Abstract
Seven T-cell subset values were measured in each of 559 mice at 8 months of age, and then again in the 494 animals that reached 18 months of age. The group included virgin males, virgin females, and mated females, and it was produced by using a four-way cross-breeding system that generates genetic heterogeneity equivalent to a very large sibship. An analysis of covariance showed that four T-cell subsets—CD4, CD4 memory, CD4 naïve, and CD4 cells expressing P-glycoprotein—were significant predictors (p< .003) of longevity when measured at 18 months of age after adjustment for the possible effects of gender and mating. The subset marked by CD4 and P-glycoprotein expression showed a significant interaction effect: this subset predicted longevity only in males. Among subsets measured when the mice were 8 months of age, only the levels of CD8 memory cells predicted longevity (p= .016); the prognostic value of this subset was largely limited to mated females. A cluster analysis that separated mice into two groups based upon similarity of T-cell subset patterns measured at 18 months showed that these two groups differed in life expectancy. Specifically, mice characterized by relatively low levels of CD4 and CD8 memory cells, high levels of CD4 naïve cells, and low levels of CD4 cells with P-glycoprotein (64% of the total) lived significantly longer (50 days = 6%; p< .0007) than mice in the other cluster. The results are consistent with the hypothesis that patterns of T-cell subsets vary among mice in a manner than can predict longevity in middle age, and they suggest that these subsets may prove to be useful for further studies of the genetics of aging and age-sensitive traits.
ANALYSES of the effects of interventions on the aging process would be facilitated by the development of assays that provide useful surrogates to longevity as the principal endpoint of the study. At present the inference that a particular diet or pharmacological agent has indeed slowed down the aging process is typically based on data showing that the intervention extends life span, and in particular some estimate of the maximal life span, such as the mean age at death of the longest-lived decile of the population. Such life-span-based experiments are expensive and time consuming even for short-lived species such as mice and rats, and all but impossible for longer-lived species. An assay or set of assays that could distinguish, among a group of individuals in the same birth cohort, those who exhibited relatively youthful results on a wide range of age-sensitive tests would deserve to be considered as a biomarker of some (hypothetical) underlying aging process that influences multiple age-dependent traits in parallel. The very wide range of effects of aging on physiological function has convinced many authorities (1)(2) that no such underlying process is likely to exist, and indeed previous attempts to extract measures of “biological age” from catalogs of age-sensitive outcomes have been justly criticized on statistical grounds (3)(4). Nonetheless, the question as to whether a set of assays can be demonstrated to predict the outcome of a wide range of other age-sensitive tests is an empirical one, and it is important enough to deserve a detailed experimental study (5).
Assays that predict subsequent longevity of apparently healthy subjects are likely to be attractive candidates as potential biomarkers of aging, because it is difficult for an intervention to produce impressive increases in longevity without a parallel deceleration of multiple diseases that could each lead to death if allowed to proceed unimpeded. A small number of prior publications have shown evidence for correlations between age-sensitive traits, measured in adult life, and longevity in mice (6)(7)(8), but the most comprehensive of these noted that the correlations seemed to apply for some but not all of the inbred mouse stocks examined.
My own prior work in this area has used four-way cross mouse stocks, bred as the progeny of two different F1 hybrid mouse stocks (9)(10)(11). This breeding scheme produces a set of mice that are, in a genetic sense, full sibs, each genetically unique, each receiving 25% of its genetic endowment, at random, from the four inbred grandparental stocks. Preliminary data, acquired on a small sample 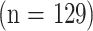 of four-way cross mice of the UM-HET3 variety, showed that longevity could be predicted by measures of CD4 memory (CD4M) T cells measured in peripheral blood cells in mice at 18 months of age (9). The association was of similar strength in both male and female mice, met a significance criterion of p < .0003 by multiple regression, and together with gender accounted for 18% of the variance in longevity among the mice. CD4M cells increase with age in many strains of mice, including UM-HET3 mice (12), and the data showed high levels of CD4M cells to be associated, as predicted, with relatively short life span. This initial report also showed suggestive evidence (p < .1) for correlations between longevity and other T-cell subsets that did not, however, meet traditional standards for statistical significance after adjustment for multiple comparisons.
of four-way cross mice of the UM-HET3 variety, showed that longevity could be predicted by measures of CD4 memory (CD4M) T cells measured in peripheral blood cells in mice at 18 months of age (9). The association was of similar strength in both male and female mice, met a significance criterion of p < .0003 by multiple regression, and together with gender accounted for 18% of the variance in longevity among the mice. CD4M cells increase with age in many strains of mice, including UM-HET3 mice (12), and the data showed high levels of CD4M cells to be associated, as predicted, with relatively short life span. This initial report also showed suggestive evidence (p < .1) for correlations between longevity and other T-cell subsets that did not, however, meet traditional standards for statistical significance after adjustment for multiple comparisons.
The current report represents a follow-up study in which seven T-cell subsets were evaluated, at ages 8 and 18 months, among 559 UM-HET3 mice for which date of death was subsequently determined. The data show significant correlations between life span and four age-sensitive T-cell subsets measured at 18 months of age.
Methods
Mice
All animals were bred at the University of Michigan, using CB6F1 mothers and C3D2F1 fathers obtained from Jackson Laboratories (Bar Harbor, ME). Mice were weaned at age 3–4 weeks into same-sex cages containing three to four pups, and the cages were maintained without replacement of animals that died. On a quarterly basis, sentinel mice (not part of the experimental groups) were exposed to pooled spent bedding material from the study population and were then later tested serologically for evidence of immunity to murine Sendai, mycoplasma, and coronavirus (mouse hepatitis virus, or MHV). In addition, the animals were examined for pinworm. All such tests were negative during the course of this study. In some cages of female mice, a male mouse was introduced at approximately 8 weeks of age to create a group of “mated females.” Litters were removed from these cages within the first week after birth, and the male mouse was removed when the females reached 6 months of age. Cages of virgin males, virgin females, and mated females were all housed within the same room.
Age at Death
Cages were examined at least daily. Mice that appeared to be clinically ill (on the grounds of poor grooming, weight loss, visible or palpable tumor, or signs of infection) were observed twice daily except on weekends. Mice judged to be so seriously ill that survival for another week was extremely unlikely were sacrificed to allow optimal necropsy analysis. Mice in this group made up 62% of those in the mated female group, and 55% of the mice in the virgin male and virgin female groups.
Exclusion Criteria
Fighting by male mice caused serious wounding in approximately 25% of the cages; in these cases all males in the cages were culled, always prior to 12 months of age and typically much earlier. The few mice dying at ages less than 8 months were not included in the study, because they did not reach the age at which the first immune assessment was conducted. The study population thus included 292 mated female mice, of which 267 survived to be reexamined at 18 months; 146 virgin females, of which 136 were reexamined at 18 months; and 121 virgin males, of which 91 were tested at 18 months of age.
Assessment of T-Cell Subset Levels in Peripheral Blood
Two-color flow cytometry analyses were conducted as previously described (9) on samples of peripheral venous blood obtained at 8 and then again at 18 months of age. Table 1 shows the definition of the seven T-cell subsets that form the subject of this report.
Table 1.
T-Cell Subsets Tested in 8- and 18-Month-Old HET3 Mice
| Subset Designation | Description | Calculation |
| CD4 | Class II restricted helper T cells | CD4(+),CD3(+) as % of CD3 |
| CD8 | Class I restricted cytotoxic T cells | CD8(+),CD3(+) as % of CD3 |
| CD4M | Memory CD4 cells | CD4(+),CD44(high) as % of CD4 |
| CD8M | Memory CD8 cells | CD8(+),CD44(high) as % of CD8 |
| CD4V | Virgin CD4 cells | CD4(+),CD45RB(low) as % of CD4 |
| CD4P | P-glycoprotein-positive CD4 cells | CD4(+), R123-extruding as % of CD4 |
| CD8P | P-glycoprotein-positive CD8 cells | CD8(+), R123-extruding as % of CD8 |
Statistical Methods
Statistical methods are described in detail in the text and figure legends as follows.
Results
Aging leads to changes in the relative proportions of several peripheral blood T-cell subsets, including increases in the proportion of CD4M, CD8M, CD4P, and CD8P cells, and decreases in CD4 and CD4V values. The central goal of this study was to determine to what extent measures of these age-sensitive T-cell subsets in the peripheral blood of adult mice could predict longevity. Because correlations among independent variables can complicate regression analyses, and because my previous work (12) on a separate population of mice had indicated substantial correlations among these subsets, I calculated the Pearson product-moment correlations among the set of T-cell assays at each of the two ages tested. Table 2 shows the results. CD4 and CD8 values were strongly and negatively correlated at each age, as expected from the mutual exclusion of these two markers on peripheral T cells. CD4M and CD8M values showed strong positive correlations at each age, consistent with my previous data and suggestions that the unknown factors regulating memory cell values in adult life affect both of these memory cell subsets in parallel. The negative correlation between CD4M and CD4V values is consistent with much previous data showing that CD4V cells give rise to CD4M progeny. The positive correlations between CD4P and CD8P values also confirm my previous data, and they indicate that accumulation of both P-glycoprotein positive subsets is regulated by individual-specific genetic or environmental factors. Table 2 shows that in 8-month-old mice, high levels of CD4 cells are significantly associated with high levels of CD4V cells and lower levels of CD4M and CD8M cells. These associations are also seen at 18 months of age, but at this older age, CD4P cells show positive correlations with CD4M and CD8M cells, as well as a negative correlation with CD4V. Factors that predispose individual mice toward increased CD4M and CD8M cells thus also seem to promote the loss with age of CD4 cells and the increase in CD4P levels.
Table 2.
Matrix of Correlation Coefficients for T-Cell Subsets
| Subset Designation | CD4 | CD8 | CD4M | CD8M | CD4V | CD4P | CD8P |
| CD4 | −0.53 | −0.26 | −0.26 | 0.19 | 0.05 | −0.06 | |
| CD8 | −0.63 | 0.07 | 0.08 | 0.00 | 0.01 | 0.05 | |
| CD4M | −0.24 | 0.07 | 0.48 | −0.15 | −0.03 | −0.17 | |
| CD8M | −0.46 | 0.35 | 0.56 | −0.36 | 0.04 | −0.02 | |
| CD4V | 0.23 | −0.11 | −0.54 | −0.50 | −0.05 | −0.08 | |
| CD4P | −0.19 | −0.03 | 0.39 | 0.29 | −0.39 | 0.38 | |
| CD8P | −0.05 | 0.05 | 0.04 | −0.04 | 0.08 | 0.29 |
Notes: Tabulated values are Pearson product-moment correlation coefficients for mice in all three groups considered together; n = 446–550 mice in each case. Values above the diagonal correspond to tests conducted when the mice were 8 months of age and values below the diagonal represent tests of 18-month-old mice. Boldface is used to highlight correlations where p < .01.
Table 3 shows the results of univariate correlations between longevity and each of the T-cell subsets in three groups of mice differing in gender and reproductive history, that is, in mated females, virgin females, and virgin males. At 18 months of age, there is for each group of mice at least one T-cell subset that is able to predict longevity to a statistically significant degree, but the strength of these correlations varies, depending on gender and reproductive history. In mated females, there are significant associations of longevity with CD4V, CD4, and CD8M, whereas in virgin females, the strongest associations are with CD4M cells; in virgin males, CD4M and CD4P cells are the strongest predictors of subsequent life span. The direction of the association is in each case consistent with the idea that mice whose immune systems most closely resemble those of aged animals tend to die first: longest life span is thus associated with relatively high levels of CD4 and CD4V cells, both of which decrease with age, but with lower levels of CD4M, CD8M, and CD4P cells, all of which increase with age.
Table 3.
Univariate Correlations Between T-Cell Subset Markers and Longevity in Different Groups of Mice
| Age = 8 mo | Age = 18 mo | |||||||||
| Subset Designation | Mated Fem. | Virgin Fem. | Virgin Males | Mated Fem. | Virgin Fem. | Virgin Males | ||||
| CD4 | 0.14 | −0.01 | −0.06 | 0.16 | 0.13 | 0.18 | ||||
| CD8 | −0.06 | 0.05 | −0.10 | −0.09 | 0.09 | −0.07 | ||||
| CD4M | −0.10 | 0.05 | −0.12 | −0.11 | −0.22 | −0.30 | ||||
| CD8M | −0.22 | 0.00 | −0.12 | − 0.14 | −0.12 | −0.05 | ||||
| CD4V | 0.13 | 0.08 | −0.03 | 0.21 | 0.15 | 0.20 | ||||
| CD4P | −0.09 | −0.06 | 0.11 | −0.11 | −0.09 | − 0.40 | ||||
| CD8P | 0.00 | 0.09 | −0.03 | 0.00 | −0.13 | 0.07 | ||||
| n | 260–292 | 102–135 | 75–121 | 259–267 | 130–136 | 85–90 | ||||
Notes : Underlining is used to indicate correlations where 0.01 < p < .05. Boldface is used to indicate .001 < p < .01. Boldface plus underline is used where p < .001; n is the number of mice for each calculation (range).
Table 3 also shows that three T-cell subsets (CD4, CD8M, and CD4V) are significantly associated with longevity when measured as early as 8 months of age, but that these associations are strong only in mated female mice.
An analysis of covariance (ANCOVA) provides a more powerful statistical test for associations between T-cell subsets and longevity, by controlling for possible effects of group variables (gender, reproductive history) and for any interactions between group variables and the immune subset of interest. Table 4 shows all significant results from a set of analyses of covariance. Among these tests, only CD4P (at 18 months) showed a significant interaction for the grouping variable. Five of the T-cell subsets exhibit a significant ability to predict longevity when tested at 18 months of age; the strongest associations are for CD4M cells 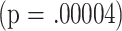 and CD4P cells
and CD4P cells 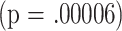 . CD8M cells measured at 18 months showed a marginal association at
. CD8M cells measured at 18 months showed a marginal association at 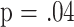 . I take this last finding as suggestive rather than definitive, because it emerged from a consideration of seven subsets, and application of the Bonferroni correction would require a significance level of
. I take this last finding as suggestive rather than definitive, because it emerged from a consideration of seven subsets, and application of the Bonferroni correction would require a significance level of 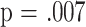 (i.e., .05/7, the number of tests considered).
(i.e., .05/7, the number of tests considered).
Table 4.
Analysis of Covariance: Prediction of Longevity by T-Cell Subset Markers After Adjustment for Interaction With Gender and Reproductive History
| T-Cell Subset | Age at Test (mo) | F | p(F) | Interaction (a) |
| CD8M | 8 | 5.8 | .016 | |
| CD4 | 18 | 9.1 | .0028 | |
| CD4M | 18 | 17.1 | .00004 | |
| CD8M | 18 | 4.2 | .04 | |
| CD4V | 18 | 13.8 | .0002 | |
| CD4P | 18 | 16.3 | .00006 | p = .03 |
Notes: Values represent F statistics (and corresponding p values) for a linear model in which longevity was predicted by the indicated T-cell subset (as a continuous variable), a grouping factor with three alternates (virgin male, virgin female, or mated female), and an interaction term (Subset × Grouping Factor). The interaction term was significant (p < .05) only for the CD4P subset.
Among the tests conducted when the mice were 8 months of age, only that for CD8M exhibited any association with subsequent longevity in the ANCOVA analysis 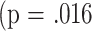 , which is not significant when compared to the Bonferroni adjusted threshold of
, which is not significant when compared to the Bonferroni adjusted threshold of 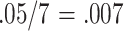 ). Although the ANCOVA analysis did not show a significant effect of group in the CD8M analysis at 8 months, a separate calculation in which mated females were contrasted to virgin females for CD8M showed a significant interaction term
). Although the ANCOVA analysis did not show a significant effect of group in the CD8M analysis at 8 months, a separate calculation in which mated females were contrasted to virgin females for CD8M showed a significant interaction term 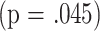 as well as a significant effect for CD8M levels
as well as a significant effect for CD8M levels 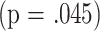 , consistent with the data in Table 3 showing a much stronger correlation between CD8M and longevity in mated females than in virgin females. The univariate correlation
, consistent with the data in Table 3 showing a much stronger correlation between CD8M and longevity in mated females than in virgin females. The univariate correlation 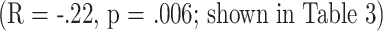 for CD8M levels versus longevity in mated female mice is also suggestive, but not definitive.
for CD8M levels versus longevity in mated female mice is also suggestive, but not definitive.
Fig. 1 presents scatterplots for the four T-cell subsets that have the strongest associations with life span when measured on 18-month-old mice. Single regression lines are shown for CD4, CD4M, and CD4V, for which the ANCOVA did not indicate a significant difference among the groups. For the CD4P scatterplot, the graph shows separate regression lines for each group, with the steepest slope corresponding to the virgin male group. Fig. 2 shows the scatterplot for CD8M cells, measured at 8 months of age, against longevity, with the steepest regression line corresponding to the data for the mated female animals. The strongest association, that is, for CD4P measured in virgin males at 18 months, corresponds to an increase in longevity of ∼60 days, that is, 7% of the mean life span, for every 10 percentage point decrease in the CD4P level. It is clear from these scatterplots, as well as from Table 3 , that variation among mice in T-cell subsets accounts for only a small, though significant, proportion of the variance among mice in longevity.
Figure 1.
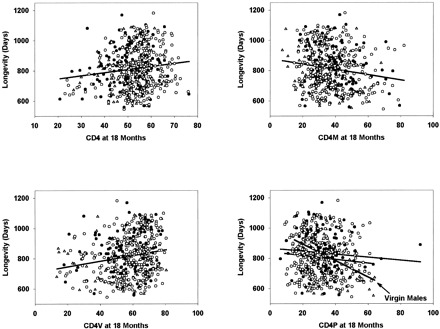
Selected scatterplots showing longevity as a function of T-cell subset values measured when the mice were 18 months of age: ▴, data from virgin males; •, virgin females; ○, mated females. Lines were calculated by least-squares regression for all mice combined, except that separate lines are shown for the CD4P scatterplot, for which a regression analysis revealed a significant interaction with group.
Figure 2.
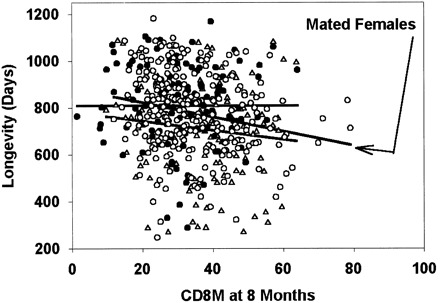
Scatterplot showing longevity related to CD8M values measured when the mice were 8 months of age. Symbols are the same as in Fig. 1. A separate regression line is shown for each group of mice; the arrow indicates the group (mated females) with the strongest association between CD8M and life span.
Stepwise multiple regression analyses were conducted to determine to what extent the T-cell subset measures might provide independent predictors of longevity. Table 5 shows the results of these analyses. For subsets measured when the mice were 8 months of age, the only significant result was obtained for mated female mice; for this group the final regression equation included two terms (CD8M and CD4), and it yielded 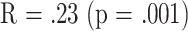 . For 18-month-old mice the calculations indicated significant regression equations for all three groups of mice, but with different optimal combinations of predictor variables.
. For 18-month-old mice the calculations indicated significant regression equations for all three groups of mice, but with different optimal combinations of predictor variables.
Table 5.
Prediction of Longevity by Using a Stepwise Linear Regression Approach
| Group | Age (mo) | R | p(F) | Subset | Beta (± SE) | p | n | Other |
| Mated females | 8 | .23 | .001 | CD8M | −.19 (.07) | .004 | 260 | CD4 |
| Mated females | 18 | .23 | .001 | CD4V | .14 (.07) | .005 | 263 | CD4 |
| Virgin females | 18 | .32 | .004 | CD8 | .26 (.10) | .02 | 130 | |
| CD4M | −.20 (.09) | .02 | 135 | |||||
| CD4 | .24 (.11) | .03 | 132 | |||||
| Virgin males | 18 | .44 | .0006 | CD4P | −.37 (.12) | .002 | 89 | CD8P, CD4M |
Notes: Forward stepwise linear regression was used to predict longevity for each group (at age 8 or 18 months) as a function of the seven T-cell subset levels, using F = 1 as an entry criterion for each successive independent variable. R shows the regression coefficient for the final equation, and p(F) provides a significance test for the regression. Each T-cell subset where p < .05 is listed along with its standardized regression coefficient beta (± its standard error), along with the p value for the subset; n is the number of mice with a measurement for the subset in that group. The “Other” column shows subsets also included in the regression equation, but with p > .05.
Cluster Analysis
I conducted a cluster analysis to determine whether the subset data would divide the mice into populations that differed in life span. This analysis used the k-means clustering algorithm, which seeks to sort individual mice into maximally disparate groups based on their position in an N-dimensional “space”—in this case the space in question has seven dimensions, one for each of the seven T-cell subsets measured when the mice were 18 months of age. The algorithm sorted the entire set of tested mice (males, virgin females, and mated females combined) into two clusters of maximal disparity of subset patterns, and it is important to note that the clusters were defined without any reference to the life-span values. Cluster 1 contained 182 mice, and Cluster 2 contained 320 mice. Fig. 3 (left panel) shows the mean values for each subset in these two clusters. Compared with the mice in Cluster 2, those in Cluster 1 tend to have relatively more CD4M, CD8M, and CD4P cells, and relatively few CD4V cells, all characteristic of relatively aged mice. To see if mice in these clusters differed in life span, I conducted an analysis of variance in which longevity was modeled as a function of cluster, mouse group, and cluster-by-group interaction. The fit to this model showed a significant 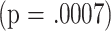 ability of cluster to predict longevity; the effect of group was marginal
ability of cluster to predict longevity; the effect of group was marginal 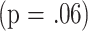 , and there was no evidence for an interaction term
, and there was no evidence for an interaction term 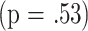 . The right panel of Fig. 3 presents life-span values for all mice and for the three groups considered individually. The mean longevity of mice in Cluster 1 (784 days; 95% confidence limits, 763–806) is significantly lower than that of mice in Cluster 2 (834 days; 95% confidence limits, 815–850), and a similar disparity is seen in each of the three groups differing in gender and reproductive history. Three cluster solutions defined by using data from 18-month-old mice did not provide additional insights, nor did two- or three-cluster solutions using data from 8-month-old animals.
. The right panel of Fig. 3 presents life-span values for all mice and for the three groups considered individually. The mean longevity of mice in Cluster 1 (784 days; 95% confidence limits, 763–806) is significantly lower than that of mice in Cluster 2 (834 days; 95% confidence limits, 815–850), and a similar disparity is seen in each of the three groups differing in gender and reproductive history. Three cluster solutions defined by using data from 18-month-old mice did not provide additional insights, nor did two- or three-cluster solutions using data from 8-month-old animals.
Figure 3.
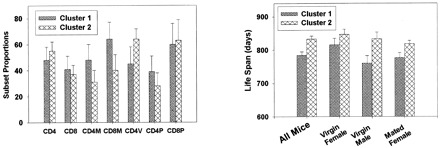
Cluster analysis using data from 18-month-old mice. The left panel shows mean values (±SEM) for each subset in the two clusters defined by a k-means clustering algorithm based on subset data alone; the right panel presents mean longevity (±SEM) for mice in each of the two clusters, first for all mice (left pair of bars), and then separately for the three groups.
Discussion
The data show clearly that measurements of peripheral blood T-cell subset proportions in 18-month-old mice provide information about individual differences in life expectancy of mice in a genetically heterogeneous population raised under specific pathogen-free conditions. After adjustment (Table 4 ) for covariates of gender and (for females) reproductive history, four of the seven tested subsets reach the Bonferroni-corrected significance threshold of 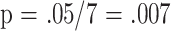 , and the three strongest associations are highly significant at p < .0002. Univariate correlations (Table 3 ) calculated separately for each of the three groups suggest that the ability of individual subset markers to predict longevity may vary somewhat with gender and after mating, but only the CD4P subset exhibited a significant interaction effect, as it was strongly associated with longevity only in male animals. Subsets measured when the mice were 8 months of age are unable to predict longevity in virgin males or females, but there is suggestive evidence that high levels of CD8M cells predict short life span in mated females (p = .006 in this subset by linear regression.)
, and the three strongest associations are highly significant at p < .0002. Univariate correlations (Table 3 ) calculated separately for each of the three groups suggest that the ability of individual subset markers to predict longevity may vary somewhat with gender and after mating, but only the CD4P subset exhibited a significant interaction effect, as it was strongly associated with longevity only in male animals. Subsets measured when the mice were 8 months of age are unable to predict longevity in virgin males or females, but there is suggestive evidence that high levels of CD8M cells predict short life span in mated females (p = .006 in this subset by linear regression.)
It is noteworthy that for each of the associations, short life span is associated with a T-cell subset level characteristic of relatively old animals. Thus aging, in these UM-HET3 mice as in nearly all mouse stocks previously examined (12)(13)(14), tends to increase CD4M, CD8M, and CD4P proportions, and to decrease CD4 and CD4V cell levels. The correlations documented in this study are thus all in an age-consistent direction, with the “older” subset values associated with early mortality. The stepwise linear regression calculations illustrated in Table 5 show that the three groups of mice differ somewhat in which sets of T-cell subsets are optimal predictors of longevity, but because these are themselves strongly correlated by the time the mice are 18 months of age (Table 2 ), the optimal set of predictors is only slightly more efficient than other sets of the independent variables when examined by best-subset regression methods (not shown).
These associations, though statistically significant, are relatively weak: no correlation in Table 3 exceeds 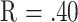 , and the scatterplots (Fig. 1 and Fig. 2) illustrate the wide ranges of life span among mice with equivalent T-cell subset values. Age at death is likely to reflect the combined influence of many genetic, environmental, and stochastic factors, and conversely patterns of immune system change are likely to be responsive to a variety of influences, not all of them connected to immune aging or late-life pathology. Thus I do not find surprising the absence of a perfect correspondence between T-cell subset levels and longevity, but I believe that my present evidence argues strongly for some form of physiological linkage between immunity and longevity.
, and the scatterplots (Fig. 1 and Fig. 2) illustrate the wide ranges of life span among mice with equivalent T-cell subset values. Age at death is likely to reflect the combined influence of many genetic, environmental, and stochastic factors, and conversely patterns of immune system change are likely to be responsive to a variety of influences, not all of them connected to immune aging or late-life pathology. Thus I do not find surprising the absence of a perfect correspondence between T-cell subset levels and longevity, but I believe that my present evidence argues strongly for some form of physiological linkage between immunity and longevity.
What is the basis for these associations? Consider three classes of hypothesis that differ in their directions of causality. The least interesting of these explanations involves the notion that illness, clinical or preclinical, leads both to early death and to altered subset proportions. This seems distinctly unlikely for several reasons, including the long delay between subset determination and death (mean of ∼8 months even for the subsets tested when the mice were 18 months of age), because of the wide range of causes of death in this heterogeneous population (9), and because the pattern of subset changes associated with diminished survival is precisely that which would be expected in accelerated aging.
Another class of models supposes that diminished immune function, as monitored by alterations in T-cell subset proportions, might in itself lead to relatively early illness and death. The third variety postulates that mice age at different rates, and that those mice in which aging is particularly rapid exhibit both earlier death and earlier changes in age-sensitive T-cell subsets. I favor the latter of these, because it is difficult to see how altered immunity per se would predispose to multiple causes of death, and because of an extensive body of work (15) testing, and failing to confirm, immune surveillance models of cancer causation. The latter model makes the prediction that youthful levels of immune status should be associated not merely with extended survival, as in the current study, but also with relatively youthful results on many other tests of age-sensitive traits, including those (e.g., patterns of liver gene expression, or collagen cross-linking, or muscle strength) unlikely to be altered by, or to alter, immune subset distributions. My colleagues and I have some preliminary data (16) suggesting an association between high CD4M levels and relatively weak muscle strength, and we are currently examining a much broader array of age-sensitive traits in immunologically characterized four-way cross mice.
A gene mapping effort now underway has already produced strong evidence (17) for a set of genetic loci whose polymorphisms variously influence levels of CD4, CD4M, CD8M, CD4V, CD4P, and CD8P T-cell subsets in UM-HET3 mice, and higher power studies, involving larger numbers of animals, are expected to extend the current understanding of how genetic variation might contribute to interanimal differences in T-cell subset levels and in longevity. Assessment of T-cell subset levels at two ages has made it possible to document quantitative trait loci that influence age-sensitive subset levels in the first 8 months of life, and other quantitative trait loci whose effect on subsets is seen only later, that is, within the 8- to 18-month interval. My colleagues and I think that both genetic and nongenetic factors are likely to contribute to interanimal variations in both immune status and life span.
The cluster analysis suggests that the effects of aging on T-cell subsets may be modulated by a relatively small number of factors. These results, illustrated in Fig. 3, showed that when mice are sorted into two groups based solely on patterns of T-cell subset levels, the groups turn out to differ significantly in life span. There is no a priori reason to expect that clusters formed on the basis of differences in T-cell subset levels should necessarily differ in longevity, and this unexpected finding therefore suggests that the immunological differences among these mice reflects some physiological dichotomy associated in a fundamental way with differences in survival and disease risks in later life.
This finding is reminiscent of the observation that tests of T-cell function conducted in relatively healthy octogenarians can be used to divide the population into three clusters, one of which was found to have a higher 2-year survival than the other two (18). In this study, improved short-term survival was associated with better T-cell proliferation, low CD8 cells, and high CD4 cells, but the protocol did not include an assessment of memory cell levels or of T-cell P-glycoprotein expression. I do not know of any previous study, in rodents or humans, in which a cluster analysis based on midlife assessments of immune status has been used to examine associations with longevity, and indeed such an analysis would be difficult to conduct on long-lived species. My data on mice suggest, however, that longitudinal studies of human populations might benefit from the inclusion of midlife assessments of age-sensitive immune status tests as potential predictors of later trajectories of age-dependent decline in multiple physiological domains.
Acknowledgments
This work was supported by National Institute on Aging Grants AG1187 and AG16699, and by core facilities supported by Grant AG08808. I thank Dr. Andrzej Galecki for statistical suggestions and Luann Linsalata and Gretchen Buehner for technical assistance.
Decision Editor: Edward J. Masoro, PhD
References
- 1.Masoro EJ, 1995. Aging: current concepts. Masoro EJ, , ed.Handbook of Physiology. Section 11: Aging 3-21. Oxford University Press, New York.
- 2.Holliday R, 1999. Understanding Ageing Cambridge University Press, Cambridge, UK.
- 3.Costa PT, McCrae RR, 1988. Measures and markers of biological aging: “a great clamoring…of fleeting significance.”. Arch Gerontol Geriatr 7:211-214. [DOI] [PubMed] [Google Scholar]
- 4.Costa PT, McCrae RR, 1995. Design and analysis of aging studies. Masoro EJ, , ed.Handbook of Physiology. Section 11: Aging 25-36. Oxford University Press, New York.
- 5.Miller RA, 1997. When will the biology of aging become useful? Future landmarks in biomedical gerontology. J Am Geriatr Soc 45:1258-1267. [DOI] [PubMed] [Google Scholar]
- 6.Harrison DE, Archer JR, 1983. Physiological assays for biological age in mice: relationship of collagen, renal function, and longevity. Exp Aging Res 9:245-251. [DOI] [PubMed] [Google Scholar]
- 7.Harrison DE, Archer JR, Sacher GA, Boyce FMI, 1978. Tail collagen aging in mice of thirteen different genotypes and two species: relationship to biological age. Exp Gerontol 12:63-73. [DOI] [PubMed] [Google Scholar]
- 8.Boersma WJA, Steinmeier FA, Haaijman JJ, 1985. Age-related changes in the relative numbers of Thy-1 and Lyt-2-bearing peripheral blood lymphocytes in mice: a longitudinal approach. Cell Immunol 93:417-430. [DOI] [PubMed] [Google Scholar]
- 9.Miller RA, Chrisp C, Galecki A, 1997. CD4 memory T cell levels predict lifespan in genetically heterogeneous mice. FASEB J 11:775-783. [DOI] [PubMed] [Google Scholar]
- 10.Miller RA, Turke P, Chrisp C, et al. 1994. Age-sensitive T cell phenotypes covary in genetically heterogeneous mice and predict early death from lymphoma. J Gerontol Biol Sci. 49:B255-B262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roderick TH, 1963. Selection for radiation resistance in mice. Genetics. 48:205-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller RA, 1997. Age-related changes in T cell surface markers: a longitudinal analysis in genetically heterogeneous mice. Mech Ageing Dev 96:181-196. [DOI] [PubMed] [Google Scholar]
- 13.Witkowski JM, Miller RA, 1993. Increased function of P-glycoprotein in T lymphocytes of aging mice. J Immunol. 150:1296-1306. [PubMed] [Google Scholar]
- 14.Miller RA, 1990. Aging and the immune response. Schneider EL, Rowe JW, , ed.Handbook of the Biology of Aging 157-180. Academic Press, San Diego, CA.
- 15.Ershler WB, 1993. The influence of an aging immune system on cancer incidence and progression. J Gerontol Biol Sci. 48:B3-B7. [DOI] [PubMed] [Google Scholar]
- 16.Miller RA, Bookstein F, van der Meulen JH, et al. 1996. Candidate biomarkers of aging: age-sensitive indices of immune and muscle function co-vary in genetically heterogeneous mice. J Gerontol Biol Sci. 52A:B39-B47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson AU, Fornes A, Galecki A, Miller RA, Burke DT, 1999. Longitudinal QTL analysis of T cell phenotypes in a population of four-way cross mice. Genetics. 151:785-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferguson FG, Wikby A, Maxson P, Olsson J, Johansson B, 1995. Immune parameters in a longitudinal study of a very old population of Swedish people: a comparison between survivors and nonsurvivors. J Gerontol Biol Sci. 50A:B378-B382. [DOI] [PubMed] [Google Scholar]


