not defined | 1 | |
| HPV-16-E7 | VQSTHVDIRTLEDLLMGTLa | DQA1*05:01–DQB1*03:01 | 2 |
| YEQLNDSSEEEDEIDa | DQB1*06:09 | 1 | |
| TPTLHEYMLDLQPETTDLYa | Not defined | 2 |
Previously published epitope sequences.
Chemoradiotherapy treatment impacts on HPV-specific T cell responses
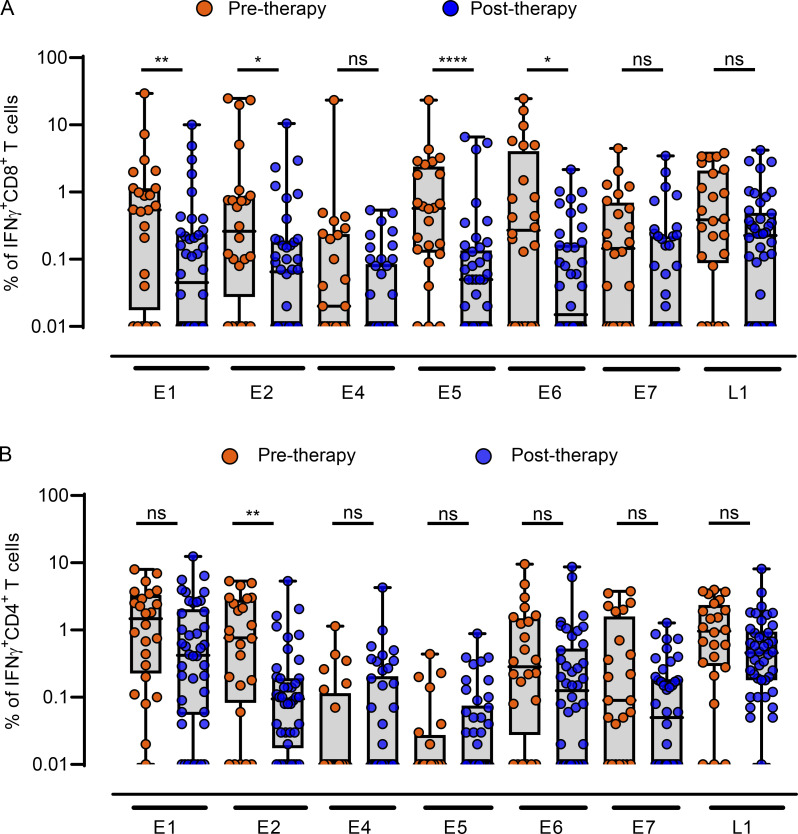
Current curative therapeutic options for OPC incorporate concurrent cisplatin and/or cetuximab with radiotherapy (Table S1). To assess the impact of curative treatment on HPV-specific T cell immunity, patients were stratified into two groups based on treatment stage (pretreatment, n = 24; post-treatment, n = 42). Interestingly, following treatment, many patients displayed a decline in HPV-specific CD8+ T cell reactivity, characterized by a significant reduction in the frequency of E1-, E2-, E5-, and E6-specific CD8+ T cells (Fig. 4 A). In contrast, in the CD4+ T cell compartment, only E2-specific responses were significantly reduced after therapy (Fig. 4 B). In-depth analysis of T cell responses above the 1% reactivity cutoff further emphasized changes in the diversity of the HPV-specific T cell response following curative therapy (Fig. 5). In this analysis, a CD4+ or CD8+ T cell response to an individual antigen was classified as a “reactivity.” Patients in the pretreatment group had up to nine reactivities, with a median of two. In contrast, the post-therapy group had up to six reactivities, with a median of one. Although the predominance of CD4+ T cell responses to E1 and L1 was evident in both patient cohorts, the proportion of responding patients decreased after treatment (E1, 50% to 33%; L1, 50% to 26%; Fig. 5, A and B). CD8+ T cell responses displayed a more dramatic reduction following treatment, with at least a 50% reduction in the proportion of patients responding to each HPV antigen (Fig. 5, A and B).
Figure 4.
HPV-16 antigen–specific T cell reactivity in PBMC from OPC patients before and after treatment. (A and B) Box-and-whisker plots showing CD8+ (A) and CD4+ (B) T cell reactivity against individual HPV-16 antigens in OPC patients before therapy (n = 24) and after therapy (n = 42). *, P < 0.05; **, P < 0.01; ****, P < 0.0001, ns, not significant; two-tailed t test with Welch’s correction was performed.
Figure 5.
Profile of HPV-specific T cell reactivity in OPC patients and the impact of curative treatment. CD8+ and CD4+ T cell responses against individual HPV antigens ≥1% IFN-γ+ were identified in 43 OPC patients, and a response matrix was derived. (A and B) The response matrix was memo sorted and plotted as response plots for patients assessed before therapy (A; n = 19) and after therapy (B; n = 24). In the response plots, each row shows the presence or absence of a CD4+ or CD8+ T cell response to an individual HPV antigen, and each column shows the response for an individual patient. The bar graph on top shows the number of antigen-specific responses observed for each patient. The percentage adjacent to each row shows the antigen-specific response frequency in the pretherapy (n = 24) and post-therapy (n = 42) patient cohorts. The bar graph on the right side shows the number of patients responding to each antigen.
Impact of OPC tumor staging and smoking history on HPV-specific T cell responses
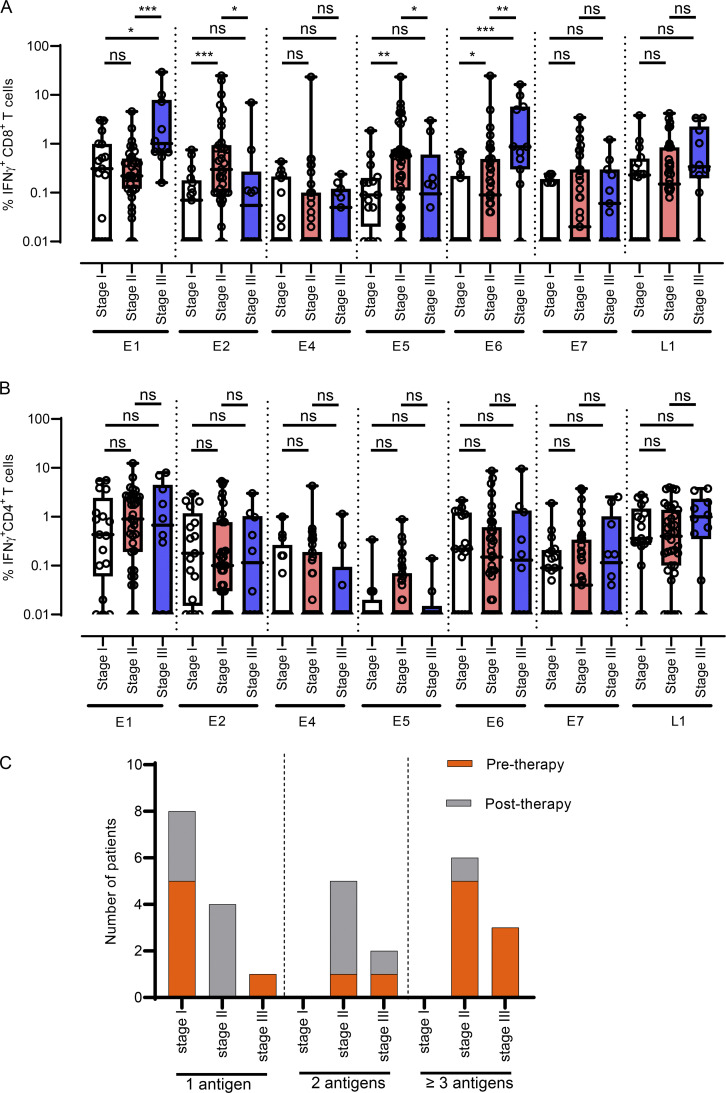
The American Joint Committee on Cancer (AJCC) tumor, node, metastasis (TNM) staging (eighth edition) is employed for categorizing OPC tumors to assist with the assessment of disease status, prognosis, and management. To explore the possibility that TNM staging at diagnosis may impact HPV-specific T cell immunity, we compared the HPV-specific CD4+ and CD8+ T cell responses of OPC patients whose tumors were classified as stage I (n = 17), stage II (n = 35), or stage III (n = 10). This revealed that HPV-specific CD8+ T cell responses directed toward E1 and E6 were significantly enhanced in patients with stage III tumors when compared with patients with stage I or II classifications (Fig. 6 A). In contrast, HPV-specific CD8+ T cell responses against E2 and E5 were significantly higher in patients with stage II tumors than in those with stage I tumors (Fig. 6 A). Patients diagnosed with different stages of OPC showed comparable levels of CD8+ T cells specific for E4, E7, and L1, whereas CD4+ T cell responses did not differ on the basis of tumor staging (Fig. 6 B). We also noted that only patients with stage II or III OPC had T cell responses against three or more antigens, whereas patients diagnosed with stage I disease only showed responses against a single antigen (Fig. 6 C).
Figure 6.
Impact of tumor staging at diagnosis on HPV-16–specific T cell reactivity in OPC patients. (A and B) Box-and-whisker plots showing IFN-γ+ CD8+ (A) and IFN-γ+ CD4+ (B) responses against individual HPV-16 antigens in OPC patients with stage I disease (n = 17), stage II disease (n = 35), and stage III disease (n = 10). (C) OPC patients were divided into groups based on their CD8+ or CD4+ response against one, two, and three or more HPV-16 antigens. A cutoff of 1% IFN-γ+ was used for this analysis, and patients were grouped according to their pretherapy or post-therapy status at the time of blood collection. *, P < 0.05; **, P < 0.01; ***, P < 0.001, ns, not significant. The Mann–Whitney U test was performed to calculate significance.
Although previous studies had recognized smoking as one of the major risk factors for high-grade HPV-associated cervical lesions (Fang et al., 2018; Mzarico et al., 2015) and OPC (Du et al., 2019), this increased risk has been proposed to be linked to poor immune control. Furthermore, it is also possible that smoking history along with disease burden and treatment status may also have an impact on the HPV-specific T cell immunity. To explore this hypothesis, we performed a multivariate analysis to determine the relationship between CD8+ and CD4+ T cell responses against individual HPV antigens and smoking history, treatment status, and tumor stage. Table 3 lists the most significant individual associations, and Table 4 shows combined associations. Overall, treatment status had the most significant impact on T cell response, followed by tumor stage and smoking history. In particular, treatment status showed a significant association with CD8+ T cell responses directed against E6 (P = 0.0164) and E1 (P = 0.0106) and CD4+ T cell responses against E2 (P = 0.007) and E7 (P = 0.0043). A combination of these factors—in particular, patients who were untreated (pretherapy), with a smoking history, and stage II or III disease—had a significant impact on CD4+ T cell responses against E2 (P = 0.031) and E7 (P = 0.0362).
Table 3. Association of CD8+ and CD4+ T cell responses with smoking history, treatment status, and tumor stage in multivariate linear regression analysis.
| Responding T cell subset | HPV antigen | Smoking history | Treatment status | Tumor stage |
|---|---|---|---|---|
| CD8+ | E1 | NS | 0.0106 | NS |
| E2 | NS | 0.0797 | NS | |
| E5 | 0.0897 | NS | NS | |
| E6 | NS | 0.0164 | 0.044 | |
| E7 | NS | NS | NS | |
| L1 | NS | 0.053 | NS | |
| CD4+ | E1 | NS | NS | NS |
| E2 | NS | 0.007 | NS | |
| E4 | NS | NS | NS | |
| E7 | NS | 0.0043 | NS |
NS, P > 0.1.
Table 4. Multivariate analysis of association of antigen-specific CD8+ and CD4+ T cell responses with smoking history, treatment status, and tumor stage.
| Responding cell | T antigen | Smoking history | Treatment status | Tumor stage | Prediction ability (multivariate P value) |
|---|---|---|---|---|---|
| CD8 | L1 | Smoker | Pretherapy | 0.0915 | |
| CD4 | E2 | Smoker | Pretherapy | II | 0.0301 |
| E7 | Pretherapy | III | 0.0515 | ||
| E7 | Smoker | Pretherapy | II | 0.0362 |
Only significant associations (P < 0.1) are shown.
Discussion
Previous studies have shown that HPV status of OPC patients is an independent prognostic factor for improved progression-free and overall survival (Ang et al., 2010). Concurrent cisplatin and/or cetuximab with radiotherapy is a curative therapeutic option for OPC; however, multimodality treatment often drastically impacts the patient’s quality of life (Xie et al., 2017). HPV-associated malignancies are an ideal target for adoptive T cell therapy because they express “foreign” viral antigens, and it is known that T cells play a vital role in suppressing and eliminating HPV-infected cells (Senba and Mori, 2012). Adoptive T cell therapy may provide a better-tolerated alternative to chemoradiotherapy in this setting. As yet, the majority of studies investigating HPV-specific T cell responses in HNSCC patients have focused on the E6 and E7 antigens (Masterson et al., 2016; Sirianni et al., 2004; Spanos et al., 2009; Wansom et al., 2010). However, recent reports have confirmed that the majority of HPV-positive HNSCC tumors contain HPV-16 in an episomal form (Arias-Pulido et al., 2006; Morgan et al., 2017), suggesting that there might be an opportunity to target other antigens with adoptive T cell therapy. Indeed, previous studies have shown that HNSCC tumors with the episomal form of the virus often express high levels of E2 (Anayannis et al., 2018). Furthermore, Anderson and colleagues demonstrated a strong serological response against HPV-16–encoded E1, E2, E4, E5, E6, and E7 in OPC patients (Anderson et al., 2015). In the present study, we have validated these observations, demonstrating that OPC patients, particularly those with advanced disease, generate T cell responses against the full array of HPV antigens.
To delineate the dynamics of HPV-specific T cell–mediated immune regulation, we recruited 66 OPC patients and 22 healthy volunteers and assessed proteome-wide CD8+ and CD4+ T cell responses against HPV. Although most of the healthy volunteers showed low or undetectable HPV-specific T cell responses, more than 60% of the OPC patients showed detectable HPV-specific T cell reactivity. We detected both CD4+ and CD8+ T cell responses against all of the antigens tested, excluding CD4+ T cell responses against E5. This lack of E5-specific CD4+ T cell reactivity is consistent with previous observations demonstrating that the lack of an E5-specific CD4+ T cell response corresponds with increasing grade of neoplasia (Gill et al., 1998). This analysis also allowed us to map a large number of novel HLA class I– and class II–restricted CD8+ and CD4+ T cell epitopes for further use in novel immunotherapeutic approaches to treating HPV-positive OPC. It is important to mention here that our T cell profiling was focused primarily on HPV-16, and it is possible that we may have missed some of the T cell responses to other high-risk HPV types which are also associated with OPC. However, we point out that we did assess the HPV-18 antigen-specific T cell response in the same cohort, and only two patients showed HPV-18–specific T cell reactivity, and one patient had a positive test result for both HPV-16 and HPV-18 (data not shown).
Given the heterogeneity of T cell responses to HPV-16–encoded proteins in OPC patients, we further evaluated the potential impact of clinical and pathological parameters on HPV-specific T cell immunity. We observed broader and higher-magnitude HPV-specific T cell responses in blood samples collected from patients before the initiation of curative therapy. A significant drop in CD8+ T cell reactivity was observed in PBMC collected after the completion of curative therapy. Although the precise reason for this is unknown, it is possible that immunosuppressive effects of chemoradiotherapy may impair HPV-specific T cell immunity (Parikh et al., 2014). It is also possible that the resolution of active disease following therapy reduces the potential source of antigen required to maintain HPV-specific T cell immunity. Our observations align with those of a previous study by van Meir et al. (2016) and colleagues, who reported a significant reduction in HPV-specific CD4+ and CD8+ T cell responses directed against E6 and E7 antigens after therapy, with altered CD4/CD8 ratios. Furthermore, a previous study has shown that an increase in myeloid-derived suppressor cells and altered CD8+/T regulatory cell ratios in patients after chemoradiotherapy can antagonize HPV-specific T cell immunity (Parikh et al., 2014).
We further extended our analysis to explore the potential impact of tumor burden and smoking history on HPV-specific T cell reactivity. Although HPV-specific CD4+ T cell responses were largely unaffected by disease burden, CD8+ T cell responses directed against each of the HPV antigens showed distinct differences in relation to OPC staging. Patients diagnosed with stage II or III OPC showed significantly larger CD8+ T cell responses to E1, E2, E5, and E6 antigens than did patients with stage I disease. However, subsequent multivariate analysis revealed that disease stage only impacted CD8+ T cell responses directed toward E6, whereas treatment status was more definitively associated with changes in the responses to multiple antigens. Nevertheless, patients with stage II or III disease were more likely to generate T cell responses against multiple antigens. This selective impact on T cell responses in the context of disease burden was unexpected, and it is difficult to delineate the precise underlying reason. It is possible that the differential expression of HPV antigens in the advanced-stage disease setting (potentially due to the presence of the episomal HPV genome) may drive the differential expansion of CD8+ T cells directed toward these antigens. A more in-depth analysis of HPV protein expression in OPC should provide further insight into T cell response dynamics in OPC patients. Smoking history has been recognized as a risk factor for supporting HPV prevalence and enhancing the risk of high-grade cervical lesions (Fang et al., 2018; Mzarico et al., 2015). This increased risk has been assumed to be linked to poor immune control. Surprisingly, our multivariate analysis revealed only minimal differences in T cell responses to HPV in OPC patients with a smoking history, suggesting that smoking does not have a dramatic impact on HPV-specific T cell immunity in OPC patients.
Taken together, this study provides comprehensive profiling of HPV-specific T cell immunity in OPC patients. Data presented here open new opportunities for the development of effective T cell therapies for the treatment of OPC. Until now, much of the focus of T cell–based therapeutics has been on targeting E6 and E7. We propose that there is an urgent need to revisit the design strategy for these cellular therapies. The detection of T cell reactivity against E1, E2, E4, and E5 in OPC patients, especially those with advanced stage disease, clearly demonstrates that targeting a broader array of antigens may provide better therapeutic outcomes. It also remains to be determined if T cells directed to E1, E2, E4, and E5 antigens can infiltrate the OPC tumor microenvironment. The data presented here strongly support the further investigation of HPV-specific T cell responses in other HPV-associated cancers. This knowledge should be exploited to develop autologous and allogeneic (“off-the-shelf”) T cell therapies to enhance current chemoradiotherapy treatment strategies.
Materials and methods
Characteristics of patients and healthy volunteers
This study was performed according to the principles of the Declaration of Helsinki. It was approved by the QIMR Berghofer Medical Research Institute Human Research Ethics Committee and the Metro South Hospital and Health Service Human Research Ethics Committee. All participants in the study provided written informed consent. 67 patients diagnosed with HPV-associated OPC were recruited from Princess Alexandra Hospital (n = 23) and Royal Brisbane and Women’s Hospital (n = 43). These patients were recruited before treatment, during treatment, or after the completion of definitive radiation therapy and/or systemic therapy. All nonconsecutively recruited patients were diagnosed with either an incisional or excisional biopsy and/or fine-needle aspiration, and p16 immunohistochemistry was performed on the tumor. Patients were considered p16 positive if >70% of tumor cells stained positive for p16. Patient demographics, tumor location, AJCC TNM staging (eighth edition), treatment received, and disease status are presented in Table S1.
PBMC isolation
Freshly drawn blood samples from OPC patients and healthy donors were collected in 10-ml BD Vacutainer tubes with K2 EDTA (BD Diagnostics). PBMC were isolated in a 50-ml SepMate tube (StemCell Technologies) according to the manufacturer’s instructions within 2 h of blood collection. After cell counting, PBMC were cryopreserved at 1 × 107 cells per 1 ml and placed inside a Nalgene Mr. Frosty Cryo 1°C Freezing Container (Thermo Fisher Scientific) at −80°C for 48 h, and the cells were later transferred to liquid nitrogen until further experiments were performed.
In vitro expansion of HPV-specific T cell lines
PBMC from patients and healthy donors were stimulated with HPV-16 OPPs from E1, E2, E4, E5, E6, E7, and L1 proteins (JPT Peptide Technologies GmbH) at a concentration of 1 µg/ml per peptide and incubated at 37°C in 6.5% CO2 for 1 h. The cells were then washed and cultured for 14 d in 24-well plates at 37°C in 6.5% CO2. These cultures were supplemented with RPMI-1640 medium (Life Technologies) containing 10% fetal bovine serum. Recombinant IL-2 (Komtur Pharmaceuticals) at 200 IU/ml was included from day 2, and medium was changed every 3 d thereafter until day 14. On day 14, a portion of the T cells was counted using trypan blue exclusion and analyzed by IFN-γ ICS following recall with individual antigens. The remaining cells were cryopreserved in culture medium containing 10% dimethyl sulfoxide (Sigma-Aldrich).
IFN-γ ICS
Cultured T cells (2 × 105) were incubated for 4 h at 37°C in 6.5% CO2 with HPV OPPs, peptide matrices, minimization peptides, or defined peptide epitopes in the presence of GolgiPlug (BD PharMingen). After incubation, the T cells were washed and labeled with anti–CD4-FITC (clone RPA-T4; BD Biosciences) and anti–CD8-PerCP-Cy5.5 (clone RPA-T8; eBioscience) antibodies and LIVE/DEAD Fixable Near-IR Dead Cell Stain (Life Technologies). Cytofix/Cytoperm solution (BD PharMingen) was used to fix and permeabilize the T cells before washing and incubation with anti–IFN-γ–PE antibody (clone B27; BD Biosciences). The T cells were washed and resuspended in phosphate-buffered saline containing 1% paraformaldehyde and acquired using a BD LSRFortessa flow cytometer (BD Biosciences). For each sample screening or minimization or all other ICS assays, an internal positive control in which T cells are stimulated with a polyclonal stimulator (PMA/ionomycin) and also appropriate negative controls (no peptide) were included. Postacquisition analysis was conducted using FlowJo software (FlowJo LLC). Antigen-specific IFN-γ+ CD4+ and CD8+ responses were calculated by subtracting the proportion of IFN-γ+ cells in the no-peptide control from each peptide-stimulated response; ≥1% IFN-γ+ was considered an antigen-specific response.
Statistical analysis
All statistical analyses were performed using Prism version 8.2.1 software (GraphPad Software). Flow cytometric data were summarized as percentages and presented as mean ± SEM. An unpaired t test with Welch’s correction was used for all categorical variable analyses. In addition, a response matrix was derived by binning the CD8+ and CD4+ T cell responses to HPV-16 antigens on the basis of T cell reactivity of ≥1% IFN-γ+ (considered a positive response) or <1% IFN-γ+ (considered a negative response). The response matrix was then memo sorted and plotted as response plots. Memo sorting arranged the HPV antigen (E1, E2, E4, E5, E6, E7, and L1) based on the number of patients who had a T cell response against each antigen. It also ordered the patients based on the number of antigens to which their T cells responded. The response matrix was used to derive Pearson’s correlations for the antigen-specific T cell responses across patients. A significance level of P ≤ 0.05 was considered statistically significant. The Pearson values were calculated by pooling together CD8+ and CD4+ T cell responses for the patient; statistical significance was computed using “corrplot” and “chart.correlation.”
Online supplemental material
Fig. S1 includes a brief outline of mapping of HPV-16 epitopes recognized by CD8+ T cells from OPC patients. Fig. S2 provides an outline for mapping HPV-16–specific CD4+ T cell epitopes in OPC patients. Table S1 includes demographic and clinical profiles of OPC patients recruited in this study.
Supplementary Material
includes demographic and clinical characteristics of patients with HNSCC and healthy volunteers
Acknowledgments
This study was supported through research and development funding provided by Atara Biotherapeutics.
Author contributions: R. Khanna, K.H. Bhatt, S. Porceddu, and C. Smith designed this study. K.H. Bhatt, R. Khanna, S. Porceddu, C. Smith, M.A. Neller, S. Srihari, B.T. Aftab, H. Liu, and L. Kenny contributed to the drafting of the manuscript and/or conducted data analysis and various experiments. P. Crooks and L. Lekieffre conducted experimental studies and also processed patient blood samples. M.A. Neller, S. Porceddu, R. Khanna, and L. Kenny contributed to the clinical study protocol drafting. S. Porceddu and L. Kenny were responsible for patient recruitment.
References
- Albers A., Abe K., Hunt J., Wang J., Lopez-Albaitero A., Schaefer C., Gooding W., Whiteside T.L., Ferrone S., DeLeo A., et al. . 2005. Antitumor activity of human papillomavirus type 16 E7-specific T cells against virally infected squamous cell carcinoma of the head and neck. Cancer Res. 65:11146–11155. 10.1158/0008-5472.CAN-05-0772 [DOI] [PubMed] [Google Scholar]
- Anayannis N.V., Schlecht N.F., Ben-Dayan M., Smith R.V., Belbin T.J., Ow T.J., Blakaj D.M., Burk R.D., Leonard S.M., Woodman C.B., et al. . 2018. Association of an intact E2 gene with higher HPV viral load, higher viral oncogene expression, and improved clinical outcome in HPV16 positive head and neck squamous cell carcinoma. PLoS One. 13 e0191581 10.1371/journal.pone.0191581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K.S., Gerber J.E., D’Souza G., Pai S.I., Cheng J.N., Alam R., Kesiraju S., Chowell D., Gross N.D., Haddad R., et al. . 2015. Biologic predictors of serologic responses to HPV in oropharyngeal cancer: The HOTSPOT study. Oral Oncol. 51:751–758. 10.1016/j.oraloncology.2015.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang K.K., Harris J., Wheeler R., Weber R., Rosenthal D.I., Nguyen-Tân P.F., Westra W.H., Chung C.H., Jordan R.C., Lu C., et al. . 2010. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 363:24–35. 10.1056/NEJMoa0912217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias-Pulido H., Peyton C.L., Joste N.E., Vargas H., and Wheeler C.M.. 2006. Human papillomavirus type 16 integration in cervical carcinoma in situ and in invasive cervical cancer. J. Clin. Microbiol. 44:1755–1762. 10.1128/JCM.44.5.1755-1762.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi A.K., Engels E.A., Pfeiffer R.M., Hernandez B.Y., Xiao W., Kim E., Jiang B., Goodman M.T., Sibug-Saber M., Cozen W., et al. . 2011. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. 29:4294–4301. 10.1200/JCO.2011.36.4596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi A.K., Anderson W.F., Lortet-Tieulent J., Curado M.P., Ferlay J., Franceschi S., Rosenberg P.S., Bray F., and Gillison M.L.. 2013. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J. Clin. Oncol. 31:4550–4559. 10.1200/JCO.2013.50.3870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Martel C., Georges D., Bray F., Ferlay J., and Clifford G.M.. 2020. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob. Health. 8:e180–e190. 10.1016/S2214-109X(19)30488-7 [DOI] [PubMed] [Google Scholar]
- Du E., Mazul A.L., Farquhar D., Brennan P., Anantharaman D., Abedi-Ardekani B., Weissler M.C., Hayes D.N., Olshan A.F., and Zevallos J.P.. 2019. Long-term survival in head and neck cancer: impact of site, stage, smoking, and human papillomavirus status. Laryngoscope. 129:2506–2513. 10.1002/lary.27807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J.H., Yu X.M., Zhang S.H., and Yang Y.. 2018. Effect of smoking on high-grade cervical cancer in women on the basis of human papillomavirus infection studies. J. Cancer Res. Ther. 14(8, Supplement):S184–S189. 10.4103/0973-1482.179190 [DOI] [PubMed] [Google Scholar]
- Gill D.K., Bible J.M., Biswas C., Kell B., Best J.M., Punchard N.A., and Cason J.. 1998. Proliferative T-cell responses to human papillomavirus type 16 E5 are decreased amongst women with high-grade neoplasia. J. Gen. Virol. 79:1971–1976. 10.1099/0022-1317-79-8-1971 [DOI] [PubMed] [Google Scholar]
- Gillison M.L., Chaturvedi A.K., Anderson W.F., and Fakhry C.. 2015. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J. Clin. Oncol. 33:3235–3242. 10.1200/JCO.2015.61.6995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillison M.L., Trotti A.M., Harris J., Eisbruch A., Harari P.M., Adelstein D.J., Jordan R.C.K., Zhao W., Sturgis E.M., Burtness B., et al. . 2019. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet. 393:40–50. 10.1016/S0140-6736(18)32779-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleber-Netto F.O., Rao X., Guo T., Xi Y., Gao M., Shen L., Erikson K., Kalu N.N., Ren S., Xu G., et al. . 2019. Variations in HPV function are associated with survival in squamous cell carcinoma. JCI Insight. 4 e124762 10.1172/jci.insight.124762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.A., Mistry P., Dalby M., Fulton-Lieuw T., Kong A.H., Dunn J., Mehanna H.M., and Gray A.M.. 2020. Concurrent cisplatin or cetuximab with radiotherapy for HPV-positive oropharyngeal cancer: Medical resource use, costs, and quality-adjusted survival from the De-ESCALaTE HPV trial. Eur. J. Cancer. 124:178–185. 10.1016/j.ejca.2019.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreimer A.R., Shiels M.S., Fakhry C., Johansson M., Pawlita M., Brennan P., Hildesheim A., and Waterboer T.. 2018. Screening for human papillomavirus-driven oropharyngeal cancer: Considerations for feasibility and strategies for research. Cancer. 124:1859–1866. 10.1002/cncr.31256 [DOI] [PubMed] [Google Scholar]
- Krishna S., Ulrich P., Wilson E., Parikh F., Narang P., Yang S., Read A.K., Kim-Schulze S., Park J.G., Posner M., et al. . 2018. Human papilloma virus specific immunogenicity and dysfunction of CD8+ T cells in head and neck cancer. Cancer Res. 78:6159–6170. 10.1158/0008-5472.CAN-18-0163 [DOI] [PubMed] [Google Scholar]
- Masterson L., Moualed D., Liu Z.W., Howard J.E., Dwivedi R.C., Tysome J.R., Benson R., Sterling J.C., Sudhoff H., Jani P., et al. . 2014. De-escalation treatment protocols for human papillomavirus-associated oropharyngeal squamous cell carcinoma: a systematic review and meta-analysis of current clinical trials. Eur. J. Cancer. 50:2636–2648. 10.1016/j.ejca.2014.07.001 [DOI] [PubMed] [Google Scholar]
- Masterson L., Lechner M., Loewenbein S., Mohammed H., Davies-Husband C., Fenton T., Sudhoff H., Jani P., Goon P., and Sterling J.. 2016. CD8+ T cell response to human papillomavirus 16 E7 is able to predict survival outcome in oropharyngeal cancer. Eur. J. Cancer. 67:141–151. 10.1016/j.ejca.2016.08.012 [DOI] [PubMed] [Google Scholar]
- Mehanna H., Robinson M., Hartley A., Kong A., Foran B., Fulton-Lieuw T., Dalby M., Mistry P., Sen M., O’Toole L., et al. ; De-ESCALaTE HPV Trial Group . 2019. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet. 393:51–60. 10.1016/S0140-6736(18)32752-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan I.M., DiNardo L.J., and Windle B.. 2017. Integration of human papillomavirus genomes in head and neck cancer: is it time to consider a paradigm shift? Viruses. 9:208 10.3390/v9080208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mzarico E., Gómez-Roig M.D., Guirado L., Lorente N., and Gonzalez-Bosquet E.. 2015. Relationship between smoking, HPV infection, and risk of Cervical cancer. Eur. J. Gynaecol. Oncol. 36:677–680. [PubMed] [Google Scholar]
- Nulton T.J., Olex A.L., Dozmorov M., Morgan I.M., and Windle B.. 2017. Analysis of The Cancer Genome Atlas sequencing data reveals novel properties of the human papillomavirus 16 genome in head and neck squamous cell carcinoma. Oncotarget. 8:17684–17699. 10.18632/oncotarget.15179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh F., Duluc D., Imai N., Clark A., Misiukiewicz K., Bonomi M., Gupta V., Patsias A., Parides M., Demicco E.G., et al. . 2014. Chemoradiotherapy-induced upregulation of PD-1 antagonizes immunity to HPV-related oropharyngeal cancer. Cancer Res. 74:7205–7216. 10.1158/0008-5472.CAN-14-1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senba M., and Mori N.. 2012. Mechanisms of virus immune evasion lead to development from chronic inflammation to cancer formation associated with human papillomavirus infection. Oncol. Rev. 6 e17 10.4081/oncol.2012.e17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirianni N., Ha P.K., Oelke M., Califano J., Gooding W., Westra W., Whiteside T.L., Koch W.M., Schneck J.P., DeLeo A., et al. . 2004. Effect of human papillomavirus-16 infection on CD8+ T-cell recognition of a wild-type sequence p53264-272 peptide in patients with squamous cell carcinoma of the head and neck. Clin. Cancer Res. 10:6929–6937. 10.1158/1078-0432.CCR-04-0672 [DOI] [PubMed] [Google Scholar]
- Spanos W.C., Nowicki P., Lee D.W., Hoover A., Hostager B., Gupta A., Anderson M.E., and Lee J.H.. 2009. Immune response during therapy with cisplatin or radiation for human papillomavirus-related head and neck cancer. Arch. Otolaryngol. Head Neck Surg. 135:1137–1146. 10.1001/archoto.2009.159 [DOI] [PubMed] [Google Scholar]
- van Meir H., Nout R.A., Welters M.J., Loof N.M., de Kam M.L., van Ham J.J., Samuels S., Kenter G.G., Cohen A.F., Melief C.J., et al. . 2016. Impact of (chemo)radiotherapy on immune cell composition and function in cervical cancer patients. OncoImmunology. 6 e1267095 10.1080/2162402X.2016.1267095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wansom D., Light E., Worden F., Prince M., Urba S., Chepeha D.B., Cordell K., Eisbruch A., Taylor J., D’Silva N., et al. . 2010. Correlation of cellular immunity with human papillomavirus 16 status and outcome in patients with advanced oropharyngeal cancer. Arch. Otolaryngol. Head Neck Surg. 136:1267–1273. 10.1001/archoto.2010.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X., O’Neill W., and Pan Q.. 2017. Immunotherapy for head and neck cancer: the future of treatment? Expert Opin. Biol. Ther. 17:701–708. 10.1080/14712598.2017.1315100 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
includes demographic and clinical characteristics of patients with HNSCC and healthy volunteers