Abstract
COVID‐19 caused by SARS‐COV‐2 first appeared in the Wuhan City of China and began to spread rapidly among people. Rapid progression of the outbreak has led to a major global public health problem of a potentially fatal disease. On January 30, 2020, WHO declared the pandemic as the sixth public health emergency of the world. Upon this, the whole country has started to take the necessary precautions. The new coronavirus uses membrane‐bound angiotensin‐converting enzyme 2 (ACE2) to enter into the cells, such as SARS‐CoV, and mostly affects the respiratory tract. Symptoms of COVID‐19 patients include fever (93%), fatigue (70%), cough (70%), anorexia (40%) and dyspnoea (34.5%). The elderly and people with underlying chronic diseases are more susceptible to infection and higher mortality. Currently, a large number of drugs and vaccines studies are ongoing. In this review, we discussed the virology, epidemiological data, the replication of the virus, and its relationship with cardiovascular diseases on COVID‐19 pandemics, treatment and vaccines. Thereby, this study aims to neatly present scientific data in light of many regarding literature that can be a clue for readers who research this disease prevention and treatment.
Significance of the study
This review summarized current information on COVID‐19 (epidemiology, pathophysiology, clinical, laboratory, cardiovascular diseases, ACE2 and pharmacological agents) for researchers and reveals guiding data for researchers, especially in the field of cardiovascular system, pharmacology, dysregulation of cellular function in disease, molecular and cell biology and physiology in the regulation of tissue function in health and disease.
Keywords: ACE2, cardiovascular disease, COVID‐19, pandemia, pharmacological agents, vaccine
1. INTRODUCTION
Coronavirus (Latin: Orthocoronavirinae) is a well‐defined virus family that causes diseases in birds and mammals and constitutes one of the two subfamilies of the Coronaviridae family. 1 Human coronaviruses were first discovered in the late 1960s. 2 It is named as corona due to its characteristic crown‐like appearance. The coronavirus subfamily is genotypically and serologically divided into four different types: α, β, γ and δ coronavirus. 3
The coronavirus is also divided into four different viral subgroups as A to D. Today, 30 different coronaviruses have been identified that infect humans, mammals, poultry, and other animals thus causing a variety of gastrointestinal, hepatic and neurological diseases, particularly in the respiratory system. 4 In humans, coronaviruses cause some cases of colds and respiratory infections that can be fatal, such as severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS) and Coronavirus disease 2019 (COVID‐19). Other types of symptoms are quite variable while they cause upper respiratory disease in chickens and diarrhoea in cows and pigs. 5
To date, six total human coronaviruses have been identified: HCoVs‐NL63, HCoVs‐229E, HCoVs‐OC43, HCoVs‐HKU1, SARS‐CoV and MERS‐CoV. 6 When looking at history; in the past 20 years, two major outbreaks have been identified, in which the transmission of animal beta coronaviruses to humans has resulted in serious diseases. In recent years, new viral infections have been detected periodically in various countries. The first epidemic was observed in 2002 to 2003 as a result of the passage of a new coronavirus of bat origin to humans through palm civet cats in Guangdong Province, China. This virus, called severe acute respiratory syndrome coronavirus, affected a total of 8422 people in China and caused 916 deaths (11% mortality). 7
Rapid economic growth in South China at that time caused an increase in demand for proteins obtained from wild animals. Numerous wild animals have been fed in crowded cages for this purpose. As a result of the lack of biosafety procedures, it facilitated the transmission of the virus from animals to humans. Viruses similar to SARS‐CoV have been detected after the resumption of the wildlife market in South China. Also, as a result of various mutations seen in these viruses, it is thought that diseases like SARS may return and cause various outbreaks. 7 , 8
The second epidemic event occurred approximately 10 years later. In 2012, the Middle East respiratory syndrome coronavirus (MERS‐CoV) emerged from bat origin through a dromedary camel in Saudi Arabia. It affected a total of 2494 people and caused 858 deaths (mortality rate of 34%). 8 All coronavirus outbreaks that have occurred in history have spread easily and caused devastating consequences. Not only did it affect people, but it also had huge losses to the economy. Nowadays, pandemic continues to affect all continents without losing speed. In our review, we will discuss the history, virology, epidemiology, clinical features, prophylaxis, treatments and precautions of SARS‐CoV‐2 infection.
1.1. History of SARS‐CoV‐2
In late December 2019, a large number of patients with unknown causes of pneumonia were reported from a seafood market in Wuhan, Hubei province, China. 6 The new coronavirus (SARS‐CoV‐2) has been identified by local hospitals using the surveillance mechanism established after the SARS outbreak that emerged in 2003 and aimed at the rapid identification of new pathogens. 9
This coronavirus was originally called the 2019 new coronavirus (2019‐nCoV) by the World Health Organization (WHO) on January 12, 2020. The Coronavirus Working Group (CSG) of the WHO and International Committee proposed to call the new virus SARS‐CoV‐2 on February 11, 2020. As a result of the samples taken from the patient, the genome sequence of the SARS‐CoV‐2 was isolated on January 7, 2020, by Chinese scientists in a short time. 10 WHO announced on February 11, 2020; that “COVID‐19” will become the official name of the disease. Tedros Adhanom Ghebreyesus, chief of the WHO, said the epidemic meant “ko,” “corona,” “vi” for “virus” and “d” for “disease” as first described on December 31, 2019. Such a name has been chosen to avoid stigmatizing a particular region, animal species or human. 11
The Wuhan strain has been identified as a new betacoronavirus strain with about 70% genetic similarity from group 2B to SARS‐CoV. 12 It is suspected that the virus has a 96% similarity to a bat coronavirus and is also caused by bats. 13 Researchers identified a patient infected with the 2019‐nCoV virus in Thailand, Japan, and Korea on January 13, 16, and 21, respectively 14 . A total of 314 patients were detected on January 22, 2020, and 6 patients were reported to have died. 14
The infection, which started to spread first in China and then in nearby countries, spreads to most countries later on. The epidemic soon reached an international dimension, affecting the whole world. As a result, the WHO considered COVID‐19 as an international public health problem and declared it as a pandemic on January 30, 2020. 15 Studies have shown that the basic reproductive number (R0) of SARS‐CoV‐2 is approximately 2.2 or more (1.4‐6.5) and, unfortunately, the outbreak is rapidly growing among the people. 16 As of August 21, 2020, at least 789.117 deaths have been confirmed due to new coronavirus infection, and more than 22.536.278 have been infected. 17 The outbreak resulted in travel restrictions and crashes across the country in many countries.
1.2. Virology of SARS‐CoV‐2
It is well known that virus particles have a diameter of around 120 nm. The envelope of the virus in electron micrographs appears as a distinct pair of electron‐dense shells. 18 Coronaviruses are single‐stranded RNA virus genomes with the largest known viral RNA genome, ranging in size from 26 to 32 kilobases. 19 The virion consists of genomic RNA embedded in phospholipid double layers and coated with two different types of nucleocapsid protein (N). The membrane (M) protein (a type III transmembrane glycoprotein) and the envelope (E) protein are among the surface (S) proteins in the virus envelope. 20 The cellular structure of the SARS‐CoV‐2 and its interaction with the target cell is shown in Figure 1. In particular, beta coronavirus subgroup A members also have a shorter spike‐like surface protein called hemagglutinin esterase (HE). 1 The lipid bilayer envelope protects membrane proteins and the nucleocapsid of the virus while outside the host cell. 21
FIGURE 1.
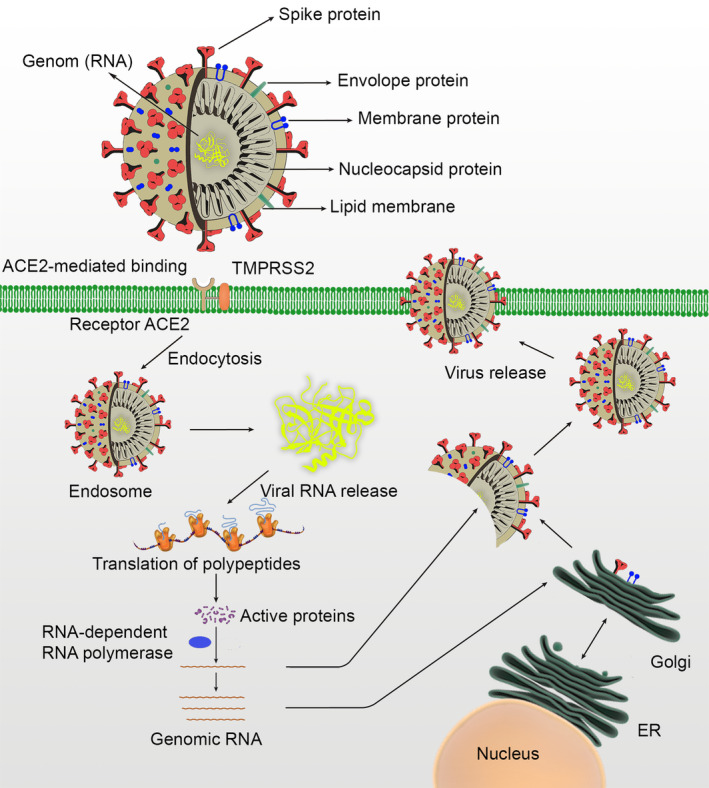
The cellular structure of the SARS‐CoV‐2, and its interaction with the target cell
Human coronavirus infections are caused only by α and β coronaviruses. 19 CoVs are very common and approximately 30% to 60% of the population in China has anti‐CoV antibodies. 20 The virus can survive at least 2 to 3 days on dry surfaces and 2 to 4 days in faeces at room temperature. 22 S protein is involved in the binding and entry of the host cell. Therefore, it is the main target for neutralizing antibodies and antiviral peptides. 23
2. EPIDEMIOLOGY
COVID‐19 has become a major pandemic, increasingly and tending to be in clusters. The first documented epidemic occurred in Wuhan, China. 24 Since December 2019, unexplained cases of pneumonia linked to a seafood‐selling market in Wuhan, China, Hubei province have been reported in a row. As a result of the further tests and analyses, it was concluded that these cases were an acute respiratory infection caused by a new coronavirus. The epidemic, which first appeared in China and its neighbours, spread to other countries in a short time and turned into a big epidemic. The fact that it was later seen in people who are not affiliated with Wuhan shows that the outbreak has increased. 25
As a result of further analysis and confirmations, COVID‐19 has already exceeded the number of cases confirmed in 2003. The fatality rate of cases reported in China is less than 4%. Accordingly, this new type of coronavirus‐induced pandemic shows that the lethality rate is lower than SARS‐CoV (fatality rate: 10%) and MERS‐CoV (fatality rate: 37%). 26 The case fatality rate varies depending on the availability of health care, the age of the population, the co‐morbidities and the number of undiagnosed persons. Preliminary research shows that the rate of case fatality rate varies between 2% and 3%. 27
According to the latest data, the cumulated attack rate (CAA) of this outbreak in China has been reported as 0.11%. However, the new type of coronavirus, which is the same as the transmission pathway of H1N1 influenza, is approximately 50 times higher than influenza. All these data show the importance of the intensive quarantine and social removal measures taken by the Huibei government. 28 According to the analysis of the data from the epidemic in China in mid‐January 2020, the increasing incidence trend has been shown to largely follow exponential growth. As a result of the conducted studies, it was determined that the average number of R0 of the epidemic was 2.24 to 3.58 and it was associated with a 2 to 8‐fold increase in the reporting rate. 29 According to another study based on data between December 31, 2019 and January 28, 2020 the R0 value was found to be 2.68, and it was found that the epidemic's doubling time was 6.4 days. 30 The incubation period for COVID‐19 and indirectly in the asymptomatic period was found to be as 2.1 to 11.1 days. 31
New updates are being released for the COVID‐19 outbreak day by day, and the available data show that the outbreak has great spreading potential. As of January 22, 2020, 571 COVID‐19 cases were reported in 25 provinces in China. 32 The Chinese National Health Commission shared details of the first 17 deaths by January 22, 2020. Another report from January 24, 2020, stated that the cumulative incidence in China is estimated to be 5502 cases. 33 On January 30, 2020, 7734 cases were detected in China; also 90 new cases were obtained from Taiwan, Thailand, Vietnam, Malaysia, Nepal, Sri Lanka, Cambodia, Japan, Singapore, Republic of Korea, United Arab Emirates, United States, The Philippines, India, Australia, Canada, Finland, France, and Germany Reported from countries other than China. According to the data obtained from all cases, the mortality rate was declared as 2.2% (170/7824). 34 The detection of the first case of COVID‐19 infection in the United States paved the way for great progress in both diagnoses, symptoms, and treatment of the disease. Also, COVID‐19's first human‐to‐human transmission case was reported in the United States on January 30, 2020. 35 The importance of screening has increased due to the transmission of human to human. For this reason, the Centres for Disease Control (CDC) has scanned more than 30 000 passengers coming to the US airports for the coronavirus and is still scanning. 35 As a result of the studies, it has been shown that there is a regular increase in the total number of COVID‐19 cases in the US and out of China. COVID‐19, which is seen in all countries of the world today, has been observed most in the USA (n = 5.477.305). The second country with the highest number of patients in Brazil (n = 3.456.652). Also, United States (n = 172.033) is the first country with the highest deaths, whereas Brazil (n = 111.100) is the second country with the highest deaths. While the outbreak initially affected Western Pacific Region countries such as China and South Korea, today the centre of the epidemic has become the European continent. According to the Taiwan CDC data in a study of 45 167 cases, the COVID‐19 mortality rate was found to be 2.5%. 36
Various studies were started to understand the source of the pandemic in a short time. On January 12, 2020, a large number of samples were taken from the wild animal market in the seafood market. As a result of the PCR study on the samples, 33 of 585 samples were tested positive because they contain 2019‐nCoV and the virus was successfully isolated from positive samples. As a result of the study, it suggested that the virus was caused by wild animals sold in the seafood market in South China. 37 The quarantine process was initiated in a short time and the epidemic was kept under control with great devotion. Later, in a study published in Lancet, they examined the first 41 infected patients hospitalized between December 16, 2019 and January 2, 2020. An epidemiological relationship was investigated between the first patient and subsequent cases, however; they concluded that there was no relationship. Also, since 13 out of 41 cases showed that they were not affiliated with the market, it was thought that the epidemic source would not be the seafood market solely. 38 As of August 21, 2020, at least 789.117 deaths have been confirmed due to new coronavirus infection, and more than 22.536.278 have been infected. 17
2.1. Age and sex
Most of the COVID‐19 cases reported in Wuhan in the early days of the outbreak were adult patients. 39 According to the study on coronavirus infection and published in the Lancet, the median age of patients is 49 years. In another study involving a total of 4021 patients in 30 different cities of China, the average age group of the people who got the disease was found to be 49, and half of the patients were found to be between the ages of 20 to 50. 40 Similarly, the New Coronavirus Pneumonia Emergency Response Epidemiology Team in China reported that 66.7% of 44 672 COVID‐19 cases of varying severity were between the ages of 20 to 60. 41 Two different epidemiological studies: these have been shown that 153 (15.1%) patients are ≥65 years old and 407 (10.1%) patients are >70 years old. 42 According to a study on children with COVID‐19; only 9 (0.9%) patients showed that they were between the ages of 0‐14. In another study, only 14 (0.35%) patients were reported to be ≤10 years old. According to the largest study in China, only 0.9% (n = 416) of patients were shown to be in the <10 age group. 41 Also, a study showed that 9 infants under the age of 1 are infected with SARS‐CoV‐2 in China. 41
According to the analysis conducted among COVID‐19 patients in the United States; showed that the fatality rate was 10% to 27% in the age group ≥85, 3% to 11% at the age of 65 to 84, 1% to 3% at the age of 55 to 64, <1% at the age of 20 to 54. Also, deaths have not been observed in people aged ≤19. 43 According to studies investigating the gender distribution of coronavirus infection, more than half of the patients are males. Studies show that 51.4% to 73.2% of patients diagnosed with COVID‐19 are male. 44 Another study found that 73% of patients with SARS‐CoV‐2 infections were male. 42 Studies have found that the male/female mortality rate is 3.25:1 and the median age of death is 75. The same study showed that the time from the first symptom to death was an average of 14 days, and this period was 20 days the age of <70, but this period was shortened to 11.5 days ≥70 age. This suggests that the disease can progress faster in the elderly than young people. 45
The rapidly growing epidemic has affected the whole world. Fortunately, there was no evidence of intrauterine infection caused by vertical conduction in women diagnosed with COVID‐19 pneumonia late in pregnancy. 46 It is thought that healthcare workers constitute a large risk group due to the transmission from person to person. Therefore, according to two different studies; 2.1% (n = 23) and 3.8% (n = 1716) of those infected are healthcare professionals. 45 , 47
2.2. Risk factors
Although the risk factors of COVID‐19 are uncertain, a large number of studies show that a significant proportion of patients have underlying risk factors or co‐morbidity. 41 , 44 A study conducted to determine risk factors; it has been shown that 50.5% of patients with SARS‐CoV2 pneumonia have cardiovascular diseases and 40.4% of patients have cerebrovascular diseases. 39 At least one disease was shown in 23.2% (n = 255) of 1099 patients with acute respiratory distress syndrome associated with SARS‐CoV‐2 infection. Especially hypertension (14.9%) and diabetes mellitus (7.4%) were found as common in these patients. 44 In another study, varying degrees of hypertension (n = 2.683, 12.8%) was found more than diabetes mellitus (n = 1.102, 5.3%), and in the third place was cardiovascular disease (n = 873, 4.2%). 41 As a result of studies conducted that patients with severe COVID‐19 disease have more co‐morbidities than mild patients (37.6% vs 20.5%). 44
Studies to determine risk factors have led to the appearance of various data. First, it was shown that most of the patients with COVID‐19 consist of middle‐aged or elderly patients, and fewer of them are children. Because risk factors or co‐morbid diseases are less in children. 47 Second, men with COVID‐19 have a higher prevalence than women. Third, SARS‐CoV‐2 infection can also be transmitted to healthcare professionals and hospitalized patients in a hospital setting. Finally, at least 20% of COVID‐19 cases have an underlying disease. Also, underlying disease and risk factors are more common in severe cases. 48
3. REPLICATION AND PATHOGENESIS
It is well known that the cell receptor for SARS‐CoV, angiotensin‐converting enzyme 2 (ACE2), is found in human lower airways. 49 It regulates both cross‐species and human‐to‐human transmission. 50 To treat COVID‐19 infection, first of all, its pathophysiology must be clarified absolutely. Studies on this subject have been done fastly. Bronchoalveolar lavage studies on patients have confirmed that SARS‐CoV‐2 uses the same cellular entry receptor ACE2 as SARS‐CoV. Subsequent studies have shown that virion S‐glycoprotein on the coronavirus surface can bind to ACE2 in the membrane of human cells. 51 S glycoprotein is divided into two subgroups as S1 and S2 52 . S1 mainly identifies the virus‐host range and cellular tropism. In contrast, S2 also exerts virus‐cell membrane fusion. Fusion occurs by binding of the virus to the ACE2 receptor in the human cell membrane. The viral genome RNA is then released into the cytoplasm. Uncoated RNA enables the production of two polyproteins, pp1a and pp1ab, that encode non‐structural proteins and form a replication‐transcription complex (RCT) in the double membrane vesicle. 53 RCT then replicates the subgenomic RNA encoding accessory and structural proteins. 54 Through newly formed genomic RNA, endoplasmic reticulum, Golgi organelles, nucleocapsid proteins and envelope glycoproteins are also synthesized, and viral particle buds are formed. 55 The vesicles containing the synthesized virion fuse with the plasma membrane and come out of the cell. The step between the SARS‐CoV‐2 Spike glycoprotein and the ACE2 receptor is the most critical point from the entry of the virus into the cell. Therefore, research on this relationship and affinity is still ongoing. The high affinity of S protein to human ACE2 can facilitate the spread of SARS‐CoV‐2 in human populations. In contrast, it has been reported that SARS‐CoV‐2 does not use other coronavirus receptors such as aminopeptidase N and dipeptidyl peptidase 4 (DPP4) to enter cells. 56
As a result of the structural analysis, it has been shown that the S protein of SARS‐CoV‐2 binds the ACE2 receptor with approximately 10 to 20 times higher affinity of SARS‐CoV. 57 In sum, similar to SARS‐CoV, SARS‐CoV‐2 also uses ACE2 as a cell entry receptor. 56 This indicates that SARS‐CoV‐2 can share the same life cycle as SARS‐CoV. Cleavage of the trimer S protein for SARS‐CoV facilitates membrane invagination for SARS‐CoV‐2 endocytosis, which is thought to be triggered by cell surface‐associated transmembrane protease serine 2 (TMPRSS2) and cathepsin. 58 Current research shows that SARS‐CoV‐2 is easily transmitted; however, it can cause less serious human infections than SARS‐CoV. Detection of viral binding and entry molecular mechanisms may explain the potential treatment and prophylaxis of SARS‐CoV‐2.
Histopathological changes in lung tissue in the SARS‐infected cases appear to have non‐specific inflammatory responses in the region such as oedema and inflammatory cell infiltration. It is also seen that there are serious exfoliation and serious damage in the alveolar septa. Pathologically, there are some non‐specific findings including necrosis, infiltration and hyperplasia. Therefore, SARS‐CoV infection is thought to cause these changes in lung tissue. SARS and MERS pathologies are not yet fully understood. Also, viral and host factors play a key role in SARS‐CoV, and MERS‐CoV infections. 59 Various immune system circumstances in the host react against the virus during infection. Pulmonary tissue damage results in functional impairment and decreased lung capacity as a result of the immune system overreacting and out of control. 20 The insufficient or excessive response of the immune system may increase viral replication and cause tissue damage. 60 The main pathological processes in the outbreak of COVID‐19 are the uncontrolled overreaction of the immune system. Increased cytokines in the respiratory tract and blood cause the acceleration of pathological processes and damage to many tissues and organs, especially pulmonary tissue.
As a result of scientific studies on patients infected with COVID‐19, it was found that there was an increase in leukocyte count, plasma pro‐inflammatory cytokine levels and abnormal breathing findings. 26 Significantly high blood levels of cytokines and chemokines were noted in patients with COVID‐19 infection that included IL1‐β, IL1RA, IL7, IL8, IL9 and IL10. 61 Studies on the potential mechanisms and pathogenic mechanisms of SARS‐CoV‐2, which have a high level of transmission while thankfully low mortality, are still ongoing.
4. CARDIOVASCULAR DISEASES, ACE2 AND SARS‐COV‐2
ACE2 is an enzyme bound to the cell membranes of the outer surface tissues in the lung, artery, heart, kidney and intestine. 62 It lowers blood pressure by catalysing the conversion of Ang 1 peptide (a vasoconstrictor) to Ang (1–7), which is known to have a vasodilating effect. 63 ACE2 has become a promising drug target in the treatment of cardiovascular diseases, both by increasing Ang (1–7) and by reducing the amount of Ang II, reducing its activity on the AT1 receptor. 64 The extracellular part of ACE2 is broken into another water‐soluble form by another enzyme known as sheddase, which is released into the blood and excreted in the urine. 65
The classical renin‐angiotensin system (RAS) pathway, which regulates the level of Ang II hormone, has affected many pathways, both the intracellular and extracellular pathways to keep many systems in balance, including the cardiovascular system. The important role and basic components of RAS in cardiovascular and kidney diseases were discovered in the early 1970s. 66 It has been highlighted in many studies that RAS causes the emergence and progression of many cardiovascular diseases such as hypertension, atherosclerosis and heart failure. 67 In particular, ACE, angiotensin II receptors (ATR), and renin blockers are among the current drug treatments frequently used in the treatment of diseases such as hypertension, heart failure, and diabetic nephropathy. 67 , 68 In addition to ACE, which Ang I to Ang II, there is also a serine protease enzyme, carboxypeptidase, chymase, and cathepsin G. It consists of Ang II aminopeptidases and Ang III, Ang IV and Ang (1‐7). It is stated that Ang (1‐7) has protective effects on vascular tissue. 69 It is also reported that ACE2 provides Ang (1‐9) formation. It is also stated that this pathway may be the counter‐regulator of Ang II formation through ACE which is dipeptidyl‐carboxypeptidase, also known as kininase II. 66 Ang II is formed by the destruction of Ang I with ACE and can be broken down into Ang III and Ang IV with aminopeptidase enzymes. It plays an important role in the regulation of blood pressure, salt, water and vascular homeostasis and is involved in the physiological and pathological processes of the vascular wall. Although its main effect is in vascular smooth muscle cells, it can also be shown by binding to ATRs found in endothelial cells. 70
It is stated that ATR1‐mediated effects cause pathological processes such as hypertension, atherosclerosis, thrombosis and inflammation, whereas binding to ATR2, Ang II increases NO release and vasodilation, reduces cell proliferation and growth. 71 ACE2 shows 42% sequence identity and 61% sequence similarity with ACE. However, ACE2 activity is not inhibited by ACE inhibitors because the active site of the enzyme in which the S2 pocket is smaller than that of the ACE. 72 Later studies show that Ang I is hydrolysed very slowly by ACE2, while Ang II hydrolyses to Ang (1‐7) with the highest catalytic efficiency seen for any of the angiotensin peptides. 72 However, ACE2 is not the only metabolic pathway to the production of Ang (1‐7), which can also be produced directly from Ang I by neprilysin (NEP), and other peptidases (prolyl endopeptidase and thimet oligopeptidase) also synthesize Ang (1‐7). Studies on ACE2 knockout mice have shown that mice develop cardiomyopathy and thereby cardiac problems occur. This circumstance indicates the importance of ACE2 activity for cardiovascular diseases. 73 It is well established that renal ACE2 gene expression is significantly found as reduced in spontaneously hypertensive rats. This result may reflect the importance of ACE2 activity in hypertension disease. 73 The definition of ACE2 is important for the prevention of hazardous effects of Ang II on the AT1 receptor, such as fibrosis, vasoconstriction and migration. Studies on the beneficial properties of Ang (1‐7), which increases as a result of ACE2 enzyme activity, are ongoing. On the other hand, in a report on this issue, Ang (1‐7) levels have been shown to decrease in patients in case of heart failure. 74
Ang (1‐7) infusions have been found to reverse cardiac dysfunction and correction of vascular endothelial dysfunction. As a result; Ang (1‐7) increased due to the activity of ACE2 was thought to have a cardioprotective effect. 75 Ang (1‐7) has been shown to have antiarrhythmic effects in experimental study after myocardial infarction. Due to the increase of ACE2 activity in the necrosis region, the level of Ang (1‐7) increased to the emergence of protective factors and tried to reduce inflammation. 76 Increased plasma Ang (1‐7) levels and cardiac ACE2 mRNA levels were demonstrated as a result of the administration of losartan or olmesartan for 28 days after coronary artery ligation. 76
As a result of recent studies, alamandin, synthesized from angiotensin A by ACE2 action, has been shown to have beneficial effects through a Mas‐associated gene receptor (MrgD). 77 Physiological effects of the RAS and its interaction with SARS‐CoV‐2 are shown in Figure 2. Also, ACE2 acts as a functional receptor for coronaviruses, including SARS‐CoV and SARS‐CoV‐2. 59 Patients exert some symptoms affecting the various system, especially the respiratory system, and these symptoms may be more severe in the population where the ACE2 level is higher compared to healthy individuals. According to the studies published on this subject, the safety and potential effects of antihypertension treatment with ACE inhibitors or ARBs in patients with COVID‐19 require careful consideration. However, it is still controversial whether COVID‐19 and patients with hypertension who take ACE inhibitors or ARBs will switch to another antihypertensive drug. 78 ARBs, which have become the most widely used antihypertensive drug class, are typically known to increase ACE2 expression by 2‐5‐fold. 79 In the COVID‐19 pandemic, there are no clear views on whether the risk of infection increases if certain antihypertensive drugs, such as ARB, are used. 80
FIGURE 2.
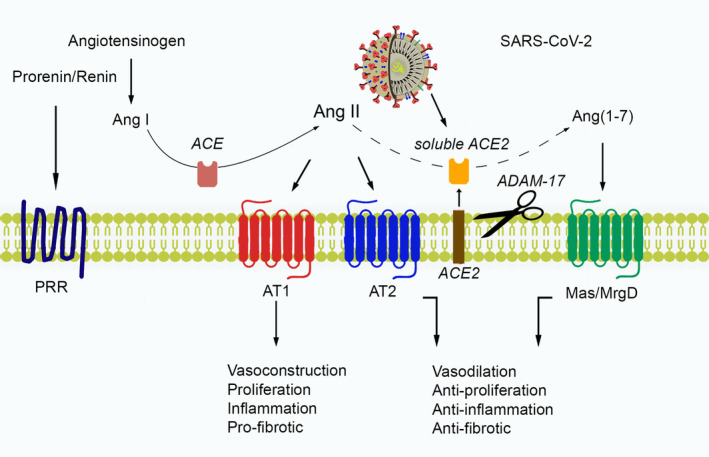
Physiological effects of the RAS, and its interaction with SARS‐CoV‐2
4.1. Patients receiving ACE inhibitors/ARBs
Patients receiving ACE inhibitors or ARBs should continue their treatment with these agents. Many guidelines state that these medications, previously prescribed for their illness, such as hypertension, should be continued. 81 , 82 , 83 , 84 , 85 There are speculations in various social media that COVID‐19 patients taking these agents may be at high risk for negative results. 78 It is known that ARBs can increase ACE2 levels because it is a receptor for SARS‐CoV‐2. 49 However, there is no study to support the increase of infection with SARS‐CoV‐2 due to the use of these drugs in patients with cardiovascular disease, hypertension, and diabetes mellitus. It should not be forgotten that stopping these agents in some patients may cause exacerbation of serious diseases accompanying cardiovascular or kidney disease and increase mortality. 86
4.2. Patients taking an immunomodulating agent
COVID‐19 infection is known to have a more severe clinical course in immunocompromised patients who have increased the risk of infection. However, patients should not stop immunomodulatory medications themselves and the necessary treatment plan should be done under the control of their physicians. COVID‐19 patients who should use antihypertensive drugs should continue to use these drugs. Also, if the drug is stopped and given later, it may cause a decrease in the treatment response. This view is supported by statements from American and other dermatology, rheumatology and gastroenterology societies. 87 , 88 , 89 , 90
4.3. Uncertainties regarding using non‐steroidal antiinflammatory drugs
Non‐steroidal antiinflammatory drugs (NSAIDs) early in the disease course may have harmful to disease outcomes. 91 These concerns have been raised by reports from a small number of patients who received early NSAIDs during infection and experienced severe disease. It should also be known that the anti‐inflammatory properties associated with NSAIDs are only at the theoretical level, which may impair the patient's immunity. Due to these concerns, it was mentioned to use acetaminophen without anti‐inflammatory properties instead of NSAIDs to reduce fever and algesia. However, both the European Medicines Agency (EMA) and WHO do not recommend avoiding NSAIDs clinically. 92 , 93 , 94
4.4. COVID‐19 and cardiovascular disease (CVD)
When looking at the patterns of patients with COVID‐19 infection, it is an undeniable fact that cardiovascular experts have very important duties. The infection can directly affect CVD. Also, pre‐existing CVD may be susceptible to infection. Current data suggest that patients with CVD may experience negative results after catching this infection. 95 Coronavirus outbreaks and cardiovascular risks are shown in Table 1. 96 It is known that drugs, which are potential treatment agents for COVID‐19 infection, also have serious side effects on the cardiovascular system. For this reason, the cardiovascular system should not be ignored in COVID‐19 patients; also, these patients with CVD should be closely monitored.
TABLE 1.
Coronavirus outbreaks and cardiovascular risks
| Outbreaks | Cardiovascular manifestations | Outcomes |
|---|---|---|
| SARS | Hypotension, tachycardia, bradycardia, cardiomegaly and arrhythmia | Mostly transient |
| Cardiac arrest | Death | |
| Sub‐clinical diastolic impairment without systolic involvement on echocardiography | Reversible on clinical recovery | |
| MERS | Acute myocarditis and acute‐onset heart failure | Recovered |
| COVID‐19 | Myocardial injury (manifesting with increased high sensitivity cardiac troponin I) in five patients | Four patients required intensive care |
| Acute cardiac injury, shock and arrhythmia | Most patients required intensive care |
4.5. Prevalence of CVD in COVID‐19 patients
It should be noted that the frequency of CVD occurrence in these patients will not be clear since it is very difficult to test all COVID‐19 patients. This causal prevalence varies from region to region. Data in the current literature suggest that there is a relationship between pre‐existing CVD and severe COVID‐19. In a meta‐analysis study on 1527 patients with COVID‐19, it was found that these patients previously had hypertension (17.1%), cardiac/cerebrovascular disease (16.4%) and diabetes mellitus (9.7%). 97 Patients who need to be admitted to the intensive care unit (ICU) are more likely to have these co‐morbidities than those without ICU. In a study involving 44 672 COVID‐19 cases, it was found that CVD (10.5%), diabetes mellitus (7.3%), hypertension (6.0%) were more common. The fatality rate in these patients was higher than other patients and reported as 2.3%. 98 Although there are differences between studies, patients with CVD are thought to have a higher risk group for COVID‐19. 99 These findings appear to be higher in patients with co‐morbid such as CVD, not only in China but also in Italy. 99 As emerging international data are available, analyses from multinational groups can help report the risk classification for serious illness, especially for patients with previous CVD. Increased frequency of adverse CVD events after COVID‐19; it shows that it can play a complex and multi‐factor role in prognosis, similar to other viral infections such as influenza. 100 , 101
4.6. Cardiovascular sequelae associated with COVID‐19
In this section, we will discuss the CVD that may occur due to COVID‐19. According to larger studies and some available reports, this infection is thought to cause cardiac problems or exacerbate the existing disease. 98
4.6.1. Myocardial injury, myocarditis and acute coronary syndromes
These cardiac problems can occur in case of severe respiratory infection or especially severe infection and acute respiratory distress due to COVID‐19 infection. Serum troponin levels increased in many patients infected with COVID‐19 and it was found that those who died due to this disease had higher levels of troponin than those discharged. 102 In a meta‐analysis of four studies involving a total of 341 patients, severe COVID‐19 related disease was found to be significantly higher in serious patients than in non‐serious patients. 103
Given the limited high‐quality data and heterogeneity of descriptions in studies, standardized data collection methods are recommended using the latest myocardial infarction definition. 104 Previous studies in other coronavirus species (MERS‐CoV) have demonstrated evidence of acute myocarditis using cardiac magnetic resonance imaging, and myocardial inflammation and damage have been reported with COVID‐19 infection. 105 In a case series of 150 patients with COVID‐19, it was shown that 33% of 68 deaths were due to myocarditis. 105 Other reports have found that a large amount of inflammatory mononuclear infiltrates are present in the myocardium of patients with COVID‐19. 106 Although pericardial involvement has not been reported yet, studies on the subject are needed.
All these data suggest that it may cause pathological cardiac conditions such as myocardial infarction due to COVID‐19 infection in both China and non‐China regions. This issue should not be ignored. As the virus continues to infect patients with cardiovascular risk factors, acute coronary syndromes due to COVID‐19 appear likely to develop. The most important mechanism in the emergence of myocardial damage is the disruption of the balance between T1 and T2 helper cells. As a result, a cytokine storm appears and causes respiratory dysfunction and hypoxemia thereby myocardial tissue is damaged. 38 Guidelines need to be followed for further advice on the care and treatment of COVID‐19 patients with myocardial injury, myocarditis and acute coronary syndromes.
4.6.2. Cardiac arrhythmia and cardiac arrest
Cardiac arrhythmias are another common CVD described in patients with COVID‐19 infection. A study has shown that 7.3% of 137 patients admitted to the hospital for COVID‐19 disease have heart palpitations. 107 Another study in China found that 16.7% of 138 COVID‐19 patients hospitalized had cardiac arrhythmias. The types of arrhythmia in patients have not yet been specified. 108 It is thought that metabolic disorder, hypoxia, neurohormonal or inflammatory stress‐related arrhythmia have developed due to viral infection with or without CVD.
4.6.3. Cardiomyopathy and heart failure
In a study on heart failure, 23.0% of COVID‐19 patients reported heart failure. Remarkably, it has been shown that heart failure is greater than acute kidney damage. Also, outpatients have been shown to develop more heart failure than patients in the hospital (51.7% vs 11.7%). 95 The cause of heart failure is thought to occur as a result of an exacerbation of pre‐existing left ventricular dysfunction due to myocarditis, however; this issue is not clear. 109
4.6.4. Venous thromboembolic disease
COVID‐19 patients likely have an increased risk of venous thromboembolism. Until now, there are no case series, however; abnormal coagulation parameters have been reported in patients with severe COVID‐19 infection. 110 In a multicentre study in China, a strong correlation was found between high D‐dimer levels (>1 g/L) in COVID‐19 patients. 111 In another study on patients with COVID‐19 infection, patients have been shown to have high d‐dimer and fibrin degradation products. In this study, it was also seen that 71.4% of patients had disseminated intravascular coagulation (DIC). 112 Thromboembolic diseases should be considered in cases of hypoxia, hemodynamic instability in patients with COVID‐19, or worsening of clinical symptoms. The most appropriate prophylactic regimen for patients under observation due to COVID‐19 related disease is unknown. For this reason, treatment protocols should be planned by following the current guidelines. 113
As a result of the studies, the importance of the relationship between COVID‐19 and CVD is increasing day by day. Therefore, patients should be followed up in terms of CVD. In this process, planning the treatments is required by following the current guidelines.
5. CLINICAL AND LABORATORY FINDINGS
As a result of analyses, the pneumonia symptoms caused by SARS‐CoV‐2 in Wuhan have started to become clear. 42 When the clinical findings of 278 patients with SARS‐CoV‐2 pneumonia were investigated, it was reported that all patients were older than 18 years old and 61.9% were men. As a result of studies on the clinical symptoms of COVID‐19 patients, the most common symptom in patients was fever (92.8%; n = 258). Other symptoms include cough (69.8%; n = 194), dyspnoea (34.5%; n = 96), myalgia (27.7%; n = 77), headache (7.2)%; n = 20) and diarrhoea (6.1%; n = 17). Also, only 4.0% of patients with relevant clinical information had rhinorrhoea, 5.1% sore throat and 17.4% pharyngalgia. 114 COVID‐19, immune system response and symptoms are shown in Figure 3.
FIGURE 3.
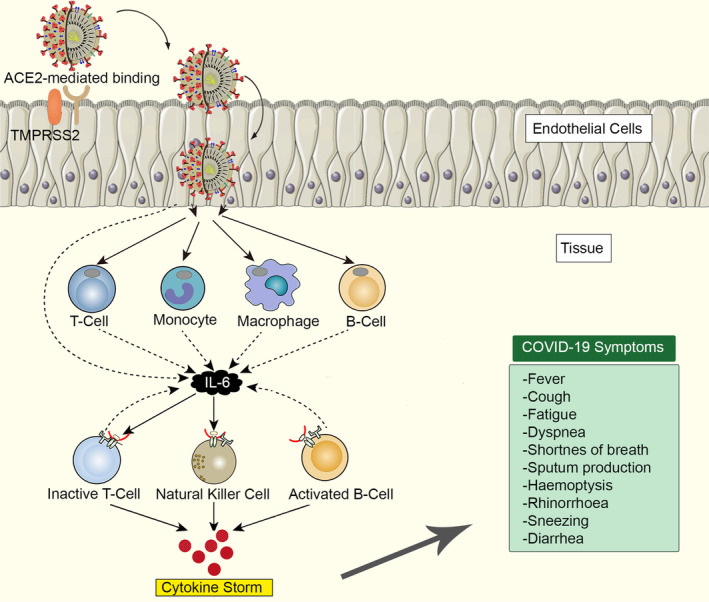
COVID‐19, immune system response and symptoms
According to the data obtained from a study containing 1099 cases, the most common symptom was detected as fever (88.7%) and this result was confirmed by a laboratory. Other symptoms were cough (67.8%), fatigue (38.1%), sputum production (33.4), shortness of breath (18.6%), sore throat (13.9%) and headache (13.6%). 115 Also, some patients were gastrointestinal symptoms such as diarrhoea (3.8%) and vomiting (5.0%). Although the patients' laboratory findings generally were normal white blood cells, 56.8% (n = 158) of the patients have leukopenia. 42 Nevertheless, in severe patients, neutrophil count, d‐dimer, blood urea and creatinine levels were found as significantly high, and lymphocyte counts were begun to decrease. 38
According to the laboratory results obtained from COVID‐19 patients, it was determined that 25% of infected patients developed leukopenia, and 63% developed lymphocytopenia. Also, the aspartate aminotransferase level increased by 37% of the patients. 38 Additionally, increased levels of IL‐2, IL‐7, IL‐10, granulocyte colony‐stimulating factor (GCSF), 10 kD interferon‐gamma‐induced protein (IP‐10), monocyte chemoattractant protein‐1 (MCP‐1), macrophage inflammatory protein 1‐α (MIP‐1α) and TNF‐α indicate that the disease‐related immune system is activated in patients. Most patients experienced elevated CK and lactate dehydrogenase. Although normal serum procalcitonin levels were seen in the majority of patients, the C reactive protein was found as increased. One‐third of the patients also had elevated d‐dimer. 116
Approximately half of the patients were found to have one or more comorbid such as hypertension, diabetes mellitus and cardiovascular disease. Other underlying diseases of elderly patients (hypertension, COPD, diabetes mellitus, cardiovascular disease) caused the patient to turn into acute respiratory distress syndrome, septic shock, metabolic acidosis and coagulation dysfunction. For this reason, the clinical symptoms in elderly patients are much more severe. 38 Patients requiring ICU have a more severe clinical symptom and have a higher rate of dyspnea. 117 In another study conducted, there were various clinical findings such as dyspnoea, respiratory frequency (≥30/min), reduced blood oxygen saturation (93%) and inspiratory oxygen rate of arterial oxygen <300 in severe patients. 98
It has been determined that an average of 8.0 days passes from the onset of the disease to dyspnoea and an average of 10.5 days to mechanical ventilation. 118 In another study, it was found that 12 (92.3%) of 13 patients had a fever and the patients had fever an average of 1.6 days before coming to the hospital. Other symptoms include cough (46.3%), upper airway obstruction (61.5%), myalgia (23.1%) and headache (23.1%). 119 In a study conducted by the China Centre for Disease Control and Prevention (41), it was found that 889 of 72 314 patients were asymptomatic cases (1.2%). 41
6. POTENTIAL TREATMENT AGENTS FOR COVID‐19
After the COVID‐19 infection affected the world in a short time and spread rapidly among people, studies focused on the treatment of infections and complications. According to our available knowledge, there is currently no specific antiviral drug or vaccine against COVID‐19 infection. 26 Currently, there are a large number of clinical trials in progress. Although the clinical effectiveness of these drugs has not yet been proven for COVID‐19, more recent studies show that some drugs may be potential drugs for COVID‐19 treatment. These potential drugs include lopinavir/ritonavir, nucleoside analogs, neuraminidase inhibitors, remdesivir, umifenovir, DNA synthesis inhibitors (tenofovir disoproxil, lamivudine), chloroquine/hydroxychloroquine, hydroxychloroquine+azithromycin, ACE2‐based peptides, 3C‐like protease (3CLpro) inhibitors. 32 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129
As of August 2020, 500 candidate therapeutics were in preclinical or a stage of phase I to IV development, with new phase II to III trials announced for hundreds of therapeutic candidates during 2020. 130 These agents are indinavir, saquinavir, lopinavir, carfilzomib, ritonavir, remdesivir, atazanavir, darunavir, tipranavir, fosamprenavir, enzaplatovir, presatovir, abacavir, bortezomib, elvitegravir, maribavir, raltegravir, montelukast, deoxyrhapontin, polydatin, chalcone, disulfiram, carmofur, shikonin, ebselen, tideglusib, PX‐12, TDZD‐8, cyclosporin A and cinanserin. 132 Pharmacological agents for the treatment of COVID‐19 are shown in Table 2. 132
TABLE 2.
Pharmacological agents for the treatment of COVID‐19
| Drugs | Feature | Mechanism of action | Indications | Status |
|---|---|---|---|---|
|
Lopinavir Ritonavir |
HIV protease inhibitor | Inhibiting HIV‐1 protease for protein cleavage, resulting in non‐infectious and immature viral particles | HIV/AIDS, SARS, MERS | Approved |
| Hydroxychloroquine/chloroquine | 9‐aminoquinolin | Increasing endosomal pH, immunomodulating and autophagy inhibitors | Malaria, autoimmunedisease | Approved,Investigational,Vet approved |
| Remdesivir | Nucleotide analog | Interfering with virus post‐entry | Ebola, SARS, MERS | Investigational |
| Nafamostat | Serine protease inhibitor | Prevents membrane fusion by reducing the release of cathepsin B | Influenza, MERS, Ebola | Investigational |
| Ribavirin | Guanosine nucleoside | Interfering with the synthesis of viral mRNA | HCV, SARS, MERS | Approved |
| Oseltamivir | Neuraminidase inhibitor | Inhibiting the activity of the viral neuraminidase enzyme, preventing budding from the host cell, viral replication and infectivity | Influenza virus A | Approved |
| PenciclovirAcyclovir | Nucleoside analog | Acyclic guanine derivative, resultingin chain termination | HSV, VZV | Approved |
| Ganciclovir | Nucleoside analog | Potent inhibitor of the HSV, CMV | AIDS‐associatedCMV infections | Approved,Investigational |
| Favipiravir | Nucleoside analog and viral RNApolymerase inhibitor | Acting on viral genetic copying to prevent its reproduction | Ebola, H1N1 | Investigational |
| Nitazoxanide | Antiprotozoal agent | Modulating the survival, growth, and proliferation of a range of extracellular and intracellular protozoa, helminths, anaerobic and microaerophilic bacteria, viruses | A wide range of viruses |
Approved,Investigational Vet approved |
AIDS, acquired immune deficiency syndrome; CMV, cytomegalovirus; MERS, Middle East respiratory syndrome; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HSV, herpes simplex virus; SARS, severe acute respiratory syndrome; VZV, varicella‐zoster virüs.
6.1. Neuraminidase inhibitors
Treatment strategies are developed for COVID‐19, based on the previous experience of combating SARS‐CoV and MERS‐CoV. 133 It has been suggested that previously used agents for influenza neuraminidase inhibitors (oseltamivir, peramivir, zanamivir, and so on), ganciclovir, acyclovir and ribavirin, and methylprednisolone should not be used against COVID‐19. 135
6.2. Remdesivir
Remdesivir is a 1'‐cyano substituted adenosine nucleotide analog prodrug. It is a broad‐spectrum antiviral drug that has activity against RNA viruses. According to the data obtained from in vitro and animal experiments, remdesivir may affect NSP12 polymerase in adjusting robust ExoN proofreading activity. 135 It is an antiviral drug candidate originally developed to treat the Ebola virus disease. 136 As a result of animal studies, remdesivir has been shown to effectively reduce the viral load in the lung tissue of MERS‐CoV‐infected mice. 137 Also, as a result of the success of remdesivir in vitro and animal experiments, the efficacy and safety of the drug were evaluated in COVID‐19 patients who started a multicentre, phase III clinical study in China. 138
It has been reported in the case report of remdesivir in the United States that it successfully treated the case of COVID‐19. 122 In a more recent study, COVID‐19 positive patients in the experimental group were given an initial dose of 200 mg by IV infusion and then 100 mg/day of remdesivir continued for nine consecutive days. The study is expected to be completed by the end of April 2020. Phase III clinical studies of remdesivir are conducted in the United States, China and Italy. 139 , 140 A clinical study in China showed that it was not effective in reducing recovery time from COVID‐19 infections or deaths. 141 On June 15, 2020, the FDA warned that the use of remdesivir and chloroquine or hydroxychloroquine may reduce the antiviral activity of remdesivir.
6.3. Umifenovir
Umifenovir is an antiviral drug used in the treatment of influenza and has been shown as a result of studies conducted in vitro that inhibit SARS‐CoV‐2 infection.
6.4. Chloroquine
Chloroquine is an antimalarial drug, which has broad‐spectrum antiviral feature was detected in 2006. 142 Chloroquine is one of the drugs with a high potential to be used to treat COVID‐19. Chloroquine has been used to treat malaria for many years, although the mechanism of action is not fully known it also exerts beneficial effects against some viral infections. 143 Chloroquine is effective in vitro against SARS‐CoV‐2 infection in previous scientific studies. 120 Various studies have been conducted on this subject, and possible mechanisms have been identified. Chloroquine can inhibit pH‐dependent steps of replication of various viruses, which has a strong effect on SARS‐CoV infection and spread. 144 Also, chloroquine has immunomodulatory effects that suppress the production/release of TNF‐α and IL‐6. It also has an autophagy inhibitor effect to prevent viral replication. 145
Several studies have shown that chloroquine plays a role in both entry and post‐entry stages of COVID‐19 infection by preventing glycosylation of SARS‐CoV cellular receptors. 120 Chloroquine and hydroxychloroquine, previously used to treat malaria, have been shown to effectively inhibit SARS‐CoV‐2 in vitro. It is reported that hydroxychloroquine also has stronger and fewer side effects than chloroquine. 146 A study has shown that chloroquine improves pathological disorders in the lung that occur due to COVID‐19 and is effective and safe in shortening the course of the disease. 147
The Guangdong Province Science and Technology Department and the Guangdong Provincial Health and Health Commission declared a report that chloroquine phosphate improves the success rate of COVID‐19 treatment and reduces the patients' hospitalization. 148 On March 17, the Italian Pharmaceuticals Agency increased its attention when it included chloroquine and hydroxychloroquine in the list of drugs with positive preliminary results for the treatment of COVID‐19. 149 Korean and Chinese Health Authorities recommend the use of chloroquine. Also, the Wuhan Virology Institute suggests a daily dose of 1 g but emphasizes that double this dose can be quite dangerous and deadly. This drug is not yet approved for COVID‐19 by the US Food and Drug Administration.
Cytokine storm, one of the pathophysiology seen in COVID‐19, causes significant complications. Studies have been published that hydroxychloroquine has anti‐cytokine storm properties. 150 In a clinical study involving 36 patients with COVID‐19, the use of hydroxychloroquine (200 mg three times a day for 10 days) was shown to decrease SARS‐CoV‐2 RNA in nasopharyngeal samples compared to the control group on day 6 (70% vs 12.5%). 151 In this study, it has been shown that the use of azithromycin in combination with hydroxychloroquine has an additional benefit, however, has not produced sufficient methodological evidence. The possibility of drug toxicity (including QTc prolongation and retinal toxicity) should be considered before using hydroxychloroquine. 152 The optimal dose scheme is uncertain; including
400 mg twice daily on day 1 then daily for five days,
400 mg twice daily on day 1 then 200 mg twice daily for four days,
600 mg twice daily on day 1 then 400 mg daily for four days various regimens are used. 98
According to data from the UK RECOVERY trial in June 2020, the use of hydroxychloroquine is beneficial for mortality in people hospitalized with severe COVID‐19 infection. 153 Also, in phase III studies for COVID‐19, multinational studies on a large number of patients have shown that adverse effects of the drug occur even at the optimal dose. 154 However, some people have had severe allergic reactions to these drugs. For this reason, national health units do not recommend the combination of hydroxychloroquine and azithromycin due to both energy and increased risk of sudden cardiac death. 155 As a result, doubts and indecision about the use of this drug still persist.
6.5. Lopinavir/ritonavir
Lopinavir/ritonavir is a drug for the human immunodeficiency virus (HIV), which is used in conjunction with other drugs to treat HIV‐1‐infected adults and children over the age of 14. 156 As a result of a study, it has been reported that lopinavir/ritonavir has anti‐SARS‐CoV activity in in vitro and clinical studies. 157 A clinical study in Korea reported that a COVID‐19 patient had significantly reduced β‐coronavirus viral loads after lopinavir/ritonavir treatment. 158 On the contrary, according to the study published in a scientifically valuable journal, lopinavir/ritonavir is ineffective in the treatment of serious diseases. 159
A study at the University of Colorado attempts to change drugs to find a compound to bind with the protease of SARS‐CoV‐2. In the UK Recovery Trial study, no clinical benefit was seen from the use of lopinavir‐ritonavir in 1596 people hospitalized with severe COVID‐19 infection for 28 days of treatment. 160 The results have not been published yet.
6.6. Darunavir
Studies on the second generation HIV‐1 protease inhibitor darunavir have been shown to inhibit SARS‐CoV‐2 infection in vitro. As a result of cell experiments, darunavir has been shown to reduce viral replication 280 times in vitro compared to the control group at 300 μM concentration. 140 Remdesivir and chloroquine combination regimen have been shown in studies to inhibit SARS‐CoV‐2 in vitro. It has been shown that protease inhibitors such as lopinavir and ritonavir can cure patients with MERS‐CoV and SARS‐CoV. 161 , 162
6.7. Nitazoxanide
Nitazoxanide (NTZ) is a drug approved in the United States for the treatment of intestinal parasite infections. 163 In recent studies, it has been shown to inhibit the replication of various viruses, including influenza. 164 Tisoxanidine, which is released as a result of its metabolism, shows its effect by inhibiting the maturation of influenza viral hemagglutinin. 165 An in vivo study has been proposed after nitazoxanide inhibits SARS‐CoV‐2 in vitro. 166
6.8. IFN‐α
IFN‐α is a broad‐spectrum antiviral used in the treatment of hepatitis B. As a result of studies, it has been shown to inhibit in vitro SARS‐CoV reproduction. 167
6.9. Ribavirin
Ribavirin is a nucleoside analogue with broad‐spectrum antiviral effects. In a study, 41 SARS patients treated with lopinavir/ritonavir and ribavirin and 111 SARS patients treated only with ribavirin were compared. According to the data obtained from the study, the effectiveness of combination therapy was found as much greater. 168
6.10. Favipiravir
Favipiravir is a drug that has an RNA‐bound RNA polymerase (RdRp) inhibitor feature. In addition to anti‐influenza virus activity, favipiravir has been shown to prevent replication of flavi‐, alpha‐, phylo‐, bunia‐, arena‐, noro‐ and other RNA viruses. Besides all these drugs, favipiravir received approval for new flu treatment on February 15, 2020, in China. 169 Favipiravir turns into a phosphorylated active form in cells. It is then recognized as a substrate by viral RNA polymerase, thereby inhibiting RNA polymerase activity. 170
According to the results obtained from these studies, favipiravir has potential efficacy on SARS‐CoV‐2. As a result of a clinical study, favipiravir gives clinical hope for the treatment of COVID‐19. Eighty patients were included in a study and it was observed that favipiravir had a stronger antiviral effect than that of lopinavir/ritonavir. As a very nice finding, there were no significant adverse reactions in the favipiravir treatment group, and even fewer side effects than the other treatment regimen. 171
6.11. Camostat
As a result of previous studies, it has been shown that CoV‐2 uses the SARS‐CoV receptor, ACE2, and cellular protease TMPRSS2 to enter target cells. 59 Accordingly, TMPRSS2 inhibitor (camostat) can be used for a therapeutic approach. 172 These findings suggest that camostat, which has been approved for another indication in Japan, maybe an effective treatment for off‐label COVID‐19. 134
6.12. Corticosteroids
Considering the large studies; to controlling the inflammatory response caused by SARS‐CoV‐2, systemic corticosteroids were given to 18.6% to 44.9% of patients. 61 , 114 According to the data obtained from a clinical study, it has been shown to reduce the MERS‐CoV RNA clearance. Also, corticosteroid use caused psychosis and diabetes mellitus in patients. 174 Therefore, the clinical use of corticosteroids in the treatment of COVID‐19 is not recommended in the interim period unless otherwise stated. 175
6.13. Polyclonal IgG (SAB‐301)
There is a clinical study in the literature investigating the safety and tolerability of human polyclonal IgG immunoglobulin (SAB‐301). 176 An experiment in the United States has shown that SAB‐301 is safe and well tolerated in single doses. Other studies on MERS‐CoV antibodies REGN3048 and REGN3051 are ongoing. 177
6.14. Tocilizumab
The interleukin‐6 receptor antagonist (tocilizumab) was approved by the FDA in 2017 for the treatment of cytokine release syndrome caused by chimeric antigen receptor (CAR) T cell therapy. 153 , 178 After studies on tocilizumab, it was included in the treatment guidelines by the Chinese National Health Commission. 179 After showing beneficial results in people with severe disease, phase II non‐randomized clinic study begun in Italy. 180
The Feinstein Northwell Health Institute announced a study on “a human antibody that can prevent activity” of IL‐6 in March 2020. 181 Also, tocilizumab is evaluated in a clinical trial for COVID‐19 patients with high IL‐6 levels in China. 182 The Australian Clinical Immunology and Allergy Association recommends that tocilizumab can be used as an off‐label treatment for those with acute respiratory distress syndrome associated with COVID‐19. 183
6.15. Vitamin D
A recent comprehensive study obtained from 20 European countries found a significantly negative correlation between mean vitamin D levels in the blood and COVID‐19 incidence/mortality in each country. 184 The mortality rate was also found as increased in northern countries, where vitamin D deficiency rates are generally high. 185 Based on this relationship, several studies have reported an association between vitamin D deficiency and the incidence of severe COVID‐19. 186 , 187 , 188 , 189
Other studies have found that individuals with dark skin are susceptible to vitamin D deficiency and therefore they exert severe COVID‐19 infection. 190 , 191 Also, different results have been reported, and it should be noted that a direct correlation between vitamin D deficiency and COVID‐19 severity is not yet clear. 191
As a result of the studies, it has been shown that the increase in the risk of acute respiratory distress syndrome and alveolar inflammation is associated with vitamin D deficiency. Vitamin D may be beneficial in minimizing alveolar damage due to COVID‐19, as it helps the regeneration of endothelial tissue. 192 Also, vitamin D may reduce the risk of infection through various mechanisms, as it enhances cellular innate immunity. 190 , 193
6.16. Passive antibody therapy
The use of antibodies produced by the immune system of patients recovering from COVID‐19 is being investigated as a method of vaccination. This strategy has been previously tested for SARS. 194 Viral neutralization is the expected mechanism of action by which passive antibody therapy can mediate defence against SARS‐CoV‐2. Other mechanisms such as antibody‐dependent cellular cytotoxicity and/or phagocytosis may be possible.
6.17. Vaccines
There is currently no vaccine that is effective against COVID‐19. Vaccine trials have not been completed yet, but trials and interventions are ongoing fastly. WHO reported that they did not expect it to be available in less than 18 months against SARS‐CoV‐2 in late February 2020. 195
Up to now, there is no cure or protective vaccine for SARS that is both safe and effective in humans. In the same way, there is no protective vaccine against MERS yet. 196 However, some vaccines continue to be developed for COVID‐19, and as of March 2020, there is a (DNA‐based) MERS vaccine that has completed phase I clinical trials in humans, all of which are viral vector vaccines, two with adenoviral vectors (ChAdOx1‐MERS, BVRS) ‐GamVac and with an MVA‐vector (MVA‐MERS‐S). 197
Many organizations use the published genomes of the virus to develop possible vaccines against SARS‐CoV‐2. 198 , 199 As of August 2020, there are 231 vaccine candidates in the development, but no candidate has completed clinical trials to prove their safety and efficacy. In August, at least 25 vaccine candidates are in clinical trials, with 6 vaccine candidates in phase III and 19 vaccine candidates still in phase I to II. 200
7. CONCLUSION
Since COVID‐19 first appeared in China, it has been a major epidemic that has affected the whole world. Because of the number of cases seen every day and their prevalence in the countries changes fastly, instead of preparing a table about scores in this review, we recommend one of the many internet addresses where these data can be followed instantly as https://covid19.who.int/. 17
WHO has declared it as a pandemic after the outbreak and scientists are doing great efforts to identify the characterization of the new coronavirus and to develop anti‐virus therapies and vaccines. In our review, we discussed the virology, epidemiological data, the replication of the virus, and its relationship with cardiovascular diseases on COVID‐19 pandemics. We also talked about COVID‐19 clinical and laboratory findings and discussed possible vaccine and pharmacological treatments.
To summarize according to the available data, first, SARS‐CoV‐2‐induced pneumonia COVID‐19 shows more infectivity but less virulence in terms of morbidity compared to SARS and MERS. Second, SARS‐CoV‐2 originating from the reservoir of bats and unknown intermediate hosts has been shown to enter the cell by binding to the ACE2 receptor with high affinity as a virus receptor to infect humans. Third, it has been observed that it affects older patients with co‐morbid diseases more. Fourth, supportive therapies with strong antiviral drugs such as remdesivir, hydroxychloroquine or lopinavir/ritonavir have begun to be used in the treatment of patients with COVID‐19. Clinical studies and vaccination studies are still ongoing fastly. Also, the pathogenesis of the virus is still not fully known, and new studies are needed in this regard. Currently, effective infection control intervention is the only way to prevent the spread of SARS‐CoV‐2. As a result of the studies to be carried out, it will reveal the mystery of the molecular mechanism(s) of viral introduction and replication, which will provide the basis for future research on the development of drugs and vaccines. Countries have to overcome great difficulties while dealing with this epidemic. We hope all of them are in a state of total war against this pandemic and that they can overcome the pandemic as quickly as possible.
ETHICS STATEMENT
No ethical approval required.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Parlakpinar H, Gunata M. SARS‐COV‐2 (COVID‐19): Cellular and biochemical properties and pharmacological insights into new therapeutic developments. Cell Biochem Funct. 2021;39:10–28. 10.1002/cbf.3591
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. De Groot R, Baker S, Baric R, et al. Family Coronaviridae. Ninth Report of the International Committee on Taxonomy of Viruses. Amsterdam: Elsevier Academic Press; 2011:806‐828.
- 2. Kahn JS, McIntosh K. History and recent advances in coronavirus discovery. Pediatr Infect Dis J 2005;24(11 Suppl):S223‐227, discussion S226. [DOI] [PubMed] [Google Scholar]
- 3.De Groot R, Baker S, Baric R, et al. Family Coronaviridae. Ninth Report of the International Committee on Taxonomy of Viruses. Amsterdam: Elsevier Academic Press; 2015;206:120‐133. [Google Scholar]
- 4. Weiss SR, Leibowitz JL. Coronavirus pathogenesis. Advances in Virus Research. Elsevier; 2011;81:85‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fan Y, Zhao K, Shi Z, Zhou P. Bat coronaviruses in China. Viruses. 2019;11:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Phan T. Novel coronavirus: from discovery to clinical diagnostics. Infect Genet Evol. 2020;79:104211‐104211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chan‐Yeung M, Xu R. SARS: epidemiology. Respirology. 2003;8(Suppl 1): p. S9‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Memish ZA, Perlman S, Van Kerkhove MD, Zumla A. Middle East respiratory syndrome. Lancet. 2020;395:1063‐1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. VANCOUVER . The coronavirus spreads racism against—and among—ethnic Chinese. February 17, 2020. https://www.economist.com/china/2020/02/17/the-coronavirus-spreads-racism-against-and-among-ethnic-chinese. Accessed March 28, 2020.
- 12. Hui DS, E IA , Madani TA, et al. The continuing 2019‐nCoV epidemic threat of novel coronaviruses to global health—the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Paraskevis D, Kostaki EG, Magiorkinis G, Panayiotakopoulos G, Sourvinos G, Tsiodras S. Full‐genome evolutionary analysis of the novel corona virus (2019‐nCoV) rejects the hypothesis of emergence as a result of a recent recombination event. Infect Genet Evol. 2020;79:104212‐104212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Organization WH . Novel coronavirus (2019‐nCoV) situation report‐2. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200122-sitrep-2-2019-ncov.pdf. Accessed January 21, 2020.
- 15. Li X, Wang W, Zhao X, et al. Transmission dynamics and evolutionary history of 2019‐nCoV. J Med Virol. 2020;92(5):501‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Riou J, Althaus CL. Pattern of early human‐to‐human transmission of Wuhan 2019 novel coronavirus (2019‐nCoV), December 2019 to January 2020. Euro Surveill. 2020;25(4):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. WHO Coronavirus Disease (COVID‐19) Dashboard . https://covid19.who.int/. Accessed August 21, 2020.
- 18.Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. Springer; 2015;1282:1‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li C, Yang Y, Ren L. Genetic evolution analysis of 2019 novel coronavirus and coronavirus from other species. Infect Genet Evol. 2020;82:104285‐104285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li G, Fan Y, Lai Y, et al. Coronavirus infections and immune responses. J Med Virol. 2020;92(4):424‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Laha S, Chakraborty J, Das S, Manna SK, Biswas S, Chatterjee R. Characterizations of SARS‐CoV‐2 mutational profile, spike protein stability and viral transmission. Infect Genet Evol. 2020;85:104445‐104445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rabenau HF, Cinatl J, Morgenstern B, Bauer G, Preiser W, Doerr HW. Stability and inactivation of SARS coronavirus. Med Microbiol Immunol. 2005;194(1–2):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tripet B, Howard MW, Jobling M, Holmes RK, Holmes KV, Hodges RS. Structural characterization of the SARS‐coronavirus spike S fusion protein core. J Biol Chem. 2004;279(20):20836‐20849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Raza H, Wahid B, Rubi G, Gulzar A. Molecular epidemiology of SARS‐CoV‐2 in Faisalabad, Pakistan: a real‐world clinical experience. Infect Genet Evol. 2020;84:104374‐104374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jin YH, Cai L, Cheng ZS, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019‐nCoV) infected pneumonia (standard version). Mil Med Res. 2020;7(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. El‐Aziz TMA, Stockand JD. Recent progress and challenges in drug development against COVID‐19 coronavirus (SARS‐CoV‐2)—an update on the status. Infect Genet Evol. 2020;83:104327‐104327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wuhan Coronavirus Death Rate. www.worldometers.info. Accessed February 2, 2020.
- 28. Wu D, Wu T, Liu Q, Yang Z. The SARS‐CoV‐2 outbreak: what we know. Int J Infect Dis. 2020;94:44‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhao S, Lin Q, Ran J, et al. Preliminary estimation of the basic reproduction number of novel coronavirus (2019‐nCoV) in China, from 2019 to 2020: a data‐driven analysis in the early phase of the outbreak. Int J Infect Dis. 2020;92:214‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019‐nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395(10225):689‐697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Backer J, Klinkenberg D, Wallinga J. Incubation period of 2019 novel coronavirus (2019‐nCoV) infections among travellers from Wuhan, China, 20‐28 January 2020. Euro Surveill. 2020;25(5):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lu H. Drug treatment options for the 2019‐new coronavirus (2019‐nCoV). Biosci Trends. 2020;14(1):69‐71. [DOI] [PubMed] [Google Scholar]
- 33. Nishiura H, Jung S‐M, Linton NM, et al. The extent of transmission of novel coronavirus in Wuhan, China, 2020. J Clin Med. 2020;9:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bassetti M, Vena A, Giacobbe DR. The novel Chinese coronavirus (2019‐nCoV) infections: challenges for fighting the storm. Eur J Clin Invest. 2020;50(3):e13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID‐19) outbreak. J Autoimmun. 2020;102433:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and corona virus disease‐2019 (COVID‐19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3):105924:1‐10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cheng ZJ, Shan J. Novel coronavirus: where we are and what we know. Infection. 2019;2020:1‐9. [Google Scholar]
- 38. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang P, Lu J, Jin Y, Zhu M, Wang L, Chen S Statistical and network analysis of 1212 COVID‐19 patients in Henan, China. Int J Infect Dis. 2020;95:391‐398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Novel CPERE . The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID‐19) in China. Zhonghua Liu Xing Bing Xue Za Zhi= Zhonghua Liuxingbingxue Zazhi. 2020;41(2):145. [DOI] [PubMed] [Google Scholar]
- 42. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID‐19. JAMA. 2020;323:1406‐1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. COVID C . Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID‐19)—United States, February 12‐March 16, 2020. [DOI] [PMC free article] [PubMed]
- 44. Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang W, Tang J, Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019‐nCoV) in Wuhan, China. J Med Virol. 2020;92(4):441‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID‐19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809‐815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Disease background of COVID‐19 . ; https://www.ecdc.europa.eu/en/2019-ncov-background-disease. Accessed March 25, 2020.
- 48. Lai CC, Liu YH, Wang CY, et al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARSCoV‐2): facts and myths. J Microbiol Immunol Infect. 2020;53:404‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Peiró C, Moncada S. Substituting angiotensin‐(1‐7) to prevent lung damage in SARS‐CoV‐2 infection? Circulation. 2020;141(21):1665‐1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade‐long structural studies of SARS coronavirus. J Virol. 2020;94(7):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tortorici MA, Veesler D. Structural insights into coronavirus entry. Adv Virus Res. 2019;105:93‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang N, Jiang S, Du L. Current advancements and potential strategies in the development of MERS‐CoV vaccines. Expert Rev Vaccines. 2014;13(6):761‐774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sawicki SG, Sawicki DL. Coronavirus transcription: a perspective. Curr Top Microbiol Immunol. 2005;287:31‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hussain S, Pan J, Chen Y, et al. Identification of novel subgenomic RNAs and noncanonical transcription initiation signals of severe acute respiratory syndrome coronavirus. J Virol. 2005;79(9):5288‐5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Perrier A, Bonnin A, Desmarets L, et al. The C‐terminal domain of the MERS coronavirus M protein contains a trans‐Golgi network localization signal. J Biol Chem. 2019;294(39):14406‐14421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wrapp D, Wang N, Corbett KS, et al. Cryo‐EM structure of the 2019‐nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260‐1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Simmons G, Gosalia DN, Rennekamp AJ, Reeves JD, Diamond SL, Bates P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc Natl Acad Sci U S A. 2005;102(33):11876‐11881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sironi M, Hasnain SE, Rosenthal B, et al. SARS‐CoV‐2 and COVID‐19: a genetic, epidemiological, and evolutionary perspective. Infect Genet Evol. 2020;84:104384‐104384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID‐19. J Pharm Anal. 2020;10(2):102‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. HuangCL W. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Parlakpinar H, Polat S, Acet HA. Pharmacological agents under investigation in the treatment of coronavirus disease 2019 and the importance of melatonin. Fundam Clin Pharmacol. 2020;1‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Parlakpinar H, Ozer MK, Acet A. Effects of captopril and angiotensin II receptor blockers (AT1, AT2) on myocardial ischemia‐reperfusion induced infarct size. Cytokine. 2011;56(3):688‐694. [DOI] [PubMed] [Google Scholar]
- 64. Chamsi‐Pasha MA, Shao Z, Tang WW. Angiotensin‐converting enzyme 2 as a therapeutic target for heart failure. Curr Heart Fail Rep. 2014;11(1):58‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Patel VB, Clarke N, Wang Z, et al. Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM‐17: a positive feedback mechanism in the RAS. J Mol Cell Cardiol. 2014;66:167‐176. [DOI] [PubMed] [Google Scholar]
- 66. Fyhrquist F, Saijonmaa O. Renin‐angiotensin system revisited. J Intern Med. 2008;264(3):224‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Luo J, Zhang WD, Du YM. Early administration of nifedipine protects against angiotensin II‐induced cardiomyocyte hypertrophy through regulating CaMKII‐SERCA2a pathway and apoptosis in rat cardiomyocytes. Cell Biochem Funct. 2016;34(3):181‐187. [DOI] [PubMed] [Google Scholar]
- 68. Ustündağ B, Canatan H, Cinkilinç N, Halifeoğlu I, Bahçecioğlu IH. Angiotensin converting enzyme (ACE) activity levels in insulin‐independent diabetes mellitus and effect of ACE levels on diabetic patients with nephropathy. Cell Biochem Funct. 2000;18(1):23‐28. [DOI] [PubMed] [Google Scholar]
- 69. Bihl JC, Zhang C, Zhao Y, et al. Angiotensin‐(1‐7) counteracts the effects of Ang II on vascular smooth muscle cells, vascular remodeling and hemorrhagic stroke: role of the NFsmall ka, CyrillicB inflammatory pathway. Vascul Pharmacol. 2015;73:115‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhang X, Gao F, Yan Y, Ruan Z, Liu Z. Combination therapy with human umbilical cord mesenchymal stem cells and angiotensin‐converting enzyme 2 is superior for the treatment of acute lung ischemia‐reperfusion injury in rats. Cell Biochem Funct. 2015;33(3):113‐120. [DOI] [PubMed] [Google Scholar]
- 71. Higuchi S, Ohtsu H, Suzuki H, Shirai H, Frank GD, Eguchi S. Angiotensin II signal transduction through the AT1 receptor: novel insights into mechanisms and pathophysiology. Clin Sci. 2007;112(8):417‐428. [DOI] [PubMed] [Google Scholar]
- 72. Rice GI, Thomas DA, Grant PJ, Turner AJ, Hooper NM. Evaluation of angiotensin‐converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J. 2004;383(Pt 1):45‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Crackower MA, Sarao R, Oudit GY, et al. Angiotensin‐converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417(6891):822‐828. [DOI] [PubMed] [Google Scholar]
- 74. Campbell DJ, Zeitz CJ, Esler MD, Horowitz JD. Evidence against a major role for angiotensin converting enzyme‐related carboxypeptidase (ACE2) in angiotensin peptide metabolism in the human coronary circulation. J Hypertens. 2004;22(10):1971‐1976. [DOI] [PubMed] [Google Scholar]
- 75. Loot AE, Roks AJ, Henning RH, et al. Angiotensin‐(1‐7) attenuates the development of heart failure after myocardial infarction in rats. Circulation. 2002;105(13):1548‐1550. [DOI] [PubMed] [Google Scholar]
- 76. Ferreira AJ, Santos RA, Almeida AP. Angiotensin‐(1‐7): cardioprotective effect in myocardial ischemia/reperfusion. Hypertension. 2001;38(3 Pt 2):665‐668. [DOI] [PubMed] [Google Scholar]
- 77. Lautner RQ, Villela DC, Fraga‐Silva RA, et al. Discovery and characterization of alamandine: a novel component of the renin‐angiotensin system. Circ Res. 2013;112(8):1104‐1111. [DOI] [PubMed] [Google Scholar]
- 78. Zheng YY, Ma Y‐T, Zhang J‐Y, Xie X. COVID‐19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin‐converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin‐converting enzyme 2. Circulation. 2005;111(20):2605‐2610. [DOI] [PubMed] [Google Scholar]
- 80. Esler M, Esler D. Can angiotensin receptor‐blocking drugs perhaps be harmful in the COVID‐19 pandemic? J Hypertens. 2020;38:781‐782. [DOI] [PubMed] [Google Scholar]
- 81. AHA . Patients taking ACE‐i and ARBs who contract COVID 19 should continue treatment unless otherwise advised by their physician. https://newsroom.heart.org/news/patients-taking-ace-i-and-arbs-who-contract-covid-19-should-continue-treatment-unless-otherwise-advised-by-their-physician. Accessed March 18, 2020.
- 82. Hypertension ESo . ESH Statement on COVID‐19. https://www.eshonline.org/spotlights/esh-statement-on-covid-19. Accessed March 18, 2020
- 83. Hypertension ISo . A statement from the International Society of Hypertension on COVID‐19. https://ish-world.com/news/a/A-statement-from-the-International-Society-of-Hypertension-on-COVID-19. Accessed March 18, 2020.
- 84. Eschner K. Position statement of the ESC council on hypertension on ACE‐inhibitors and angiotensin receptor blockers. https://www.escardio.org/Councils/Council-on-Hypertension(CHT)/News/position-statement-of-the-ESC-Council-on-hypertension-on-ace-inhibitors-and-ang. Accessed March 18, 2020.
- 85. Canada H. Hypertension Canada‐statement on COVID‐19. https://hypertension.ca/wp-content/uploads/2020/03/2020-30-15-Hypertension-Canada‐Statement‐on‐COVID‐19‐ACEi‐ARB.pdf. Accessed on March 18, 2020.
- 86. Qiao Y, Shin JI, Chen TK, et al. Association between renin‐angiotensin system blockade discontinuation and all‐cause mortality among persons with low estimated glomerular filtration rate. JAMA Intern Med. 2020;180:718‐726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Joint GI society message: COVID‐19 clinical insights for our community of gastroenterologists and gastroenterology care providers. https://www.gastro.org/press-release/joint-gi-societymessage-covid-19-clinical-insights-for-our-community-of-gastroenterologists-andgastroenterology-care-providers. Accessed March 18, 2020.
- 88. Rheumatism TELA. EULAR Guidance for the patient's COVID‐19 outbreak. https://www.eular.org/eular_guidance_for_patients_covid19_outbreak.cfm. Accessed March 18, 2020.
- 89. Dermatology TAAo . Dry Skin Relief From Covid‐19 Handwashing. https://www.aad.org/public/everyday-care/skin-care-basics/dry/coronavirus-handwashing. Accessed March 18, 2020.
- 90. Rheumatology ACo . March 18, 2020.
- 91. Day M. Covid‐19: Ibuprofen Should Not Be Used for Managing Symptoms, Say Doctors and Scientists. British Medical Journal Publishing Group; 2020;1‐1. [DOI] [PubMed] [Google Scholar]
- 92. Alert S. Updated: WHO Now Doesn't Recommend Avoiding Ibuprofen For COVID‐19 Symptoms. https://www.sciencealert.com/who-recommends-to-avoid-taking-ibuprofen-for-covid-19-symptoms. Accessed March 19, 2020.
- 93. Agency EM. EMA advises on the use of non‐steroidal anti‐inflammatories for COVID‐19. https://www.ema.europa.eu/en/news/ema-gives-advice-use-non-steroidal-anti-inflammatories-covid-19. Accessed March 19, 2020.
- 94. Lemaitre F, Solas C, Gregoire M, et al. Potential drug‐drug interactions associated with drugs currently proposed for COVID‐19 treatment in patients receiving other treatments. Fundam Clin Pharmacol. 2020;34:530‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Xiong TY, Redwood S, Prendergast B, Chen M. Coronaviruses and the cardiovascular system: acute and long‐term implications. Eur Heart J. 2020;41:1798‐1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Li B, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID‐19 in China. Clin Res Cardiol. 2020;109(5):531‐538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239‐1242. [DOI] [PubMed] [Google Scholar]
- 99. Guan W‐J, Ni Z‐Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. New Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kwong JC, Schwartz KL, Campitelli MA, et al. Acute myocardial infarction after laboratory‐confirmed influenza infection. New Engl J Med. 2018;378(4):345‐353. [DOI] [PubMed] [Google Scholar]
- 101. Davis MM, Taubert K, Benin AL, et al. Influenza vaccination as secondary prevention for cardiovascular disease: a science advisory from the American Heart Association/American College of Cardiology. J Am Coll Cardiol. 2006;48(7):1498‐1502. [DOI] [PubMed] [Google Scholar]
- 102. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8:475‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lippi G, Lavie CJ, Sanchis‐Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID‐19): evidence from a meta‐analysis. Prog Cardiovasc Dis. 2020;63:390‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). Eur Heart J. 2019;40(3):237‐269. [DOI] [PubMed] [Google Scholar]
- 105. Alhogbani T. Acute myocarditis associated with novel Middle East respiratory syndrome coronavirus. Ann Saudi Med. 2016;36(1):78‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu K, Fang YY, Deng Y, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl). 2020;133(9):1025‐1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Buzon J, Roignot O, Lemoine S, et al. Takotsubo cardiomyopathy triggered by influenza A virus. Intern Med. 2015;54(16):2017‐2019. [DOI] [PubMed] [Google Scholar]
- 110. Driggin E, Madhavan MV, Bikdeli B, et al. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID‐19) pandemic. J Am Coll Cardiol. 2020;75(18):2352‐2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Witt DM, Nieuwlaat R, Clark NP, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv. 2018;2(22):3257‐3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID‐19. JAMA. 2020;323:1406‐1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Wang D, Hu B, Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Chang LM, Wei L, et al. Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. JAMA. 2020;323:1092‐1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Res. 2020;30(3):269‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Morse JS, Lalonde T, Xu S, Liu WR. Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019‐nCoV. Chembiochem. 2020;215(5):730‐738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Holshue ML, DeBolt C, Lindquist S, et al. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382(10):929‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Ko WC, Rolain JM, Lee NY, et al. Arguments in favor of remdesivir for treating SARS‐CoV‐2 infections. Int J Antimicrob Agents. 2020;55:105933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Colson P, Rolain JM, Raoult D. Chloroquine for the 2019 novel coronavirus SARS‐CoV‐2. Int J Antimicrob Agents. 2020;55(3):105923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Chang R, Sun WZ. Repositioning Chloroquine as Ideal Antiviral Prophylactic against COVID‐19‐Time is Now. 2020. [DOI] [PMC free article] [PubMed]
- 126. Szűcs Z, Kelemen V, Le Thai S, et al. Structure‐activity relationship studies of lipophilic teicoplanin pseudoaglycon derivatives as new anti‐influenza virus agents. Eur J Med Chem. 2018;157:1017‐1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Zhou N, Pan T, Zhang J, et al. Glycopeptide antibiotics potently inhibit Cathepsin L in the late endosome/lysosome and block the entry of Ebola virus, Middle East respiratory syndrome coronavirus (MERS‐CoV), and severe acute respiratory syndrome coronavirus (SARS‐CoV). J Biol Chem. 2016;291(17):9218‐9232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Balzarini J, Keyaerts E, Vijgen L, et al. Inhibition of feline (FIPV) and human (SARS) coronavirus by semisynthetic derivatives of glycopeptide antibiotics. Antiviral Res. 2006;72(1):20‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Rahimkhoei V, Jabbari N, Nourani A, Sharifi S, Akbari A. Potential small‐molecule drugs as available weapons to fight novel coronavirus (2019‐nCoV): a review. Cell Biochem Funct. 2020;1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Biopharma products in development for COVID‐19. https://www.bioworld.com/COVID19products. Accessed August 3, 2020.
- 131. Medica SIoM . Chinese Academy of Sciences. A joint research team of the Shanghai Institute of Materia Medica and Shanghai Tech University discover a group of old and traditional Chinese medicines that may be efficacious in treating the novel form of pneumonia. http://www.simm.ac.cn/xwzx/kydt/202001/t20200125_5494417.html. Accessed February 22, 2020.
- 132. Guo Y‐R, Cao Q‐D, Hong Z‐S, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID‐19) outbreak–an update on the status. Mil Med Res. 2020;7(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Zumla A, Chan JF, Azhar EI, Hui DS, Yuen K‐Y. Coronaviruses—drug discovery and therapeutic options. Nat Rev Drug Discov. 2016;15(5):327‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Li H, Wang Y, Xu J, Cao B. Potential antiviral therapeutics for 2019 novel coronavirus. Zhonghua Jie he he Hu Xi Za Zhi= Zhonghua Jiehe he Huxi Zazhi= Chinese J Tuberc Respir Dis. 2020;43:E002‐E002. [DOI] [PubMed] [Google Scholar]
- 135. Agostini ML, Andres EL, Sims AC, et al. Coronavirus susceptibility to the antiviral remdesivir (GS‐5734) is mediated by the viral polymerase and the proofreading exoribonuclease. MBio. 2018;9(2):e00221‐e00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Warren TK, Jordan R, Lo MK, et al. Therapeutic efficacy of the small molecule GS‐5734 against Ebola virus in rhesus monkeys. Nature. 2016;531(7594):381‐385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Sheahan TP, Sims AC, Leist SR, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS‐CoV. Nat Commun. 2020;11(1):222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Rosa SGV, Santos WC. Clinical trials on drug repositioning for COVID‐19 treatment. [DOI] [PMC free article] [PubMed]
- 139. Beeching NJ FT, Fowler R. Update on COVID‐19 pandemic. https://bestpractice.bmj.com/topics/en-gb/3000168#important-update. Accessed March 2020.
- 140. Sun T. News: Abidol and darunavir can effectively inhibit coronavirus. http://www.sd.chinanews.com/2/2020/0205/70145.html. Accessed February 21, 2020.
- 141. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID‐19: a randomised, double‐blind, placebo‐controlled, multicentre trial. Lancet. 2020;395(10236):1569‐1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Savarino A, Di Trani L, Donatelli I, Cauda R, Cassone A. New insights into the antiviral effects of chloroquine. Lancet Infect Dis. 2006;6(2):67‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Aguiar AC, Murce E, Cortopassi WA, et al. Chloroquine analogs as antimalarial candidates with potent in vitro and in vivo activity. Int J Parasitol Drugs Drug Resist. 2018;8(3):459‐464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases? Lancet Infect Dis. 2003;3(11):722‐727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Golden EB, Cho HY, Hofman FM, Louie SG, Schonthal AH, Chen TC. Quinoline‐based antimalarial drugs: a novel class of autophagy inhibitors. Neurosurg Focus. 2015;38(3):E12. [DOI] [PubMed] [Google Scholar]
- 146. Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Clin Infect Dis. 2020;71:732‐739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID‐19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72‐73. [DOI] [PubMed] [Google Scholar]
- 148. Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID‐19. J Crit Care. 2020;57:279‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Farmaco AId . Azioni intraprese per favorire la ricerca e l'accesso ai nuovi farmaci per il trattamento del COVID‐19. https://www.aifa.gov.it/-/azioni-intraprese-per-favorire-la-ricerca-e-l-accesso-ai-nuovi-farmaci-per-il-trattamento-del-covid-19. Accessed March 27, 2020.
- 150. Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing sesign of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Clin Infect Dis. 2020;71:732‐739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial. Int J Antimicrob Age. 2020;56(1):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 152. Lemaitre F, Solas C, Grégoire M, et al. Potential drug‐drug interactions associated with drugs currently proposed for COVID‐19 treatment in patients receiving other treatments. Fundam Clin Pharmacol. 2020;34:530‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.No clinical benefit from use of hydroxychloroquine in hospitalised patients with COVID‐19. https://www.recoverytrial.net/news/statement-from-the-chief-investigators-of-the-randomised-evaluation-of-covid-19-therapy-recovery-trial-on-hydroxychloroquine-5-june-2020-no-clinical-benefit-from-use-of-hydroxychloroquine-in-hospitalised-patients-with-covid-19. Accessed June 5, 2020.
- 154. Mullard A. Flooded by the torrent: the COVID‐19 drug pipeline. Lancet. 2020;395(10232):1245‐1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Palca J. "NIH Panel Recommends Against Drug Combination Promoted By Trump For COVID‐19. https://www.npr.org/sections/coronavirus-live-updates/2020/04/21/840341224/nih-panel-recommends-against-drug-combination-trump-has-promoted-for-covid-19. April 21, 2020.
- 156. Su B, Wang Y, Zhou R, et al. Efficacy and tolerability of Lopinavir/ritonavir‐and Efavirenz‐based initial antiretroviral therapy in HIV‐1‐infected patients in a Tertiary Care Hospital in Beijing, China. Front Pharmacol. 2019;10:1472:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Chu C, Cheng V, Hung I, et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59(3):252‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Lim J, Jeon S, Shin HY, et al. Case of the index patient who caused tertiary transmission of COVID‐19 infection in Korea: the application of lopinavir/ritonavir for the treatment of COVID‐19 infected pneumonia monitored by quantitative RT‐PCR. J Korean Med Sci. 2020;35(6):e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Cao B, Wang Y, Wen D, et al. A trial of lopinavir‐ritonavir in adults hospitalized with severe Covid‐19. N Engl J Med. 2020;382:1787‐1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.No clinical benefit from use of lopinavir‐ritonavir in hospitalised COVID‐19 patients studied in RECOVERY. https://www.recoverytrial.net/files/lopinavir-ritonavir-recovery-statement-29062020_final.pdf. Accessed June 29, 2020.
- 161. Cvetkovic RS, Goa KL. Lopinavir/ritonavir: a review of its use in the management of HIV infection. Drugs. 2003;63(8):769‐802. [DOI] [PubMed] [Google Scholar]
- 162. Arabi YM, Alothman A, Balkhy HH, et al. Treatment of Middle East respiratory syndrome with a combination of lopinavir‐ritonavir and interferon‐beta1b (MIRACLE trial): study protocol for a randomized controlled trial. Trials. 2018;19(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Amadi B, Mwiya M, Musuku J, et al. Effect of nitazoxanide on morbidity and mortality in Zambian children with cryptosporidiosis: a randomised controlled trial. Lancet. 2002;360(9343):1375‐1380. [DOI] [PubMed] [Google Scholar]
- 164. Rossignol JF. Nitazoxanide: a first‐in‐class broad‐spectrum antiviral agent. Antiviral Res. 2014;110:94‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Rossignol JF, La Frazia S, Chiappa L, Ciucci A, Santoro MG. Thiazolides, a new class of anti‐influenza molecules targeting viral hemagglutinin at the post‐translational level. J Biol Chem. 2009;284(43):29798‐29808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Res. 2020;30(3):269‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3(9):e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Chu CM, Cheng VC, Hung IF, et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59(3):252‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Delang L, Abdelnabi R, Neyts J. Favipiravir as a potential countermeasure against neglected and emerging RNA viruses. Antiviral Res. 2018;153:85‐94. [DOI] [PubMed] [Google Scholar]
- 170. Furuta Y, Komeno T, Nakamura T. Favipiravir (T‐705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(7):449‐463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID‐19). Drug Discov Ther. 2020;14(1):58‐60. [DOI] [PubMed] [Google Scholar]
- 172. Hoffmann M, Kleine‐Weber H, Krüger N, Mueller MA, Drosten C, Pöhlmann S. The novel coronavirus 2019 (2019‐nCoV) uses the SARS‐coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. BioRxiv. 2020;181(2):271‐280. [Google Scholar]
- 173. Iwata‐Yoshikawa N, Okamura T, Shimizu Y, Hasegawa H, Takeda M, Nagata N. TMPRSS2 contributes to virus spread and immunopathology in the airways of murine models after coronavirus infection. J Virol. 2019;93(6):e01815‐e01818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Xiao JZ, Ma L, Gao J, et al. Glucocorticoid‐induced diabetes in severe acute respiratory syndrome: the impact of high dosage and duration of methylprednisolone therapy. Zhonghua Nei Ke Za Zhi. 2004;43(3):179‐182. [PubMed] [Google Scholar]
- 175. Li G, Fan Y, Lai Y, et al. Coronavirus infections and immune responses. J Med Virol. 2020;92(4):424‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Beigel JH, Voell J, Kumar P, et al. Safety and tolerability of a novel, polyclonal human anti‐MERS coronavirus antibody produced from transchromosomic cattle: a phase 1 randomised, double‐blind, single‐dose‐escalation study. Lancet Infect Dis. 2018;18(4):410‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Pang J, Wang MX, Ang IYH, et al. Potential rapid diagnostics, vaccine and therapeutics for 2019 novel coronavirus (2019‐nCoV): a systematic review. J Clin Med. 2020;9(3):623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Liu A. China turns Roche arthritis drug Actemra against COVID‐19 in new treatment guidelines. https://www.fiercepharma.com/pharma-asia/china-turns-roche-arthritis-drug-actemra-against-covid-19-new-treatment-guidelines. Accessed March 4, 2020.
- 179. Xu X, Han M, Li T, et al. Effective treatment of severe COVID‐19 patients with Tocilizumab. ChinaXiV. 2020;202003:v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.3 patients get better on arthritis drug. http://www.ansa.it/english/news/general_news/2020/03/13/3-patients-get-better-on-arthritis-drug_90d4764d-d93f-463e-ab07-168b34b084d0.html. Accessed March 5, 2020.
- 181. Solnik C. Northwell Health Initiates Clinical Trials of 2 COVID‐19 Drugs. https://www.longislandpress.com/2020/03/21/northwell-health-initiates-clinical-trials-of-2-covid-19-drugs/. Accessed March 21, 2020.
- 182. McIntosh K, Hirsch MS, Bloom A. Coronavirus disease 2019 (COVID‐19). UpToDate Hirsch MS Bloom. 2020;5. [Google Scholar]
- 183. Grainger S. ASCIA Position Statement: Specific Treatments for COVID‐19. https://allergy.org.au/hp/papers/specific-treatments-for-covid-19. Accessed July 03, 2020.
- 184. Ilie PC, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res. 2020;32(7):1195‐1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Rhodes JM, Subramanian S, Laird E, Kenny RA. Low population mortality from COVID‐19 in countries south of latitude 35 degrees north supports vitamin D as a factor determining severity. Aliment Pharmacol Ther. 2020;51(12):1434‐1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. De Smet D, De Smet K, Herroelen P, Gryspeerdt S, Martens GA. Vitamin D deficiency as risk factor for severe COVID‐19: a convergence of two pandemics. MedRxiv. 2020;1‐23. [Google Scholar]
- 187.Lau FH, Majumder R, Torabi R, et al. Vitamin D insufficiency is prevalent in severe COVID‐19. medRxiv. 2020;1‐14. [Google Scholar]
- 188. Meltzer DO, Best TJ, Zhang H, Vokes T, Arora V, Solway J. Association of vitamin D deficiency and treatment with COVID‐19 incidence. medRxiv. 2020;1‐22. [Google Scholar]
- 189. Daneshkhah A, Agrawal V, Eshein A, Subramanian H, Roy HK, Backman V. Evidence for possible association of vitamin D status with cytokine storm and unregulated inflammation in COVID‐19 patients. Aging Clin Exp Res. 2020;1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Mitchell F. Vitamin‐D and COVID‐19: do deficient risk a poorer outcome? Lancet Diabetes Endocrinol. 2020;8:570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Lee J, Van Hecke O, Roberts N. Vitamin D: a rapid review of the evidence for treatment or prevention in COVID‐19. Centre for Evidence‐Based Medicine Website 2020.
- 192. Kakodkar P, Kaka N, Baig M. A comprehensive literature review on the clinical presentation, and management of the pandemic coronavirus disease 2019 (COVID‐19). Cureus. 2020;12(4):e7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Grant WB, Lahore H, McDonnell SL, et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID‐19 infections and deaths. Nutrients. 2020;12(4):988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Casadevall A, Pirofski LA. The convalescent sera option for containing COVID‐19. J Clin Invest. 2020;130(4):1545‐1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Grenfell R, Trevor D. Here's why it's taking so long to develop a vaccine for the new coronavirus. https://www.sciencealert.com/who-says-a-coronavirus-vaccine-is-18-months-away. Accessed February 28, 2020.
- 196. Mahmoud M, Shehata MRG, Mohamed AA, et al. Middle East respiratory syndrome coronavirus: a comprehensive review. Front Med. 2016;10(2):120‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Yong CY, Ong HK, Yeap SK, Ho KL, Tan WS. Recent advances in the vaccine development against Middle East respiratory syndrome‐coronavirus. Front Microbiol. 2019;10:1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Duddu P. Coronavirus outbreak: vaccines/drugs in the pipeline for Covid‐19. https://www.clinicaltrialsarena.com/analysis/coronavirus‐mers‐cov‐drugs/. Accessed February 19, 2020.
- 199. Lee J. These nine companies are working on coronavirus treatments or vaccines—here's where things stand. https://www.marketwatch.com/story/these-nine-companies-are-working-on-coronavirus-treatments-or-vaccines-heres-where-things-stand-2020-03-06. Accessed March 7, 2020.
- 200. COVID‐19 vaccine development pipeline . 2020. https://vac-lshtm.shinyapps.io/ncov_vaccine_landscape/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


