1. INTRODUCTION
Due to the fear of transmission of the new coronavirus disease 2019 (COVID‐19), many cancer patients have had their diagnosis and treatment affected. 1 Specifically, the rapid spread of the virus has led to a global impact on elective surgical procedures, including cancer centers. 2 Effects of these changes are uncertain, as is the safety of electively operating on patients while the risk of postoperative COVID‐19 pneumonia remains unknown. 3 This becomes even more challenging with cancer patients, since delaying their treatment for long periods of time due to the pandemic may worsen prognosis. To prevent both cancer mortality, as well as mortality due to COVID‐19, operational efficiency, quick and shared decisions, as well as predictability, are needed. Measures to reduce the circulation of individuals in hospitals and unique attention to patients according to regional and local contagion phases are critical for adequate risk management. It becomes essential to have a plan in place to be able to safely treat patients during the current pandemic. We demonstrate how a cancer center in the largest city in Brazil has managed to maintain the safe treatment of breast cancer patients during the COVID‐19 pandemic. This is a retrospective cohort trend study of patients receiving surgical breast cancer treatment at the AC Camargo Breast Cancer Reference Center between March 9 and May 21, 2020, during the COVID‐19 pandemic. In January 2020, the AC Camargo Cancer Center established a crisis team as a preventative measure to monitor the numbers and actions taken by other countries and to foresee and plan necessary measurements to help control cases in our Institution. The first cases in Brazil were subsequently recorded in February 2020.
By March 13, 2020 (Figure 1), the AC Camargo Cancer Center had implemented measures to provide the best protection to patients, caregivers, and health professionals, while simultaneously providing a safe and best possible cancer treatment. A key goal was to restrict the circulation of patients with severe acute respiratory syndrome‐related coronavirus symptoms within the institution by designating specific COVID‐19 emergency and admission units for these patients. Additional measures included phone contact before patients' appointments to the outpatient unit to provide instructions and triage of signs and symptoms, in addition to reading body temperature at the hospital's entrances. Appointments were rescheduled, personal protective equipment was employed, all personnel was excluded from the operating room (OR) during orotracheal intubation, and both ORs and hospital rooms were designated for COVID‐19‐positive patients. A contingency plan was also developed by the Breast Surgery Department. This plan was flexible according to the number of COVID‐19‐positive patients admitted. For example, surgical procedures could be postponed or canceled if hospital beds for COVID‐19‐positive patients were needed. This plan was developed by a multidisciplinary team of doctors from the breast cancer reference center and it followed local best practice procedures adapted to the Brazilian population. Recommendations of other countries or societies were adapted to our reality, and implementation of a contingency plan was not necessary since COVID‐19 maximum hospital bed occupancy (i.e., daily rate of 7%) was not reached. Preoperative testing of patients with COVID‐19 real‐time polymerase chain reaction (RT‐PCR) was implemented. Between March 9 and May 30, 2020, breast surgeons had 3021 outpatient appointments scheduled. During the same period in 2019, 6115 outpatient appointments were scheduled. Thus, a 49.4% decrease in outpatient appointments occurred during the current pandemic. In addition, a 13.17% decrease in the number of patients who underwent breast surgery was observed for this time period compared with 2019. Between March 9 and May 21, 2020, a total of 146 patients underwent breast surgical procedures. Among these patients, 68 were tested for COVID‐19 and 3 received positive results. The mean age of this cohort was 51 years and 55.48% (n = 81) had comorbidities. Patients who tested positive for COVID‐19 had their surgeries rescheduled 21 days later to accommodate an expected recovery period of 15 days plus an additional week for retesting and confirmation of a negative RT‐PCR result.
Figure 1.
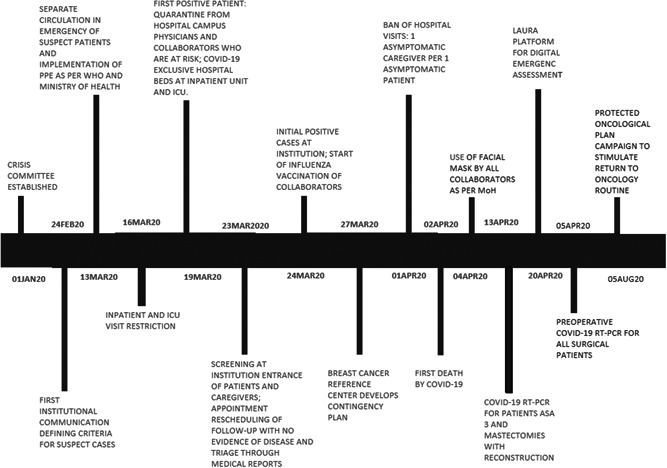
Timeline of adopted measures for safe anticancer treatment at the AC Camargo Cancer Center. COVID‐19, coronavirus disease 2019; ICU, intensive care unit; MoH, Ministry of Health; RT‐PCR, real‐time polymerase chain reaction
2. DISCUSSION
With the pandemic currently in the spotlight, we are faced with increased mortality in other diseases, either because their importance is being downgraded or patients are having difficulties accessing health care. For cancer, reduced screenings and/or limited access to ancillary exams and biopsies have delayed both diagnosis and treatment, thereby leading to a negative impact on survival. To preserve cancer diagnosis and treatments, the AC Camargo Cancer Center, as well as other sites, 2 , 4 , 5 , 6 have developed strategies for managing patient care by maximizing the potential of a site's organization and structure. A pivotal point in this process at our institute was the development of a multidisciplinary surgical and clinical crisis committee. With this committee in place, streamlined and coordinated strategies have been reviewed since January 2020, months before the COVID‐19 index case in Brazil. A critical objective for our Breast Surgery Department was the development of an efficient plan to reduce surgery load without adversely impacting patient prognosis. Surgeries involving curable tumors, including in situ tumors with an indication of delay by other protocols, were neither suspended nor postponed, taking into account the fact that surgery was the best treatment for these patients. These considerations were differently addressed in comparison to American and European guidelines. 1 , 4 , 5 , 6 Worldwide, cancer treatment centers have closed their doors either partially or totally during the current pandemic. However, at the AC Camargo Cancer Center, we have been able to sustain treatment of cancer patients, reduce the number of deaths, and the number of COVID‐19‐related postoperative complications. We have achieved this by maintaining communication between the crisis team and clinical staff and by developing clear and streamlined strategies to resume outpatient visits, surgical procedures, and chemotherapy treatments according to a safe oncological plan. Furthermore, we have achieved this with no breach of the COVID‐19 transmission protocols of the Brazilian Vigilance and Sanitary Agency, the Ministry of Health, and the World Health Organization. Thus, while the implementation of a contingency plan at the AC Camargo Cancer Center has differed from those suggested globally, 1 , 2 , 5 , 6 the Institutional guidelines which have been implemented have allowed oncological surgeries to be performed without complications. Amidst the COVID‐19 pandemic, we advocate that it is feasible to establish and implement safety measures which still allow the safe diagnosis and continuous treatment of cancer patients without delay, which otherwise could further increase cancer prognosis and incidence in Brazil.
We advocate that similar measures be implemented at other institutions according to their current reality, and according to the types of tumor needing treatment, so that safe anticancer treatments can continue to improve patients' lives.
3. ETHICS STATEMENT
This study was approved by the appropriate institutional research in Ethics Committee (Comitê de Ética em Pesquisa do Hospital AC Camargo) and has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards as well as local legislation on research. In view of the retrospective nature of the study and as procedures performed were part of the routine care, the Ethics Committee waived the need for written informed consent
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
All authors are familiar with the contents of the report and agree to its publication.
Leite FPM, Curi C, Sanches SM, et al. How to maintain elective treatment of breast cancer during the COVID‐19 pandemic—A cancer center experience. J Surg Oncol. 2021;123:9‐11. 10.1002/jso.26233
DATA AVAILABILITY STATEMENT
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Dietz JR, Moran MS, Isakoff SJ, et al. Recommendations for prioritization, treatment, and triage of breast cancer patients during the COVID‐19 pandemic. The COVID‐19 pandemic breast cancer consortium. Breast Cancer Res Treat. 2020;181(3):487‐497. 10.1007/s10549-020-05644-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pramesh CS, Badwe RA. Cancer management in India during COVID‐19. N Engl J Med. 2020;382:e61. 10.1056/nejmc2011595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Makdissi FB, Leite FPM, Peres SV, et al. Breast cancer survival in a Brazilian cancer center: a cohort study of 5,095 patients. Mastology. 2019;29:37‐46. [Google Scholar]
- 4. Fregatti P, Gipponi M., Giacchino M., et al. Breast cancer surgery during the COVID‐19 pandemic: an observational clinical study of the Breast Surgery Clinic at Ospedale Policlinico San Martino—Genoa, Italy. In Vivo. 2020;34:1667‐1673. 10.21873/invivo.11959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. ESMO . ESMO Clinical Practice Guidelines: Breast Cancer. https://www.esmo.org/guidelines/breast-cancer. Accessed June 30, 2020.
- 6. American College of Surgeons . COVID‐19 Guidelines for Triage of Breast Cancer Patients. https://www.facs.org/covid-19/clinical-guidance/elective-case/breast-cancer. Accessed June 30, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.


