This report describes a COVID‐19 patient treated with nafamostat and heparin to prevent circuit thrombosis during extracorporeal membrane oxygenation (ECMO) support. Combination with nafamostat and heparin might prevent circuit thrombosis in ECMO.

Keywords: Circuit thrombosis, COVID‐19, ECMO, nafamostat
Abstract
Background
Preventing thrombosis in extracorporeal membrane oxygenation (ECMO) can be life‐saving in cases of coronavirus disease (COVID‐19); however, circuit thrombosis is a complication. This report describes a COVID‐19 patient treated with nafamostat and heparin to prevent circuit thrombosis during ECMO support.
Case presentation
A 63‐year‐old man was transferred to our hospital with respiratory failure due to COVID‐19 pneumonia. He was provided venous‐venous ECMO to maintain oxygenation. During ECMO support, occlusive circuit thrombosis developed despite systemic anticoagulation therapy with heparin. He was subsequently given combination therapy with nafamostat and heparin. Although the combination therapy could prevent circuit thrombosis, it was converted to heparin monotherapy because of hyperkalemia and hemothorax. After tracheostomy and a gradual improvement in oxygenation, ECMO was discontinued. He was transferred to another hospital for further rehabilitation.
Conclusion
Combination therapy with nafamostat and heparin can prevent circuit thrombosis during ECMO. However, bleeding can still develop with this combination therapy during ECMO.
INTRODUCTION
Coronavirus disease (COVID‐19) is caused by severe acute respiratory syndrome coronavirus 2 and could lead to coagulopathy. 1 More pronounced coagulation activation seems to be correlated with a severe disease course. 2 During hypoxic respiratory failure due to COVID‐19 pneumonia, venous‐venous extracorporeal membrane oxygenation (V‐V ECMO) has been shown to be life‐saving when undertaken with appropriate anticoagulation therapy to prevent circuit thrombosis. 3 Nafamostat, a serine protease inhibitor, is an anticoagulant that was recently shown to have antiviral properties against COVID‐19. 4 Combination therapy with nafamostat and heparin has been hypothesized to be beneficial for patients with COVID‐19. 5 However, clinical evidence for this is lacking; therefore, we present a case involving a patient with COVID‐19 who was treated with nafamostat and heparin to prevent circuit thrombosis during ECMO support.
CASE REPORT
A 63‐year‐old Japanese man with a history of hypertension was transferred to our hospital with acute respiratory failure due to COVID‐19 pneumonia. On arrival, his blood pressure was 126/70 mmHg, pulse rate was 90 b.p.m., temperature was 37.7°C, and O2 saturation was 91% on ventilation with the following settings: 15 cmH2O positive end expiratory pressure (PEEP) and 65% fraction of inspiratory oxygen (FiO2). His body mass index was 26.8 kg/m2. On admission, computed tomography of the chest showed a crazy paving pattern (Fig. 1). His Sequential Organ Failure Assessment score and disseminated intravascular coagulation score were 4 and 0, respectively.
Fig. 1.
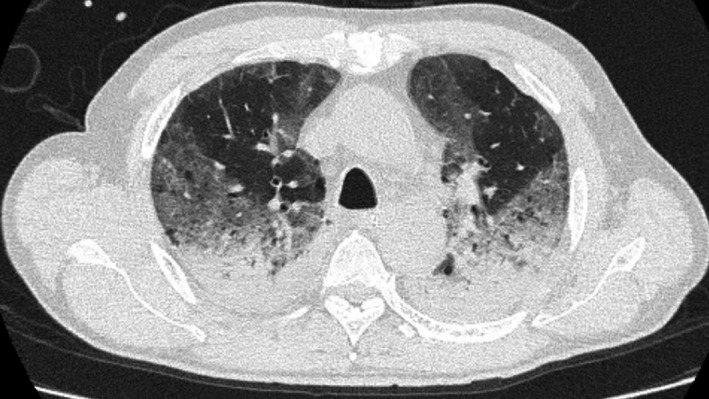
Computed tomography of a 63‐year‐old man on the day of admission for respiratory failure due to COVID‐19 pneumonia. The scan of the chest shows a crazy paving pattern.
After 5 days in the prone position, the patient’s respiratory failure worsened; the partial pressure of arterial oxygen was 66 mmHg with ventilation settings of 16 cmH2O PEEP and 80% FiO2. Venous‐venous ECMO was provided through the right jugular vein and right common femoral vein.
During ECMO support, the dose of i.v. heparin was controlled by targeting an activated clotting time value of 160–180 s. Although the activated partial thromboplastin time was 88 s, circuit thrombosis appeared during ECMO therapy. Because V‐V ECMO could not oxygenate his blood, replacement of the ECMO membrane was required 4 days after the initial V‐V ECMO insertion (Fig. 2, first black triangle). Just after the new membrane insertion, circuit thrombosis rapidly developed.
Fig. 2.
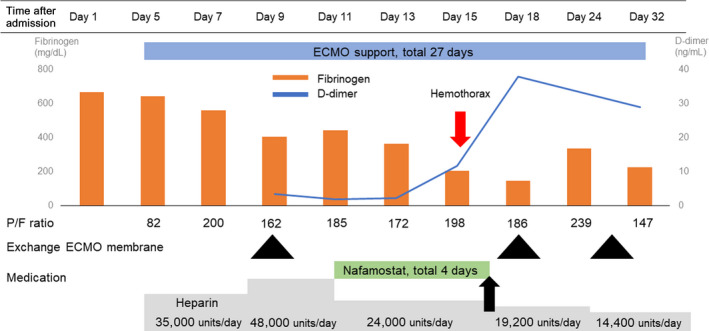
Timeline of clinical features and laboratory findings in a 63‐year‐old man with respiratory failure due to COVID‐19 pneumonia. Nafamostat was discontinued after 4 days (black arrow) due to hyperkalemia and hemothorax. ECMO, extracorporeal membrane oxygenation.
On day 11, nafamostat at 0.06 mg/kg/h was continuously infused into ECMO, in combination with i.v. heparin, to prevent circuit thrombosis. During this combination therapy, activated clotting time was well controlled and remained between 180 and 220 s with appropriate adjustment of the heparin dose. During the combination therapy, circuit thrombosis did not develop. Blood tests showed that fibrinogen had decreased while D‐dimer had not increased with the combination therapy (Fig. 2).
On day 15, the patient developed hyperkalemia (potassium, 6.5 mEq/L) and hemothorax; thus, nafamostat infusion was stopped (Fig. 2, black arrow). Rapid blood loss due to the hemothorax necessitated transfusion of 16 units of red blood cells and 12 units of fresh‐frozen plasma over a 48‐h period. Treatment with heparin was continued. However, ECMO membrane exchange was needed on days 17 and 28 (Fig. 2).
On day 32, V‐V ECMO was successfully discontinued because oxygenation was maintained without V‐V ECMO support. Tracheostomy was carried out when oxygenation substantially recovered. The patient was transferred to another hospital for rehabilitation on day 52.
DISCUSSION
We presented a patient with COVID‐19 who was treated with a combination of nafamostat and heparin during V‐V ECMO. The results showed that combination therapy could prevent circuit thrombosis more effectively than could heparin monotherapy in short period of time. However, bleeding can still develop with this combination therapy during V‐V ECMO.
Helms et al. 6 observed that two of 12 patients with COVID‐19 treated with V‐V ECMO experienced occlusions of the centrifugal pump due to circuit thrombosis, and their membranes needed prompt replacement. Circuit thrombosis occurred despite systemic anticoagulation by continuous infusion of heparin. This might have been due to the high level of fibrinogen, resulting in high concentrations inside the dialyzer capillaries. 7
Nafamostat is reportedly associated with fewer bleeding complications during ECMO without increasing the incidence of thromboembolic episodes. 8 Another recent report showed that nafamostat not only had anticoagulant effects but also potential anti‐inflammatory and antiviral effects against COVID‐19. 4 In the present case, circuit thrombosis was suppressed with the combination therapy, probably because of these anticoagulant and anti‐inflammatory effects.
Bleeding can develop with combination therapy during V‐V ECMO. Bleeding related to systemic heparinization during V‐V ECMO was observed in up to 60% of patients and found to directly affect the prognosis in a previous study. 9 In this case, after combination therapy, increased bleeding due to hemothorax was observed, and the treatment was switched to heparin monotherapy. Figure 2 shows decreased fibrinogen and increased D‐dimer levels during the combination therapy with heparin and nafamostat, indicating potential bleeding caused by the hemothorax. After the occurrence of this complication, the coagulation abnormality continued to worsen.
CONCLUSIONS
The findings from this case suggest that combination therapy with nafamostat and heparin is more effective than heparin monotherapy in preventing circuit thrombosis during V‐V ECMO support.
DISCLOSURE
Approval of the research protocol: N/A.
Informed consent: Informed consent was obtained from the patient.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
Conflict of interest: None.
Acknowledgments
We are deeply grateful to all health‐care professionals of St. Marianna university hospital who have been fighting against the COVID‐19 pandemic. We would like to thank Editage for English language editing.
Funding information
No funding information provided.
REFERENCES
- 1. Jean MC, Jerrold HL. COVID‐19 and its implications for thrombosis and anticoagulation. Blood 2020; 135: 2033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zou Y, Guo H, Zhang Y et al Analysis of coagulation parameters in patients with COVID‐19 in Shanghai, China. Biosci. Trends 2020; 14: 285–9. [DOI] [PubMed] [Google Scholar]
- 3. Michael CS, Eric S, Laurance L, Eddy F, Hussein DK. Anticoagulation practices during venovenous extracorporeal membrane oxygenation for respiratory failure: a systematic review. Ann. Am. Thorac. Soc. 2016; 12: 2242–50. [DOI] [PubMed] [Google Scholar]
- 4. Hoffmann M, Schroeder S, Kleine‐Weber H, Müller MA, Drosten C, Pöhlmann S. Nafamostat mesylate blocks activation of SARS‐CoV‐2: new treatment option for COVID‐19. Antimicrob. Agents Chemother. 2020; 64: e00754–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Asakura H, Ogawa H. Potential of heparin and nafamostat combination therapy for COVID‐19. J. Thromb. Haemost. 2020; 18: 1521–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Helms J, Tacquard C, Severac F et al High risk of thrombosis in patients with severe SARS‐CoV‐2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020; 4: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oudemans‐van Straaten HM. Hemostasis and thrombosis in continuous renal replacement treatment. Semin. Thromb. Hemost. 2015; 41: 91–8. [DOI] [PubMed] [Google Scholar]
- 8. Han W, San Bok J, Cho HJ et al Single‐center experience of extracorporeal membrane oxygenation mainly anticoagulated with nafamostat mesilate. J. Thorac. Dis. 2019; 11: 2861–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aubron C, DePuydt J, Belon F et al Predictive factors of bleeding events in adults undergoing extracorporeal membrane oxygenation. Ann. Intensive Care 2016; 6: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]


