Abstract
Introduction
Delayed cerebral ischaemia (DCI) due to cerebral vasospasm (cVS) remains the foremost contributor to morbidity and mortality following aneurysmal subarachnoid haemorrhage (aSAH). Past efforts in preventing and treating DCI have failed to make any significant progress. To date, our most effective treatment involves the use of nimodipine, a calcium channel blocker. Recent studies have suggested that cilostazol, a platelet aggregation inhibitor, may prevent cVS. Thus far, no study has evaluated the effect of cilostazol plus nimodipine on the rate of DCI following aSAH.
Methods and analysis
This is a multicentre, double-blinded, randomised, placebo-controlled superiority trial investigating the effect of cilostazol on DCI. Data concerning rates of DCI, symptomatic and radiographic vasospasm, length of intensive care unit stay, and long-term functional and quality-of-life (QoL) outcomes will be recorded. All data will be collected with the aim of demonstrating that the use of cilostazol plus nimodipine will safely decrease the incidence of DCI, and decrease the rates of both radiographic and symptomatic vasospasm with subsequent improvement in long-term functional and QoL outcomes when compared with nimodipine alone.
Ethics and dissemination
Ethical approval was obtained from all participating hospitals by the Ascension Providence Hospital Institutional Review Board. The results of this study will be submitted for publication in peer-reviewed journals.
Trial registration number
Keywords: adult intensive & critical care, neurology, stroke medicine, neurological injury, stroke, neurosurgery
Strengths and limitations of this study.
First randomised controlled trial in the USA to evaluate the effect of cilostazol and nimodipine on delayed cerebral ischaemia (DCI) and cerebral vasospasm.
Adequately powered study.
Includes both objective outcomes (DCI and angiographic vasospasm) and subjective patient-reported outcomes.
We limited the study population to anterior circulation aneurysmal subarachnoid haemorrhage, thereby limiting the generalisability of the results to other patient populations.
Introduction
Aneurysmal subarachnoid haemorrhage (aSAH) is a devastating condition which affects approximately 9 in 100 000 people annually around the world.1 2 Much advance has been made in the treatment of ruptured aneurysms; however, there has been little progress in the treatment and prevention of delayed cerebral ischaemia (DCI) due to cerebral vasospasm (cVS).1 2 cVS and subsequent DCI remain to be the most prominent cause of morbidity and mortality following aSAH.3 Although the mechanism and pathogenesis of cVS is not fully understood, it is considered a vital underlying mechanism in DCI.4 5 cVS is known to take effect between days 3 and 21 post-aSAH with a peak incidence between days 6 and 10.2 Current therapy includes definitive treatment of the ruptured aneurysm through either open clipping or endovascular therapy followed by a 21-day course of nimodipine after the onset of SAH.6–8 Nimodipine is a dihydropyridine calcium channel blocker which is recommended for the postprocedural treatment of aSAH for the prevention of DCI.8 9 This regimen has shown long-term outcome improvement following aSAH.7 10 Multiple other modalities have been investigated for the treatment and/or prevention of cVS including mechanical removal of blood, cisternal irrigation, Rho kinase inhibitors, triple-H therapy and numerous endovascular treatments—all of which demonstrated minimal efficacy or limited use.11–18 Despite years of investigation and improvement, the risk of symptomatic and radiographic vasospasm remains unacceptably high between 20% and 50%11 19–21 and as high as 80%, respectively.11 21–23 This also continues to be prevalent at our institution as we observed rates of symptomatic vasospasm and DCI to be 40% and 60%, respectively.
Cilostazol, a platelet aggregation inhibitor used for the treatment of symptomatic intermittent claudication, is a selective phosphodiesterase-3 inhibitor that exerts a vasodilatory and antithrombotic effect.9 This vasodilatory effect has been demonstrated on healthy cerebral arteries,24 and shown to prevent cVS in SAH animal models.25 26 Subsequent human trials have demonstrated cilostazol to be safe and effective at decreasing both radiographic and symptomatic cVS, with no serious adverse reactions.9 27–30 In addition, two recent systematic reviews and meta-analyses both concluded that cilostazol effectively reduced incidences of vasospasm, new cerebral infarction and poor outcomes in patients following aSAH.31 32 However, to date, no randomised controlled trial has evaluated the combined application of nimodipine and cilostazol. This combination therapy of nimodipine and cilostazol with possible synergistic effect requires further investigation.31
Our randomised superiority trial seeks to investigate the combined effect of cilostazol plus nimodipine on cVS, rates of DCI and functional neurological outcome when compared with nimodipine alone.
Study goals and objectives
Our goal is to demonstrate that cilostazol plus nimodipine is safe and superior to nimodipine alone in the prevention of DCI in patients with aSAH.
Primary objective
To demonstrate that the combined use of cilostazol plus nimodipine when compared with nimodipine alone will decrease the rate of DCI in patients following aSAH.
Secondary objectives
To demonstrate that the combined use of cilostazol plus nimodipine is not associated with increased drug-related serious adverse events (SAE).
To demonstrate that the combined use of cilostazol plus nimodipine will decrease rates of symptomatic and radiographic vasospasm.
To demonstrate that the combined use of cilostazol plus nimodipine will decrease the average length of intensive care unit (ICU) stay.
To demonstrate that the combined use of cilostazol plus nimodipine will decrease the incidence of secondary endovascular intervention (intra-arterial verapamil or angioplasty).
To demonstrate that the combined use of cilostazol plus nimodipine will improve modified Rankin Scores (mRS) and quality-of-life (QoL) outcomes at 6 months.
Methods and analysis
This is a multicentre, double-blinded, randomised, placebo-controlled superiority trial in adults in accordance with Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines. This study will have a two-arm parallel design without cross-over and equal randomisation per arm. Figure 1 outlines the Consolidated Standards of Reporting Trials flow chart.
Figure 1.
Study design Consolidated Standards of Reporting Trials (CONSORT) flow diagram.
Table 1 provides details to the inclusion, exclusion and withdrawal criteria. This protocol was approved by the Ascension Providence Hospital Institutional Review Board (IRB) and published on ClinicalTrials.gov.
Table 1.
Inclusion, exclusion and withdrawal criteria
| Inclusion | Exclusion |
| 18 years of age or older | Non-aneurysmal subarachnoid haemorrhage. |
| Anterior circulation aneurysm rupture | Multiple ruptured aneurysms. |
| Patients who have undergone surgical intervention | Patients with congestive heart failure. |
| Absence of rebleeding or new intracranial haemorrhage on postintervention CT scan | Severe aneurysmal subarachnoid haemorrhage (Hunt-Hess grade V). |
| Consent to study participation | Active pathological bleeding. |
| Allergy to cilostazol. | |
| Positive pregnancy test. | |
| Coagulopathy not caused by anticoagulant use. | |
| History of haemorrhagic complications (gastrointestinal bleeding, and so on). | |
| Uncontrolled or severe comorbidity that would qualify as an absolute contraindication for cilostazol. | |
| Patients requiring anticoagulant treatment following intervention (eg, stent-assisted coiling or flow-diverting stent obliteration of aneurysm). Exception: patients who require the use of aspirin as determined by the staff member performing the coil embolisation for thromboembolic event protection due to a small amount of coil extrusion from the aneurysm neck will be allowed to be included into the trial. |
Criteria for discontinuing follow-up: subject wishing to terminate participation in the study at any time throughout his/her participation.
Over a 3-year period, consecutive adult patients over the age of 18 who present to our tertiary care institution with aSAH diagnosed on CT and CT angiography (CTA) will be assessed for eligibility. Recruitment of participants is based on those adults who are diagnosed with aSAH due to ruptured anterior circulation aneurysm(s). Patients with posterior circulation aSAH are known to be lower risk for developing symptomatic vasospasm and were therefore excluded to avoid bias.33 After satisfying inclusion/exclusion criteria, patients/family members are consented for full participation in the trial. Once consented, patients are randomised to receive either placebo or intervention with a centralised treatment allocation mechanism and block randomisation to assure the two arms achieve equal proportion of patients over time.
All patients, treatment providers, investigators and statisticians are blinded to the allocation. Blinding is achieved by allocation sequence being concealed to personnel involved in the enrolling, care and evaluation of the patient. The study coordinator will keep the randomisation schedule in a cloud-based, secure and encrypted database. Only the study coordinator who monitors the trial, the pharmacist who executes the allocation, the supervising investigator who is not involved in the patients’ care or enrolment will have access to the randomisation schedule. Pharmacy will prepare identical appearing tablets/capsules/syringes as placebo which will conceal the identity of the medications.
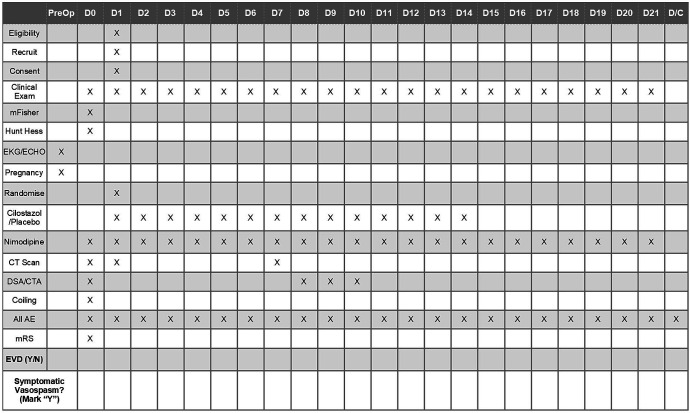
All participating patients, after undergoing treatment of their ruptured aneurysm (open clipping vs endovascular coiling) and confirmation of a stable head CT 24 hours postintervention will be randomised and scheduled to receive their allocation within 48 hours of surgery/intervention for a total of 14 days. In addition to their randomised allocation, all patients will receive a standard aSAH treatment protocol,7 including 21 days of nimodipine as endorsed by the Congress of Neurological Surgeons. The standardised treatment regimen is summarised in table 2. Each patient is followed according to the data collection schedule (figure 2). While in the hospital, the patients are monitored frequently (every hour while in the ICU and every 4 hours while on the medical/surgical unit) for any adverse event (AE)/SAE (table 3). AE and SAE are defined using a validated classification scheme (table 4).34 SAEs are defined as a grade 2 or higher (table 4). All unexpected SAEs related or possibly related to the study medication will be recorded and reported immediately to the principal investigator (PI) and the IRB within 24 hours. In addition to cessation of the intervention, SAEs may present a situation in which knowledge of the allocation will aid in the clinical management of the patient and therefore warrant unblinding of the allocation.
Table 2.
Standardised treatment regimen
| Location | Treatment |
| NSICU and floor | Intervention group
|
Control group
|
CTA, CT angiography; DSA, digital subtraction angiography; NSICU, neurosurgical intensive care unit; PO, postoperative; POD, postoperative day.;
Figure 2.
Data collection schedule and timeline. AE, adverse event; CTA, CT angiography; EVD, external ventricular drain; DSA, digital subtraction angiography; mRS, modified Rankin Score.
Table 3.
List of adverse or serious adverse events
| Cilostazol | Nimodipine | ||
| Adverse events | Headache Diarrhoea Abnormal stools Palpitations Dizziness Peripheral oedema Dyspepsia Abdominal pain Tachycardia |
Adverse events | Hypotension (mild) Diarrhoea Dyspepsia Rash Headache Flushing |
| Serious adverse events | Hypotension Bleeding Stevens-Johnson syndrome Anaphylaxis Hypersensitivity reaction Leucopenia Thrombocytopenia Tachyarrhythmias Myocardial infarction Angina |
Serious adverse events | Hypotension (severe) EKG changes CHF Thromboembolism Thrombocytopenia Anaemia GI bleeding Ileus Intestinal obstruction |
CHF, congestive heart failure; GI, gastrointestinal.
Table 4.
Definition and classification of surgical complications
| Grade | Definition |
| Grade 1 | Any deviation from the normal postoperative course without the need for pharmacological or surgical, endoscopic and radiological interventions. |
| Grade 2 | Requiring pharmacological treatment with drugs other than such allowed for grade 1 complication. Blood transfusions and total parenteral nutrition are also included. |
| Grade 3 | Requiring surgical, endoscopic or radiological intervention. |
| 3a | Intervention not under general anaesthesia. |
| 3b | Intervention under general anaesthesia. |
| Grade 4 | Life-threatening complication (including CNS complications)* requiring IC/ICU management. |
| 4a | Single-organ dysfunction (including dialysis). |
| 4b | Multiorgan dysfunction. |
| Grade 5 | Death of a patient |
| Suffix | If the patient suffers from a complication at the time of discharge, the suffix ‘d’ is added to the respective grade of complication. This label indicates the need for a follow-up to fully evaluate the complication. |
*Cerebral haemorrhage, ischaemic stroke, subarachnoid haemorrhage, but excluding transient ischaemic attacks.
CNS, central nervous system; IC, intermediate care; ICU, intensive care unit.
The primary outcome will be defined as new ischaemic areas on CT performed at 1 month following initial presentation and not observed on postoperative CT (24 hours postintervention) determined by blinded neuroradiologists.35 Ischaemic areas or low-density areas on CT performed the day after intervention will be defined as rupture-related or procedural-related infarctions and/or brain injury (such as ventriculostomy tract, craniotomy changes, and so on). Secondary outcomes including symptomatic vasospasm, angiographic vasospasm, length of ICU stay, QoL and mRS at 1, 3 and 6 months postoperatively will be prospectively collected. Length of ICU stay will be determined by standardised discharge criteria. Rates of symptomatic vasospasm will be collected and defined as development of a new focal or global neurological deficit or deterioration of at least 2 points on the Glasgow Coma Scale,36 which was not explained by initial haemorrhage, rebleeding, hydrocephalus, surgical complications, fever, infections, or electrolyte or metabolic disturbances regardless of cerebral infarctions on CT scanning or MRI and angiographic vasospasm on diagnostic cerebral angiogram (digital subtraction angiography (DSA)) or CTA.14 37 38 Radiographic vasospasm will be assessed by either CTA or DSA between 7 and 10 days postoperatively. Radiographic vasospasm with be defined as arterial narrowing not attributable to atherosclerosis, catheter-induced vasospasm, or vessel hypoplasia as a ratio of stenosis compared with previous baseline CTA or DSA as determined by blinded neuroradiologists.14 In each patient, the smallest diameters of 10 arterial segments of the bilateral distal internal carotid arteries, M1 and M2 segments of the middle cerebral artery and A1 and A2 segments of the anterior cerebral artery will be measured. Severity of the radiographic vasospasm will be categorised as none or mild (0%–25% decrease in vessel diameter from baseline), moderate (25%–50% decrease in vessel diameter from baseline) or severe (greater than 50% decrease in vessel diameter from baseline). The most affected segment will be used to determine severity of radiographic vasospasm.
To demonstrate superiority, an 80% power is used to minimise chances of false negatives. Assuming a relative effect size of 29% with the use of cilostazol and a baseline rate of DCI of 50%, a total sample size was estimated to be 100 patients with an alpha of 0.05. In anticipation for any unforeseen events and those lost to follow-up, we plan to enrol a total of 120 patients.
Patient and public involvement
At the time of 1-month postoperative follow-up, patients or their families will be asked to participate as study advisers in our data monitoring and safety committee. There will be two to four patient advisers at any given time during the study period, each with a term of 6 months. These patient advisers will share their experience regarding the recruitment process, surgery and postoperative care in order to help ensure patient safety and satisfaction throughout the study.
Trial status
At the time of manuscript submission, the trial is ongoing.
Safety considerations
All study-related AEs are recorded and reported immediately to the PI and subsequently to the IRB within 24 hours of the event as previously stated. All AEs will be logged in an adverse outcome reporting log as needed. The institutional data safety monitoring board (DSMB) will be responsible for monitoring the clinical and surgical safety of the study and review AEs reported to the IRB to determine risk and benefits. Any SAE related to the study medication represents a circumstance under which unblinding is permissible in order to ensure the safety of the participant. At that time, the intervention will be stopped, and any clinical intervention required at the discretion of the attending surgeon will ensue and documented and presented to the IRB and DSMB. Members of the DSMB will be surgeons and related experts who will meet to review the results and any AEs biannually to evaluate study safety.
Follow-up
Postoperatively, patients will be followed according to the data collection schedule (figure 2). Data will be prospectively collected using a standardised specific adverse outcome and clinical report form (CRF). Once discharged from the hospital, patients will be scheduled follow-up visits at 1, 3 and 6 months. Any additional follow-up will be designated at the discretion of the treating attending physician.
Data management and statistical analysis
During the first 2 weeks of the trial, the PI, clinical research coordinator (CRC), will observe all the steps of the intervention and data collection to ensure proper execution. The progress of data entry, follow-up and recruitment are logged and monitored regularly by the CRC. The CRF will be entered into the database within 24 hours of the patient’s discharge and the database will be maintained to within 1 week of the data collection. CRC will coordinate the postoperative follow-up and evaluate the capture rate for QoL and mRS at 1, 3 and 6 months.
Comparability between groups will be evaluated by descriptive and univariate analyses. Multivariate, stratified or subgroup analyses will be used in case of confounders imbalance. A p value less than or equal to 0.05 will be considered statistically significant. Bonferroni’s correction will be applied when appropriate. Descriptive statistics will be used in each arm for proportion who did not receive allocated intervention, lost to follow-up, excluded from primary analysis and drug-related complications. Intention-to-treat, per-protocol and sensitivity analyses will be performed. An interim analysis will be conducted quarterly during the trial, that is, after a total of 25, 50 and 75 patients have been enrolled. This is a superiority trial. Early discontinuation of the study will be dependent on overwhelming positive results for the primary outcome. We will discontinue the trial if we achieve p<0.001 threshold at the time of interim analysis.39
Quality assurance
Standardised medication orders will conceal the treatment allocation. The study coordinator will be responsible for managing the quality of patient data recorded in the study. All participating research staff will be trained and given written copies of a standard operating procedure to ensure consistency during recruitment, consent, handling of data and follow-up evaluation. The study coordinator along with the PI will check weekly the content of the forms and database to ensure accurate and timely entry. Compliance at all study timepoints including enrolment, randomisation, intervention, data and outcome collection will be documented daily on a compliance monitoring sheet by the investigator. The recorded data will be entered into a cloud-based, secure and encrypted database by the research staff. Access to the database will be restricted. Data validation tool has been embedded in the database. Data entered will undergo monthly verification with the source document.
Expected outcome of the study
This study is intended to demonstrate that the use of cilostazol plus nimodipine is safe and superior to nimodipine alone in the prevention of DCI in patients who have aSAH. We expect to identify any immediate drug-related adverse effects as listed in table 3. Additionally, we aim to demonstrate that cilostazol plus nimodipine decreases rates of both symptomatic and radiographic vasospasm.
Duration of the project
Given our institutional volume, we anticipate a study period of 1–3 years assuming 50% of eligible patients agree to participate. Interim analysis will be performed quarterly as previously described and subject to discontinuation if all previously defined criteria are met.
Project management
Neurosurgery staff will counsel and recruit subjects according to their initial screening to participate in this trial. The neurosurgery staff will check for eligibility using inclusion and exclusion criteria listed in table 1. They will also explain the study principles, including the detailed experimental in-hospital and postoperative protocol, investigational treatment, potential risks and benefits. Subsequent detailed written consent will be obtained by the staff and placed in a cloud-based, secure and encrypted database (see online supplemental material). The designated lead pharmacists will execute the randomised allocation assignment according to the block randomisation schedule to maintain masking of allocation. The neuroscience ICU charge nurse will be responsible for overseeing and monitoring administration of the study medication. The neuroscience-trained intensive care nursing staff will administer the study medication to the study participants. The PI and support staff will record all perioperative and postoperative data including study-related AEs. The study coordinator will ensure and maintain follow-up visits for postoperative secondary outcomes. The neuroradiologists will evaluate and determine primary and secondary outcomes of DCI and vasospasm, respectively. The clinical research methodologist will function as the CRC, supervise the overall execution of the study and participate in the writing of the protocol and manuscript.
bmjopen-2019-036217supp001.pdf (100.7KB, pdf)
Ethics and dissemination
The study will be conducted according to the Helsinki Declaration,40 the NIH Human Subjects guidelines and the International Conference on Harmonization E6 Guideline for Good Clinical Practice.41 This protocol is written following the SPIRIT 2013 guidelines and was approved by Ascension Providence Hospital IRB. The results of this study will be submitted for publication in peer-reviewed journals and the key findings will be presented at national conferences.
Supplementary Material
Footnotes
Contributors: TD, CFC, SR, BR and DT contributed substantially to the conception and design of this trial including organisation and execution over two hospital campuses. DS contributed to the design and execution of the study drug protocol including randomisation, blinding and placebo. MB and LG contributed substantially to the acquisition of data, ensuring accurate and standard operating procedures, and maintaining quality assurance among study participants and their subsequent care over two hospital campuses. TD, CFC, SR, DS, MB, LG, PK, BR, TMS and DT contributed to the drafting of the original manuscript, participated in critically revising the manuscript and agree to be accountable for all aspects of the work.
Funding: This trial is supported by the Ascension Providence Hospital Institutional Research Grant (1478832-5).
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The protocol was approved by the Ascension Providence Hospital Institutional Review Board (IRB).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.D'Souza S. Aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol 2015;27:222–40. 10.1097/ANA.0000000000000130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dabus G, Nogueira RG. Current options for the management of aneurysmal subarachnoid hemorrhage-induced cerebral vasospasm: a comprehensive review of the literature. Interv Neurol 2013;2:30–51. 10.1159/000354755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kassell NF, Torner JC, Haley EC, et al. . The International cooperative study on the timing of aneurysm surgery. Part 1: overall management results. J Neurosurg 1990;73:18–36. 10.3171/jns.1990.73.1.0018 [DOI] [PubMed] [Google Scholar]
- 4.Budohoski KP, Guilfoyle M, Helmy A, et al. . The pathophysiology and treatment of delayed cerebral ischaemia following subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry 2014;85:1343–53. 10.1136/jnnp-2014-307711 [DOI] [PubMed] [Google Scholar]
- 5.Francoeur CL, Mayer SA. Management of delayed cerebral ischemia after subarachnoid hemorrhage. Crit Care 2016;20:277. 10.1186/s13054-016-1447-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen GS, Ahn HS, Preziosi TJ, et al. . Cerebral arterial spasm--a controlled trial of nimodipine in patients with subarachnoid hemorrhage. N Engl J Med 1983;308:619–24. 10.1056/NEJM198303173081103 [DOI] [PubMed] [Google Scholar]
- 7.Connolly ES, Rabinstein AA, Carhuapoma JR, et al. . Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American heart Association/american stroke association. Stroke 2012;43:1711–37. 10.1161/STR.0b013e3182587839 [DOI] [PubMed] [Google Scholar]
- 8.Petruk KC, West M, Mohr G, et al. . Nimodipine treatment in poor-grade aneurysm patients. Results of a multicenter double-blind placebo-controlled trial. J Neurosurg 1988;68:505–17. 10.3171/jns.1988.68.4.0505 [DOI] [PubMed] [Google Scholar]
- 9.Veldeman M, Höllig A, Clusmann H, et al. . Delayed cerebral ischaemia prevention and treatment after aneurysmal subarachnoid haemorrhage: a systematic review. Br J Anaesth 2016;117:17–40. 10.1093/bja/aew095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haley EC, Kassell NF, Torner JC. A randomized controlled trial of high-dose intravenous nicardipine in aneurysmal subarachnoid hemorrhage. A report of the cooperative aneurysm study. J Neurosurg 1993;78:537–47. 10.3171/jns.1993.78.4.0537 [DOI] [PubMed] [Google Scholar]
- 11.Findlay JM, Kassell NF, Weir BK, et al. . A randomized trial of intraoperative, intracisternal tissue plasminogen activator for the prevention of vasospasm. Neurosurgery 1995;37:168–78. 10.1227/00006123-199507000-00041 [DOI] [PubMed] [Google Scholar]
- 12.Findlay JM, Weir BK, Kassell NF, et al. . Intracisternal recombinant tissue plasminogen activator after aneurysmal subarachnoid hemorrhage. J Neurosurg 1991;75:181–8. 10.3171/jns.1991.75.2.0181 [DOI] [PubMed] [Google Scholar]
- 13.Origitano TC, Wascher TM, Reichman OH, et al. . Sustained increased cerebral blood flow with prophylactic hypertensive hypervolemic hemodilution ("triple-H" therapy) after subarachnoid hemorrhage. Neurosurgery 1990;27:729–40. 10.1227/00006123-199011000-00010 [DOI] [PubMed] [Google Scholar]
- 14.Shibuya M, Suzuki Y, Sugita K, et al. . Effect of AT877 on cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Results of a prospective placebo-controlled double-blind trial. J Neurosurg 1992;76:571–7. 10.3171/jns.1992.76.4.0571 [DOI] [PubMed] [Google Scholar]
- 15.Eskridge JM, McAuliffe W, Song JK, et al. . Balloon angioplasty for the treatment of vasospasm: results of first 50 cases. Neurosurgery 1998;42:510–6. 10.1097/00006123-199803000-00016 [DOI] [PubMed] [Google Scholar]
- 16.Musahl C, Henkes H, Vajda Z, et al. . Continuous local intra-arterial nimodipine administration in severe symptomatic vasospasm after subarachnoid hemorrhage. Neurosurgery 2011;68:1541–7. 10.1227/NEU.0b013e31820edd46 [DOI] [PubMed] [Google Scholar]
- 17.Sawada M, Hashimoto N, Tsukahara T, et al. . Effectiveness of intra-arterially infused papaverine solutions of various concentrations for the treatment of cerebral vasospasm. Acta Neurochir 1997;139:706–11. 10.1007/BF01420042 [DOI] [PubMed] [Google Scholar]
- 18.Tachibana E, Harada T, Shibuya M, et al. . Intra-Arterial infusion of fasudil hydrochloride for treating vasospasm following subarachnoid haemorrhage. Acta Neurochir 1999;141:13–19. 10.1007/s007010050260 [DOI] [PubMed] [Google Scholar]
- 19.Charpentier C, Audibert G, Guillemin F, et al. . Multivariate analysis of predictors of cerebral vasospasm occurrence after aneurysmal subarachnoid hemorrhage. Stroke 1999;30:1402–8. 10.1161/01.STR.30.7.1402 [DOI] [PubMed] [Google Scholar]
- 20.Solenski NJ, Haley EC, Kassell NF, et al. . Medical complications of aneurysmal subarachnoid hemorrhage: a report of the multicenter, cooperative aneurysm study. participants of the multicenter cooperative aneurysm study. Crit Care Med 1995;23:1007–17. 10.1097/00003246-199506000-00004 [DOI] [PubMed] [Google Scholar]
- 21.Vajkoczy P, Meyer B, Weidauer S, et al. . Clazosentan (AXV-034343), a selective endothelin A receptor antagonist, in the prevention of cerebral vasospasm following severe aneurysmal subarachnoid hemorrhage: results of a randomized, double-blind, placebo-controlled, multicenter phase IIA study. J Neurosurg 2005;103:9–17. 10.3171/jns.2005.103.1.0009 [DOI] [PubMed] [Google Scholar]
- 22.Bederson JB, Connolly ES, Batjer HH, et al. . Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the stroke Council, American heart association. Stroke 2009;40:994–1025. 10.1161/STROKEAHA.108.191395 [DOI] [PubMed] [Google Scholar]
- 23.Weidauer S, Lanfermann H, Raabe A, et al. . Impairment of cerebral perfusion and infarct patterns attributable to vasospasm after aneurysmal subarachnoid hemorrhage: a prospective MRI and DSA study. Stroke 2007;38:1831–6. 10.1161/STROKEAHA.106.477976 [DOI] [PubMed] [Google Scholar]
- 24.Birk S, Kruuse C, Petersen KA, et al. . The phosphodiesterase 3 inhibitor cilostazol dilates large cerebral arteries in humans without affecting regional cerebral blood flow. J Cereb Blood Flow Metab 2004;24:1352–8. 10.1097/01.WCB.0000143536.22131.D7 [DOI] [PubMed] [Google Scholar]
- 25.Ito H, Fukunaga M, Suzuki H, et al. . Effect of cilostazol on delayed cerebral vasospasm after subarachnoid hemorrhage in rats: evaluation using black blood magnetic resonance imaging. Neurobiol Dis 2008;32:157–61. 10.1016/j.nbd.2008.07.004 [DOI] [PubMed] [Google Scholar]
- 26.Yamaguchi-Okada M, Nishizawa S, Mizutani A, et al. . Multifaceted effects of selective inhibitor of phosphodiesterase III, cilostazol, for cerebral vasospasm after subarachnoid hemorrhage in a dog model. Cerebrovasc Dis 2009;28:135–42. 10.1159/000223439 [DOI] [PubMed] [Google Scholar]
- 27.Senbokuya N, Kinouchi H, Kanemaru K, et al. . Effects of cilostazol on cerebral vasospasm after aneurysmal subarachnoid hemorrhage: a multicenter prospective, randomized, open-label blinded end point trial. J Neurosurg 2013;118:121–30. 10.3171/2012.9.JNS12492 [DOI] [PubMed] [Google Scholar]
- 28.Yoshimoto T, Shirasaka T, Fujimoto S, et al. . Cilostazol may prevent cerebral vasospasm following subarachnoid hemorrhage. Neurol Med Chir 2009;49:235–41. 10.2176/nmc.49.235 [DOI] [PubMed] [Google Scholar]
- 29.Shinohara Y, Katayama Y, Uchiyama S, et al. . Cilostazol for prevention of secondary stroke (CSPS 2): an aspirin-controlled, double-blind, randomised non-inferiority trial. Lancet Neurol 2010;9:959–68. 10.1016/S1474-4422(10)70198-8 [DOI] [PubMed] [Google Scholar]
- 30.Gotoh F, Tohgi H, Hirai S, et al. . Cilostazol stroke prevention study: a placebo-controlled double-blind trial for secondary prevention of cerebral infarction. J Stroke Cerebrovasc Dis 2000;9:147–57. 10.1053/jscd.2000.7216 [DOI] [PubMed] [Google Scholar]
- 31.Shan T, Zhang T, Qian W, et al. . Effectiveness and feasibility of cilostazol in patients with aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. J Neurol 2020;267:1577–84. 10.1007/s00415-019-09198-z [DOI] [PubMed] [Google Scholar]
- 32.Saber H, Desai A, Palla M, et al. . Efficacy of cilostazol in prevention of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage: a meta-analysis. J Stroke Cerebrovasc Dis 2018;27:2979–85. 10.1016/j.jstrokecerebrovasdis.2018.06.027 [DOI] [PubMed] [Google Scholar]
- 33.Hirashima Y, Kurimoto M, Hori E, et al. . Lower incidence of symptomatic vasospasm after subarachnoid hemorrhage owing to ruptured vertebrobasilar aneurysms. Neurosurgery 2005;57:1110–6. 10.1227/01.NEU.0000185632.69374.C9 [DOI] [PubMed] [Google Scholar]
- 34.Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–13. 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vergouwen MDI, Vermeulen M, van Gijn J, et al. . Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary Research Group. Stroke 2010;41:2391–5. 10.1161/STROKEAHA.110.589275 [DOI] [PubMed] [Google Scholar]
- 36.Voldby B, Enevoldsen EM, Jensen FT. Regional CBF, intraventricular pressure, and cerebral metabolism in patients with ruptured intracranial aneurysms. J Neurosurg 1985;62:48–58. 10.3171/jns.1985.62.1.0048 [DOI] [PubMed] [Google Scholar]
- 37.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. The Lancet 1974;304:81–4. 10.1016/S0140-6736(74)91639-0 [DOI] [PubMed] [Google Scholar]
- 38.Frontera JA, Fernandez A, Schmidt JM, et al. . Defining vasospasm after subarachnoid hemorrhage: what is the most clinically relevant definition? Stroke 2009;40:1963–8. 10.1161/STROKEAHA.108.544700 [DOI] [PubMed] [Google Scholar]
- 39.Zannad F, Gattis Stough W, McMurray JJV, et al. . When to stop a clinical trial early for benefit: lessons learned and future approaches. Circ Heart Fail 2012;5:294–302. 10.1161/CIRCHEARTFAILURE.111.965707 [DOI] [PubMed] [Google Scholar]
- 40.Puri KS, Suresh KR, Gogtay NJ, et al. . Declaration of Helsinki, 2008: implications for stakeholders in research. J Postgrad Med 2009;55:131–4. 10.4103/0022-3859.52846 [DOI] [PubMed] [Google Scholar]
- 41.Abraham J. International Conference On Harmonisation Of Technical Requirements For Registration Of Pharmaceuticals For Human Use : Brouder A, Tietje C, Handbook of transnational economic governance regimes. Brill, 2009: 1041–54. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-036217supp001.pdf (100.7KB, pdf)