Abstract
Background
Elderly patients are susceptible to general anesthetics, with a higher bispectral index (BIS) at loss of consciousness (LOC) achieved by propofol infusion compared with young patients. Overexposure to general anesthetics can have adverse effects such as inadequate emergence and postoperative delirium (PD). This study aimed to compare the effects of BIS-guided individualized anesthesia with standard general anesthesia on emergence and delirium after esophagectomy.
Material/Methods
Data on 161 elderly patients undergoing esophagectomy for cancer were retrospectively obtained from electronic medical records. We performed propensity score matching analysis between patients receiving individualized anesthesia (BIS value maintained at about 10 less than the value at LOC) and those receiving standard anesthesia (BIS value maintained at 40–60). In addition, we conducted univariate and multivariate logistic analyses in the entire cohort.
Results
Patients receiving individualized anesthesia had higher BIS values and a lower propofol requirement during surgery than those receiving standard general anesthesia (P<0.05). The overall incidences of inadequate emergence and PD were 37.9% and 18.0% (n=161), respectively. Logistic regression analysis revealed that the independent risk factors for PD were organic brain disease (odds ratio [OR] 6.308; 95% confidence interval [CI] 2.458–16.187) and inadequate emergence (OR 4.063; 95% CI 1.645–10.033).
Conclusions
BIS-guided individualized anesthesia (lighter) does not reduce inadequate emergence or PD compared with standard general anesthesia in elderly patients undergoing esophagectomy. Independent risk factors for PD include organic brain disease and inadequate emergence.
MeSH Keywords: Anesthesia, General; Delayed Emergence from Anesthesia; Delirium; Esophagectomy; Postoperative Complications
Background
As the world population ages, the proportion of elderly patients requiring surgical services and anesthesia is increasing rapidly [1,2]. Less anesthetic is required to achieve the same hypnotic state in elderly patients compared with younger patients [3]. The lower requirement for anesthetics in elderly patients is attributed to age-related declines in cardiovascular, respiratory, hepatic, and renal function [4]. However, the primary action sites of general anesthetics are in the central nervous system. The anatomy and physiology of brain also show age-related changes, such as cortical thinning [5], decreased numbers of dendritic spines of the pyramidal neurons [6], loss of white matter and ventricle enlargement [7], declines in neuroprotection and neurogenesis mechanisms [8], and increased susceptibility to oxidative stress and inflammation [9]. Therefore, it is not surprising that elderly patients are susceptible to adverse effects from general anesthetics. However, the neurotoxicity of general anesthetics is unclear [10–12].
The bispectral index (BIS) serves as an objective indicator based on electroencephalographic (EEG) analysis. It is widely used to track the level of unconsciousness and to guide anesthetic administration, so as to reduce the risk of awareness and to improve postoperative recovery [13,14]. Overexposure to general anesthetics can have adverse effects such as inadequate emergence and postoperative delirium (PD). Prior studies have found a reduced incidence of PD and cognitive decline [12,15–18], and even reduced morbidity and mortality [19–22], when anesthesia is performed with the guidance of BIS monitoring or when the BIS is targeted to a higher value. PD occurs frequently, affecting up to 50% of elderly patients (≥65 years), and it is associated with short- and long-term negative outcomes, such as increased length of hospital stay, cognitive decline, and postdischarge mortality [22–25]. Nonetheless, the effects of depth of anesthesia on postoperative cognition are controversial.
BIS values of 40–60 have been suggested to be appropriate for surgical anesthesia. Previous studies demonstrated a higher BIS value at loss of consciousness (LOC) achieved by propofol infusion in elderly patients compared with young patients [26,27]. However, standard general anesthesia maintained at BIS values of 40–60 may be deeper or too deep in elderly patients. A BIS-guided individualized anesthesia technique was used in elderly patients undergoing esophagectomy for cancer in our institute to avoid unexpected deep anesthesia and to improve postoperative emergence. Compared with standard general anesthesia, the BIS-guided individualized anesthesia was based on maintaining the BIS value at about 10 less than the value at LOC as determined by the combined Observer’s Assessment of Alertness/Sedation Scale (OAA/S) in each patient. However, little is known about the benefits of this individualized anesthesia in elderly patients. Thus, we performed a retrospective cohort study with propensity score-matched analysis in these patients to evaluate the effects of the BIS-guided individualized anesthesia on inadequate emergence from anesthesia and PD.
Material and Methods
Participants and study design
This retrospective cohort study was approved by the Ethics Committee of Jining No. 1 people’s hospital on October 16, 2018 (#2018-006). The informed written consent was waived because the study was only analyzing data from previous medical records obtained between January 2015 and August 2018. Eligibility criteria for the current study were esophagectomy for cancer, age ≥65 years, total intravenous anesthesia, and BIS monitoring. Patients with missing data or perioperative administration of hypnotics were excluded. Data and patient identification were processed anonymously before analysis. The patients were divided into 2 groups based on how the targeted BIS values were obtained: standard general anesthesia group (group S, BIS value maintained at 40–60) and individualized anesthesia group (group I, BIS value maintained at about 10 less than the value at LOC). The group division was not blindly randomized. Patients included in the final analysis in group I were from those undergoing esophagectomy, following the acquisition of fund support and ethical approval for individualized anesthesia in 2017, while patients in group S were mainly from before then.
Anesthetic management
The applicability of thoracic paravertebral block or epidural anesthesia was determined by the anesthesiologists in charge. One or the other was performed at the level of T7/8 before induction with propofol. All patients received BIS-guided total intravenous anesthesia. BIS was continuously monitored with a BeneView T8 Mindray monitoring device (Mindray International Medical Co., Ltd., Shenzhen, China). A BIS value was developed by a built-in EEG analysis algorithm every 15 s and automatically recorded at an interval of 10 min.
In group I, the BIS value at LOC achieved with propofol administration was determined first. LOC was defined as no response to verbal commands or mild shaking, score 1 on the OAA/S scale. Propofol (Fresenius Kabi Austria GmbH, Graz, Austria) 0.5 mg/kg was injected intravenously every 15 s, and meanwhile, the patient was asked to open his or her eyes by verbal command and mild shaking of the shoulder until LOC. The number shown on the BIS monitor immediately after unconsciousness was defined as the BIS value at LOC. After that, propofol infusion was started at 3 to 5 mg/kg/h to achieve a BIS value of about 10 less than the value at LOC, and then sufentanil (Yichang Humanwell Pharmaceutical Co., Ltd, Yichang, China) 0.3 μg/kg and cisatracurium (Jiangsu Hengrui Pharmaceutical Co., Ltd, Lianyungang, China) 0.2 mg/kg were administered intravenously. Two minutes after cisatracurium administration, an endotracheal tube was placed into the trachea. The rate of propofol infusion was adjusted to maintain the real BIS value during surgery at about 10 less than the BIS value at LOC. In group S, anesthesia was induced with propofol 1–2 mg/kg, sufentanil 0.3 μg/kg, and cisatracurium 0.2 mg/kg for endotracheal intubation, and maintained at a BIS value of around 50 with propofol infusion during surgery. If the real BIS deviated from the setpoint by more than 10 during surgery, the rate of propofol infusion was modified in both groups. Cisatracurium 0.1–0.2 mg/kg/h and remifentanil (Yichang Humanwell Pharmaceutical Co., Ltd, Yichang, China) 3–5 μg/kg/h were infused following intubation. Cisatracurium 5 mg was administered to improve muscle relaxation, and sufentanil 10 μg was used to improve intraoperative analgesia, as necessary. Cisatracurium and remifentanil were discontinued, and sufentanil 0.1 μg/kg was administered about 30 min before the end of surgery. Propofol infusion was discontinued at the end of the skin suture. Neostigmine (Shanghai Xinyi Pharmaceutical Co., Ltd, Shanghai, China) 0.04 mg/kg and atropine (Tianjin Jinyao Pharmaceutical Co., Ltd, Tianjin, China) 0.02 mg/kg were given to reverse residual muscle relaxation at the end of surgery. Postoperative analgesia was provided with either intravenous sufentanil 0.06 μg/kg/h or epidural ropivacaine (AstraZeneca AB, Sodertalje, Sweden) 0.2% at 2 mL/h via a patient-controlled analgesia pump (Jiangsu Aipeng Medical Technology Co. LTD, Nantong, China).
Data collection
All evaluated parameters were obtained by reviewing the electronic medical records, including demographics, BIS values and anesthetic requirements, emergence after anesthesia and PD, and perioperative adverse events. The primary outcomes included inadequate emergence and PD. The secondary outcomes were the BIS values during surgery, anesthetic requirements, awareness, and other perioperative adverse events. Inadequate emergence, PD, and awareness were assessed using the Riker sedation-agitation scale (SAS) in the postanesthetic care unit, the confusion assessment method every day within 7 postoperative days, and the Brice Questionnaire immediately before discharge from the postanesthetic care unit and on the 7th postoperative day by a nurse anesthetist who had undergone training on the use of these evaluation tools and was not involved in the management of the patients. Inadequate emergence included 2 types of emergence agitation and hypoactive emergence, as defined in previous studies [28,29]. Emergence agitation was defined with an SAS score of ≥5 in this study, and hypoactive emergence was defined with an SAS score of ≤2 maintaining ≥30 min from termination of propofol infusion as in our previous study [30].
Statistical analysis
All statistical tests were performed using SPSS (version 19.0; SPSS Inc.). The normality of the distribution was determined using the Kolmogorov-Smirnov test. Data were expressed as means±standard deviation (SD) for quantitative variables with normal distribution (e.g., age, weight, height), median (interquartile range, IQR) for quantitative variables with non-normal distribution (e.g., duration of surgery, BIS values at baseline, requirement of propofol for induction), and number (percent) for categorical variables (e.g., sex, ASA classification, comorbidities). The unpaired t test, Mann-Whitney U-test, and chi-square test or Fisher’s exact test were performed for statistical analysis as appropriate. Propensity score matching analysis was used to allow an unbiased comparison. Propensity score was calculated using a logistic regression model based on anesthetic type (0=standard general anesthesia, 1=individualized anesthesia) as the binary treatment indicator, with the following covariates: age, sex, body mass index, ASA classification, anesthetic technique, minimally invasive approach, and duration of surgery. A 1: 1 match between individualized anesthesia and standard general anesthesia groups was achieved using the nearest neighbor matching algorithm with a caliper definition of 0.02. The demographic and intervening variables associated with PD were analyzed by univariate analysis. Variables with a P-value <0.1 in univariate analysis, including age, ASA classification, organic brain disease, BIS baseline, inadequate emergence, and perioperative blood transfusion, were entered in multivariate logistic regression model. Odds ratios (OR) and 95% confidence intervals (CI) were calculated as estimates of relative risk. A value of P<0.05 was considered statistically significant.
Results
Study population
One hundred sixty-one patients were eligible for this cohort analysis, out of 551 patients who received esophagectomy during the study period. After propensity score matching, 58 patients in each group were selected (Figure 1). The demographic variables before and after propensity matching are presented according to the anesthetic type in Table 1. Although there were significant differences in duration of surgery between groups before propensity score matching, no significant difference was observed after matching (Table 1).
Figure 1.
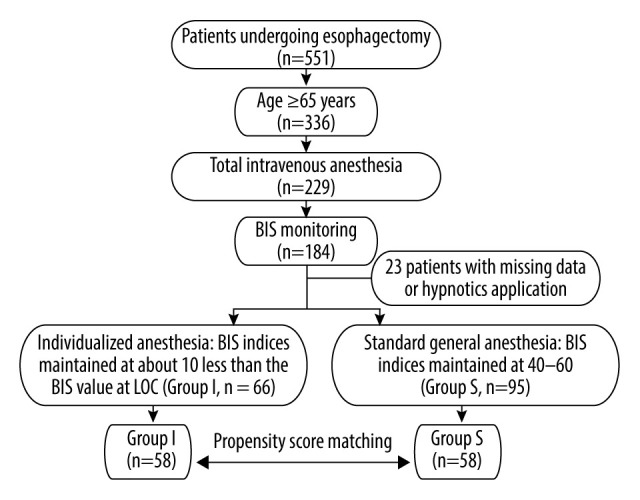
Flow diagram of patient enrollment in this study. BIS – bispectral index; LOC – loss of consciousness.
Table 1.
Patient demographics between groups before and after propensity score matching.
| Variable | Before matching | After matching | ||||
|---|---|---|---|---|---|---|
| Group I (n=66) | Group S (n=95) | P-value | Group I (n=58) | Group S (n=58) | P-value | |
| Age, yr | 70.2±4.3 | 70.3±4.1 | 0.856 | 70.2±4.1 | 70.2±3.7 | 0.962 |
| Male, n (%) | 43 (65.2) | 67 (70.5) | 0.471 | 39 (67.2) | 39 (67.2) | 1.000 |
| Weight, kg | 60.4±10.1 | 61.6±12.1 | 0.504 | 61.0±10.2 | 58.9±9.9 | 0.249 |
| Height, cm | 164.6±7.5 | 164.3±7.9 | 0.756 | 165.0±7.4 | 163.2±7.0 | 0.176 |
| BMI | 22.2±3.0 | 22.7±3.1 | 0.365 | 22.3±3.0 | 22.0±3.0 | 0.584 |
| ASA classification ≥3, n (%) | 32 (48.5) | 53 (55.8) | 0.361 | 28 (48.3) | 29 (50.0) | 1.000 |
| Comorbidities | ||||||
| Hypertension, n (%) | 23 (34.8) | 31 (32.6) | 0.770 | 19 (32.8) | 16 (27.6) | 0.544 |
| Coronary artery disease, n (%) | 25 (37.9) | 30 (31.6) | 0.407 | 22 (37.9) | 21 (36.2) | 0.848 |
| Diabetes mellitus, n (%) | 9 (13.6) | 5 (5.3) | 0.064 | 8 (13.8) | 4 (6.9) | 0.361 |
| COPD, n (%) | 17 (25.8) | 22 (23.2) | 0.705 | 14 (24.1) | 9 (15.5) | 0.244 |
| Organic brain disease, n (%) | 9 (13.6) | 22 (23.2) | 0.132 | 8 (13.8) | 13 (22.4) | 0.228 |
| Previous malignancy, n (%) | 4 (6.1) | 1 (1.1) | 0.180 | 4 (6.9) | 0 (0) | 0.119 |
| Anesthtic technique | ||||||
| TIVA only, n (%) | 17 (25.8) | 23 (24.2) | 17 (29.3) | 13 (22.4) | ||
| TIVA with PVB, n (%) | 34 (51.5) | 60 (63.2) | 0.191 | 30 (51.7) | 37 (63.8) | 0.419 |
| TIVA with EA, n (%) | 15 (22.7) | 12 (12.6) | 11 (19.0) | 8 (13.8) | ||
| Minimally invasive approach, n (%) | 19 (13.6) | 38 (0.40) | 0.143 | 18 (31.0) | 20 (34.5) | 0.692 |
| Type of surgical procedure | ||||||
| Sweet, n (%) | 44 (66.7) | 51 (53.7) | 37 (63.8) | 33 (56.9) | ||
| McKeown, n (%) | 21 (31.8) | 42 (44.2) | 0.257 | 20 (34.5) | 24 (41.4) | 0.744 |
| Ivor-Lewis, n (%) | 1 (1.5) | 2 (2.1) | 1 (1.7) | 1 (1.7) | ||
| Duration of anesthesia1, min | 252.2±52.5 | 268.2±60.6 | 0.084 | 251.2±54.5 | 268.7±65.2 | 0.119 |
| Duration of surgery2, min | 221 (200–272) | 240 (212–283) | 0.045 | 220 (196–274) | 239 (209–278) | 0.104 |
| Intraoperative fluid infusion, ml | 2164±481 | 2292±611 | 0.142 | 2178±501 | 2213±580 | 0.731 |
Values are expressed as mean±SD, n (%), or median (IQR). Groups: I, BIS indices maintained at about 10 less than the BIS value at LOC; S, BIS indices maintained at 40–60. LOC – loss of consciousness; BMI – body weight index; BIS – bispectral index; ASA – American Society of Anesthesiologist; COPD – chronic obstructive pulmonary disease; TIVA – total intravenous anesthesia; PVB – paravertebral block; EA, epidural anesthesia.
Duration of anesthesia: the time from anesthetic induction to the termination of propofol infusion;
Duration of surgery: the time from skin incision to skin suture.
Depth of anesthesia and requirements of anesthetics
Patients in group I had higher BIS values during surgery and a lower requirement for propofol for induction and maintenance, compared with those in group S, regardless of propensity matching (Table 2). No significant difference was observed in consumption of sufentanil, remifentanil, or cis-atracurium during surgery between groups, regardless of propensity matching (Table 2).
Table 2.
Comparison of bispectral values and anesthetic requirements between groups before and after propensity score matching.
| Variable | Before matching | After matching | ||||
|---|---|---|---|---|---|---|
| Group I (n=66) | Group S (n=95) | P-value | Group I (n=58) | Group S (n=58) | P-value | |
| BIS at baseline | 97 (94–97) | 97 (94–98) | 0.489 | 97 (94–97) | 97 (94–98) | 0.800 |
| BIS at LOC | 75.0±5.5 | n.a. | n.a. | 74.8±5.6 | n.a. | n.a. |
| BIS minimal during surgery | 47.6±6.5 | 38.0±7.3 | 0.000 | 48.1±6.6 | 38.1±7.8 | 0.000 |
| BIS maximal during surgery | 68.2±6.2 | 60.0±6.3 | 0.000 | 68.4±6.4 | 60.3±5.0 | 0.000 |
| BIS average during surgery | 57.9±5.4 | 49.3±5.4 | 0.000 | 58.3±5.6 | 49.2±5.4 | 0.000 |
| Requirements of anesthetics during surgery | ||||||
| Propofol for induction, mg | 75 (57.5–100) | 100 (75–120) | 0.000 | 75 (60–100) | 100 (75–120) | 0.003 |
| Propofol for maintenance, mg·kg−1·h−1 | 3.6±1.4 | 4.5±1.9 | 0.001 | 3.7±1.4 | 4.4±1.9 | 0.013 |
| Sufentanil, μg/kg | 0.62±0.14 | 0.67±0.16 | 0.159 | 0.62±0.14 | 0.66±0.16 | 0.160 |
| Remifentanil, μg·kg−1·h−1 | 4.20 (4.11–4.28) | 4.15 (4.06–4.23) | 0.088 | 4.20 (4.11–4.27) | 4.15 (4.04–4.24) | 0.177 |
| Cisatracurium, mg·kg−1·h−1 | 0.126 (0.124–0.128) | 0.125 (0.123–0.128) | 0.413 | 0.126 (0.124–0.130) | 0.125 (0.123–0.127) | 0.235 |
Values are expressed as median (IQR) or mean±SD. Groups: I, BIS indices maintained at about 10 less than the BIS value at LOC; S, BIS indices maintained at 40–60. LOC – loss of consciousness; BIS – bispectral index; n.a. – not available.
Emergence after anesthesia and PD
The eye-opening time and the extubation time were lower in group I than in group S, regardless of propensity matching (Table 3). No significant difference was found between groups in inadequate emergence (including emergence agitation and delayed emergence) and the occurrence of PD, regardless of propensity matching (Table 3).
Table 3.
Comparison of emergence after anesthesia and postoperative delirium (PD) between groups before and after propensity score matching.
| Variable | Before matching | After matching | ||||
|---|---|---|---|---|---|---|
| Group I (n=66) | Group S (n=95) | P-value | Group I (n=58) | Group S (n=58) | P-value | |
| Eye-opening time1, min | 11 (8–15) | 15 (12–20) | 0.000 | 11 (8–15) | 16 (12–20) | 0.000 |
| Extubation time2, min | 12.5 (9–16) | 17 (13–22) | 0.000 | 13 (9–17) | 17 (14–20) | 0.000 |
| Inadequate emergence | 20 (30.3) | 41 (43.2) | 0.098 | 18 (31.0) | 25 (43.1) | 0.178 |
| Emergence agitation3, n (%) | 18 (27.3) | 35 (36.8) | 0.204 | 14 (24.1) | 20 (34.5) | 0.221 |
| Delayed emergence4, n (%) | 4 (6.1) | 6 (6.3) | 1.000 | 4 (6.9) | 5 (8.6) | 1.000 |
| Postoperative delirium, n (%) | 9 (13.6) | 20 (21.1) | 0.228 | 7 (12.1) | 12 (20.7) | 0.210 |
Values are expressed as median (IQR) or n (%). Groups: I, BIS indices maintained at about 10 less than the BIS value at LOC; S, BIS indices maintained at 40–60. PD – postoperative delirium; BIS – bispectral index; LOC – loss of consciousness, SAS – Riker sedation-agitation scale; PACU – postanesthetic care unit.
Eye-opening time: the time from the termination of propofol infusion to eye-opening on verbal command;
Extubation time: the time from the termination of propofol infusion to the tube removal out of the tracheal;
Emergence agitation: SAS score ≥5 at 1 min after extubation and in PACU;
Delayed emergence: duration ≥30 min with SAS score ≤2 from termination of propofol infusion.
Adverse events
No significant difference was found between groups in intraoperative cardiovascular events (including hypertension and hypotension), perioperative blood transfusion, intensive care unit admission, secondary surgery, and mortality in hospital, whether propensity matching or not (Table 4). Possible awareness occurred in only one patient in group I, who received an epidural anesthesia with total intravenous anesthesia for surgery (Table 4).
Table 4.
Comparison of adverse events between groups before and after propensity score matching.
| Variable | Before matching | After matching | ||||
|---|---|---|---|---|---|---|
| Group I (n=66) | Group S (n=95) | P-value | Group I (n=58) | Group S (n=58) | P-value | |
| Hypertension1, n (%) | 28 (42.4) | 38 (0.40) | 0.758 | 24 (41.4) | 25 (43.1) | 0.851 |
| Hypotension2, n (%) | 21 (31.8) | 21 (22.1) | 0.167 | 18 (31.0) | 12 (20.7) | 0.203 |
| Blood transfusion3, n (%) | 7 (10.6) | 13 (13.7) | 0.560 | 6 (10.3) | 6 (10.3) | 1.000 |
| ICU admission, n (%) | 1 (1.5) | 4 (4.2) | 0.649 | 1 (1.7) | 3 (5.2) | 0.618 |
| Second surgery, n (%) | 1 (1.5) | 0 (0) | 0.410 | 1 (1.7) | 0 (0) | 1.000 |
| In-hospital mortality, n (%) | 1 (1.5) | 1 (1.1) | 1.000 | 1 (1.7) | 1 (1.7) | 1.000 |
| Awareness, n (%) | 1 (1.5) | 0 (0) | 0.410 | 0 (0) | 0 (0) | 1.000 |
Values are expressed as mean±standard deviation or number of patients (n, %). Groups: I, BIS indices maintained at about 10 less than the BIS value at LOC; S, BIS indices maintained at 40–60. ICU – Intensive Care Unit; BIS – bispectral index; LOC – loss of consciousness.
Hypertension: use of any vasodilator during surgery to maintain systolic blood pressure no more than 140 mmHg;
Hypotension: use of any vasopressor during surgery to maintain systolic blood pressure no less than 90 mmHg;
Blood transfusion: the number of patients receiving blood transfusion during surgery and/or after surgery.
Risk factors for PD
The incidence of PD was similar between groups before and after propensity score matching. The overall incidence of PD was 18.0% (n=161, Table 3). Table 5 summarizes the results of both the univariate and multivariate analyses of the risk factors for PD. On multivariate analysis, the independent risk factors for PD were organic brain disease (OR 6.308; 95% CI 2.458–16.187) and inadequate emergence (OR 4.063; 95% CI 1.645–10.033).
Table 5.
Univariate and multivariate analyses of factors associated with postoperative delirium (PD) in the entire cohort.
| variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Individualized vs. standard anesthesia | 0.592 | 0.251–1.398 | 0.232 | |||
| Age | 1.113 | 1.016–1.220 | 0.021 | |||
| BMI | 0.992 | 0.869–1.132 | 0.902 | |||
| Gender (Male vs. Female) | 0.711 | 0.308–1.643 | 0.425 | |||
| ASA classification ≥3 | 4.328 | 1.655–11.318 | 0.003 | |||
| Organic brain disease | 6.767 | 2.761–16.585 | 0.000 | 6.308 | 2.458–16.187 | 0.000 |
| Minimally invasive approach | 0.522 | 0.208–1.310 | 0.166 | |||
| Anesthesia technique | ||||||
| GA | 1 | Referent | ||||
| GA+EA | 0.570 | 0.231–1.406 | 0.222 | |||
| GA+PVB | 0.916 | 0.277–3..031 | 0.886 | |||
| Duration of anesthesia | 1.001 | 0.995–1.008 | 0.685 | |||
| Duration of surgery | 1.002 | 0.995–1.009 | 0.623 | |||
| Intraoperative fluid infusion | 1.000 | 0.999–1.001 | 0.983 | |||
| BIS baseline | 0.887 | 0.797–0.989 | 0.030 | |||
| BIS minimal during surgery | 1.026 | 0.978–1.076 | 0.296 | |||
| BIS maximal during surgery | 0.989 | 0.938–1.043 | 0.688 | |||
| Hypertension during surgery | 0.592 | 0.251–1.398 | 0.232 | |||
| Hypotension during surgery | 1.991 | 0.850–4.664 | 0.113 | |||
| Inadequate emergence | 4.370 | 1.866–10.234 | 0.001 | 4.063 | 1.645–10.033 | 0.002 |
| Perioperative blood transfusion | 2.913 | 1.045–8.120 | 0.041 | |||
PD – postoperative delirium; BMI – body weight index; ASA – American Society of Anesthesiologist; GA – general anesthesia; PVB – paravertebral block; EA – epidural anesthesia; BIS – bispectral index; OR – odd ratios; CI – confidence intervals.
Discussion
This retrospective study explored the effects of individualized anesthesia on postoperative emergence and PD, guided by the BIS value at LOC. As the BIS values during surgery showed, anesthetic depth was lighter in patients receiving individualized anesthesia than those receiving standard anesthesia. Individualized anesthesia reduced propofol requirement and shortened emergence from anesthesia compared with standard general anesthesia. However, no significant difference was observed in emergence quality and the occurrence of PD between the 2 anesthetic regimens, in either the entire cohort or the propensity-matched cohort analysis.
Behavioral disturbances are not uncommon in the perioperative period. Inadequate emergence occurs in 8.2% to 15% of a population regardless of age, surgical type, or anesthetic type [28,29]. However, Neufeld and colleagues [23] described a higher incidence of emergence agitation, up to 45%, in patients aged ≥70 years undergoing major surgical procedures. In this study, emergence agitation occurred in 32.9% of the patients overall. Hypoactive emergence, which was considered to be the equivalent of delayed emergence as in our previous study [30], occurred in 6.2% of the patients overall. However, as our results showed, individualized anesthesia did not reduce the risk of inadequate emergence compared with standard general anesthesia. A recent study demonstrated that BIS-guided closed-loop intravenous anesthesia markedly decreased emergence delirium due to a higher adequate level of anesthesia [31].
In previous studies, PD was associated with deeper anesthesia or burst depression of ECG, and BIS-guided anesthesia reduced the occurrence of PD [15–18]. However, these conclusions were not supported in several recent studies [32,33]. The brain of elderly patients is vulnerable to general anesthetics. Theoretically, BIS-guided individualized anesthesia could be helpful to provide an appropriate depth of anesthesia (not too deep or too light). Prior studies found a higher BIS value at LOC in elderly patients [26,27]. In the current study, we found that the BIS value at LOC was 75.0±5.5 in elderly patients aged ≥65 years, similar to that reported by Lysakowski and colleagues [26]. Despite the anesthetic depth being individualized and possibly more appropriate in elderly patients receiving individualized anesthesia than in those receiving standard anesthesia, our results showed no difference in the occurrence of PD between the 2 anesthetic regimens. Intraoperative awareness was an adverse event during individualized anesthesia due to possible awareness occurring in one patient. In the current study, the setpoint of anesthesia depth was maintained at about 10 less than the BIS value at LOC in patients receiving individualized anesthesia, thus reducing the fluctuation of the BIS value above that at LOC and the risk of accidental awareness due to study design. A previous study reported that no awareness occurred in patients aged ≥60 years, who received light anesthesia with an average BIS value of 62.6±1.5, with the maximum BIS value set at 65 [34], which was similar to the average BIS value of 57.9±5.4 in elderly patients receiving individualized anesthesia in the current study. One patient with possible awareness did not indicate that the BIS-guided individualized anesthesia was associated with an increased likelihood of awareness.
PD is a common surgical complication in patients undergoing esophagectomy, and it is associated with negative outcomes, such as increased incidence of postoperative complications and mortality after discharge [35]. Two recent studies reported that the incidence of delirium after esophagectomy ranged from 16.9% to 32% [36,37], which may not be significantly different from that of 9.2% to 26% and up to 50% reported in several earlier studies [35,38,39]. In the present study, PD occurred in 18.0% of the patients overall. We confirmed that 2 predisposing factors were associated with delirium after esophagectomy, including organic brain disease and inadequate emergence. Presurgical cognitive impairment is an independent risk factor for delirium development after multiple types of surgical procedures including esophagectomy [40], and patients with organic brain disease have an increased likelihood of preexisting cognitive decline. However, presurgical assessment of cognitive function is not routinely conducted in our clinical practice. Emergence agitation, one type of inadequate emergence, is also identified as a factor associated with PD [23]. In contrast to delirious children who present as hyperactive with crying and agitation, elderly patients with PD in postoperative intensive care unit present primarily with a hypoactive form [41,42]. It is still debated whether hypoactive emergence is a form of delirium or simply delayed emergence from anesthesia. Our study found no association of duration of surgery or perioperative blood transfusion with PD, consistent with the results reported by Takeuchi and colleagues [38]. Our findings also showed that minimally invasive surgery and combined nerve block were not protective against PD, contrary to the report by Dezube and colleagues [37]. Delirium is also thought to be the sequela of other complications, such as pneumonia and respiratory complications [21,35], but the relevant data were lacking for many patients in our medical records system.
Several limitations should be considered when interpreting the results from our analysis. First, this study had a retrospective design. Certain confounding factors may affect the results of our study despite our attempt to address the possibility through a propensity score matching analysis. Second, this study was based on data from a single center. BIS-guided individualized anesthesia was an attempt in our institute to provide appropriate anesthesia, not lighter anesthesia in elderly patients. Our small sample size could have reduced the power to detect clinically important differences in some of the outcomes, such as individualized anesthesia (lighter) on PD. Two recent randomized trials still demonstrated the potential benefits of lighter anesthesia [34,43]. In one of these studies, lighter sedation (BIS values of 82.3±9.4) reduced the development of delirium in elderly patients with a Charlson comorbidity index of 0, compared with heavier sedation (BIS values of 57.0±14.8) by subgroup analysis [43]. In the other study, lighter anesthesia (BIS values of 62.6±1.5) decreased the development of postoperative cognitive dysfunction in elderly patients receiving total knee replacement [34]. Moreover, because the BIS value developed by a built-in EEG analysis algorithm supposes the same level of consciousness independent of the anesthetic agent, age, and preexisting cognitive status of the patient, it is widely considered to be inherently inconsistent with the real level of unconsciousness [42]. Different anesthetic agents produce different EEG patterns [44], and the EEG pattern produced by the same anesthetic agent can vary among individuals owing to age and neurodegenerative disease status [45]. Therefore, an inconsistency between the BIS value and the real level of unconsciousness could mask the beneficial effects of individualized anesthesia or anesthetic depth on PD. A recent study found that BIS-guided deep anesthesia reduced early postoperative cognitive decline and inhibited postoperative peripheral inflammation in elderly patients [46]. A third limitation involves pain severity, which is a known factor associated with PD [47]. The quality and the type of analgesia were not taken into account in our study because all patients were given sufentanil 0.1 μg/kg at about 30 min before the end of the surgery, patient-controlled intravenous or epidural analgesia, and additional analgesics as needed. In addition to the small study sample size used to compare 2 regimens of anesthesia, this retrospective study did not include the use of propensity score matching to study additional variables, such as perioperative laboratory findings, fluid balance, and surgical procedures. Further large-scale, multicenter prospective studies are required to determine whether BIS-guided individualized anesthesia can be recommended for elderly patients undergoing esophagectomy.
Conclusions
In conclusion, BIS-guided individualized anesthesia (lighter) does not reduce inadequate emergence or PD in elderly patients undergoing esophagectomy compared with standard general anesthesia. Independent risk factors for PD include organic brain disease and inadequate emergence. The potential benefits of BIS-guided individualized anesthesia in elderly patients warrant exploration in further studies.
Footnotes
Source of support: This research was supported by Jining City Science and Technology Promoting plan on New and Old Kinetic Energy Conversion grant 2017SMNS0009
References
- 1.Dall TM, Gallo PD, Chakrabarti R, et al. An aging population and growing disease burden will require a large and specialized health care workforce by 2025. Health Aff (Millwood) 2013;32:2013–20. doi: 10.1377/hlthaff.2013.0714. [DOI] [PubMed] [Google Scholar]
- 2.Etzioni DA, Liu JH, Maggard MA, Ko CY. The aging population and its impact on the surgery workforce. Ann Surg. 2003;238:170–77. doi: 10.1097/01.SLA.0000081085.98792.3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin G, Glass PS, Breslin DS, et al. A study of anesthetic drug utilization in different age groups. J Clin Anesth. 2003;15:194–200. doi: 10.1016/s0952-8180(03)00030-8. [DOI] [PubMed] [Google Scholar]
- 4.Sear JW. Implication of aging on anesthetic drugs. Curr Opin Anaesthesiol. 2003;16:373–78. doi: 10.1097/01.aco.0000084481.59960.10. [DOI] [PubMed] [Google Scholar]
- 5.McGinnis SM, Brickhouse M, Pascual B, Dickerson BC. Age-related changes in the thickness of cortical zones in humans. Brain Topogr. 2011;24:279–91. doi: 10.1007/s10548-011-0198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrison JH, Baxter MG. The ageing cortical synapse: Hallmarks and implications for cognitive decline. Nat Rev Neurosci. 2012;13:240–50. doi: 10.1038/nrn3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen CC, Tung YY, Chang C. A lifespan MRI evaluation of ventricular enlargement in normal aging mice. Neurobiol Aging. 2011;32:2299–307. doi: 10.1016/j.neurobiolaging.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Marr RA, Thomas RM, Peterson DA. Insights into neurogenesis and aging: Potential therapy for degenerative disease? Future Neurol. 2010;5:527–41. doi: 10.2217/FNL.10.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joseph JA, Shukitt-Hale B, Casadesus G, Fisher D. Oxidative stress and inflammation in brain aging: nutritional considerations. Neurochem Res. 2005;30:927–35. doi: 10.1007/s11064-005-6967-4. [DOI] [PubMed] [Google Scholar]
- 10.Bilotta F, Evered LA, Gruenbaum SE. Neurotoxicity of anesthetic drugs: An update. Curr Opin Anaesthesiol. 2017;30:452–57. doi: 10.1097/ACO.0000000000000482. [DOI] [PubMed] [Google Scholar]
- 11.Wu L, Zhao H, Weng H, Ma D. Lasting effects of general anesthetics on the brain in the young and elderly: “Mixed picture” of neurotoxicity, neuroprotection and cognitive impairment. J Anesth. 2019;33:321–25. doi: 10.1007/s00540-019-02623-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mutch WAC, El-Gabalawy RM, Graham MR. Postoperative delirium, learning, and anesthetic neurotoxicity: some perspectives and directions. Front Neurol. 2018;9:177. doi: 10.3389/fneur.2018.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao WW, He YH, Liu L, et al. BIS monitoring on intraoperative awareness: A meta-analysis. Curr Med Sci. 2018;38:349–53. doi: 10.1007/s11596-018-1886-1. [DOI] [PubMed] [Google Scholar]
- 14.Lewis SR, Pritchard MW, Fawcett LJ, Punjasawadwong Y. Bispectral index for improving intraoperative awareness and early postoperative recovery in adults. Cochrane Database Syst Rev. 2019;9:CD003843. doi: 10.1002/14651858.CD003843.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radtke FM, Franck M, Lendner J, et al. Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br J Anaesth. 2013;110:i98–105. doi: 10.1093/bja/aet055. [DOI] [PubMed] [Google Scholar]
- 16.Whitlock EL, Torres BA, Lin N, et al. Postoperative delirium in a substudy of cardiothoracic surgical patients in the BAG-RECALL clinical trial. Anesth Analg. 2014;118:809–17. doi: 10.1213/ANE.0000000000000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sieber FE, Zakriya KJ, Gottschalk A, et al. Sedation depth during spinal anesthesia and the development of postoperative delirium in elderly patients undergoing hip fracture repair. Mayo Clin Proc. 2010;85:18–26. doi: 10.4065/mcp.2009.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan MT, Cheng BC, Lee TM, Gin T CODA Trial Group. BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol. 2013;25:33–42. doi: 10.1097/ANA.0b013e3182712fba. [DOI] [PubMed] [Google Scholar]
- 19.Monk TG, Saini V, Weldon BC, Sigl JC. Anesthetic management and one-year mortality after noncardiac surgery. Anesth Analg. 2005;100:4–10. doi: 10.1213/01.ANE.0000147519.82841.5E. [DOI] [PubMed] [Google Scholar]
- 20.Lindholm ML, Träff S, Granath F, et al. Mortality within 2 years after surgery in relation to low intraoperative bispectral index values and preexisting malignant disease. Anesth Analg. 2009;108:508–12. doi: 10.1213/ane.0b013e31818f603c. [DOI] [PubMed] [Google Scholar]
- 21.Leslie K, Myles PS, Forbes A, Chan MT. The effect of bispectral index monitoring on long-term survival in the B-aware trial. Anesth Analg. 2010;110:816–22. doi: 10.1213/ANE.0b013e3181c3bfb2. [DOI] [PubMed] [Google Scholar]
- 22.Sieber F, Neufeld KJ, Gottschalk A, et al. Depth of sedation as an interventional target to reduce postoperative delirium: mortality and functional outcomes of the Strategy to Reduce the Incidence of Postoperative Delirium in Elderly Patients randomised clinical trial. Br J Anaesth. 2019;122:480–89. doi: 10.1016/j.bja.2018.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neufeld KJ, Leoutsakos JM, Sieber FE, et al. Outcomes of early delirium diagnosis after general anesthesia in the elderly. Anesth Analg. 2013;117:471–78. doi: 10.1213/ANE.0b013e3182973650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Witlox J, Eurelings LS, de Jonghe JF, et al. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: A meta-analysis. JAMA. 2010;304:443–51. doi: 10.1001/jama.2010.1013. [DOI] [PubMed] [Google Scholar]
- 25.Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367:30–39. doi: 10.1056/NEJMoa1112923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lysakowski C, Elia N, Czarnetzki C, et al. Bispectral and spectral entropy indices at propofol-induced loss of consciousness in young and elderly patients. Br J Anaesth. 2009;103:387–93. doi: 10.1093/bja/aep162. [DOI] [PubMed] [Google Scholar]
- 27.Yang N, Yue Y, Pan JZ, et al. Changes in the bispectral index in response to loss of consciousness and no somatic movement to nociceptive stimuli in elderly patients. Chin Med J (Engl) 2016;129:410–16. doi: 10.4103/0366-6999.176083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xará D, Silva A, Mendonça J, Abelha F. Inadequate emergence after anesthesia: Emergence delirium and hypoactive emergence in the postanesthesia care unit. J Clin Anesth. 2013;25:439–46. doi: 10.1016/j.jclinane.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 29.Radtke FM, Franck M, Hagemann L, et al. Risk factors for inadequate emergence after anesthesia: Emergence delirium and hypoactive emergence. Minerva Anestesiol. 2010;76:394–403. [PubMed] [Google Scholar]
- 30.Li CW, Shi JH, Wang K, et al. [Risk factors for inadequate emergence after non-cardiac thoracic surgery]. J Clin Anesthesiol. 2016;32:33–37. [in Chinese] [Google Scholar]
- 31.Cotoia A, Mirabella L, Beck R, et al. Effects of closed-loop intravenous anesthesia guided by Bispectral Index in adult patients on emergence delirium: Arandomized controlled study. Minerva Anestesiol. 2018;84:437–46. doi: 10.23736/S0375-9393.17.11915-2. [DOI] [PubMed] [Google Scholar]
- 32.Chan MTV, Hedrick TL, Egan TD, et al. American Society for Enhanced Recovery and perioperative quality initiative joint consensus statement on the role of neuromonitoring in perioperative outcomes: Electroencephalography. Anesth Analg. 2020;130:1278–91. doi: 10.1213/ANE.0000000000004502. [DOI] [PubMed] [Google Scholar]
- 33.Bocskai T, Kovács M, Szakács Z, et al. Is the bispectral index monitoring protective against postoperative cognitive decline? A systematic review with meta-analysis. PLoS One. 2020;15:e0229018. doi: 10.1371/journal.pone.0229018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hou R, Wang H, Chen L, et al. POCD in patients receiving total knee replacement under deep vs. light anesthesia: A randomized controlled trial. Brain Behav. 2018;8:e00910. doi: 10.1002/brb3.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Markar SR, Smith IA, Karthikesalingam A, Low DE. The clinical and economic costs of delirium after surgical resection for esophageal malignancy. Ann Surg. 2013;258:77–81. doi: 10.1097/SLA.0b013e31828545c1. [DOI] [PubMed] [Google Scholar]
- 36.Fuchita M, Khan SH, Perkins AJ, et al. Perioperative risk factors for postoperative delirium in patients undergoing esophagectomy. Ann Thorac Surg. 2019;108:190–95. doi: 10.1016/j.athoracsur.2019.01.040. [DOI] [PubMed] [Google Scholar]
- 37.Dezube AR, Bravo-Iñiguez CE, Yelamanchili N, et al. Risk factors for delirium after esophagectomy. J Surg Oncol. 2020;121:645–53. doi: 10.1002/jso.25835. [DOI] [PubMed] [Google Scholar]
- 38.Takeuchi M, Takeuchi H, Fujisawa D, et al. Incidence and risk factors of postoperative delirium in patients with esophageal cancer. Ann Surg Oncol. 2012;19:3963–70. doi: 10.1245/s10434-012-2432-1. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto M, Yamasaki M, Sugimoto K, et al. Risk evaluation of postoperative delirium using comprehensive geriatric assessment in elderly patients with esophageal cancer. World J Surg. 2016;40:2705–12. doi: 10.1007/s00268-016-3602-2. [DOI] [PubMed] [Google Scholar]
- 40.Knaak C, Brockhaus WR, Spies C, et al. Presurgical cognitive impairment is associated with postoperative delirium and postoperative cognitive dysfunction. Minerva Anestesiol. 2020;86:394–403. doi: 10.23736/S0375-9393.20.13903-8. [DOI] [PubMed] [Google Scholar]
- 41.Robinson TN, Raeburn CD, Tran ZV, et al. Motor subtypes of postoperative delirium in older adults. Arch Surg. 2011;146:295–300. doi: 10.1001/archsurg.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koch S, Spies C. Neuromonitoring in the elderly. Curr Opin Anaesthesiol. 2019;32:101–7. doi: 10.1097/ACO.0000000000000677. [DOI] [PubMed] [Google Scholar]
- 43.Sieber FE, Neufeld KJ, Gottschalk A, et al. Effect of depth of sedation in older patients undergoing hip fracture repair on postoperative delirium: The STRIDE randomized clinical trial. JAMA Surg. 2018;153:987–95. doi: 10.1001/jamasurg.2018.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Purdon PL, Sampson A, Pavone KJ, Brown EN. Clinical electroencephalography for anesthesiologists: Part I: Background and basic signatures. Anesthesiology. 2015;123:937–60. doi: 10.1097/ALN.0000000000000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Purdon PL, Pavone KJ, Akeju O, et al. The ageing brain: Age-dependent changes in the electroencephalogram during propofol and sevoflurane general anaesthesia. Br J Anaesth. 2015;115:i46–57. doi: 10.1093/bja/aev213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quan C, Chen J, Luo Y, et al. BIS-guided deep anesthesia decreases short-term postoperative cognitive dysfunction and peripheral inflammation in elderly patients undergoing abdominal surgery. Brain Behav. 2019;9:e01238. doi: 10.1002/brb3.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Denny DL, Such TL. Exploration of relationships between postoperative pain and subsyndromal delirium in older adults. Nurs Res. 2018;67:421–29. doi: 10.1097/NNR.0000000000000305. [DOI] [PubMed] [Google Scholar]


