Abstract
Introduction
Severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2)–infected patients commonly have elevated troponin and D‐dimer levels, but limited imaging exists to support most likely etiologies in efforts to avoid staff exposure. The purpose of this study was to report transthoracic echocardiographic (TTE) findings in SARS‐CoV‐2 patients with correlating troponin and D‐dimer levels.
Methods
We identified 66 SARS‐CoV‐2 patients (mean age 60 ± 15.7 years) admitted within a large, eight‐hospital healthcare system over a 6‐week period with a TTE performed. TTE readers were blinded to laboratory data with intra‐observer and inter‐observer analysis assessed.
Results
Sixty‐six of 1780 SARS‐CoV‐2 patients were included and represented a high‐risk population as 38 (57.6%) were ICU‐admitted, 47 (71.2%) had elevated D‐dimer, 41 (62.1%) had elevated troponin, and 25 (37.9%) died. Right ventricular (RV) dilation was present in 49 (74.2%) patients. The incidence and average D‐dimer elevation was similar between moderate/severe vs. mild/no RV dilation (69.6% vs 67.6%, P = 1.0; 3736 ± 2986 vs 4141 ± 3351 ng/mL, P = .679). Increased left ventricular (LV) wall thickness was present in 46 (69.7%) with similar incidence of elevated troponin and average troponin levels compared to normal wall thickness (66.7% vs 52.4%, P = .231; 0.88 ± 1.9 vs 1.36 ± 2.4 ng/mL, P = .772). LV dilation was rare (n = 6, 9.1%), as was newly reduced LV ejection fraction (n = 2, 3.0%).
Conclusion
TTE in SARS‐CoV‐2 patients is scarce, technically difficult, and reserved for high‐risk patients. RV dilation is common in SARS‐CoV‐2 but does not correlate with elevated D‐dimer levels. Increased LV wall thickness is common, while newly reduced LV ejection fraction is rare, and neither correlates with troponin levels.
Keywords: D‐Dimer, SARS‐CoV‐2, transthoracic echocardiography, troponin
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) has caused an upheaval in healthcare delivery by limiting imaging, invasive therapies, and face‐to‐face encounters despite abnormal vital signs and laboratory data. Acute cardiac injury has been reported in 7%–28% of cases, largely defined by elevated troponin levels. 1 , 2 Elevated D‐dimer levels have been reported in up to 68% of cases with anecdotal reports of hypercoagulability. 3 However, diagnostic abilities are hindered by limited testing to avoid personnel exposure and preserve personal protective equipment. Transthoracic echocardiography (TTE) is a readily available, high‐yield modality that can aid in these cases, but literature for TTE in SARS‐CoV‐2 is scarce. 4 The purpose of this correspondence was to describe TTE findings in patients with SARS‐CoV‐2. A secondary aim was to associate these TTE finding with D‐dimer and troponin levels.
2. METHODS
2.1. Population
Patients were included (n = 66, mean age 60 ± 15.7 years) if admitted with SARS‐CoV‐2 and underwent a TTE within Beaumont Health's eight‐hospital system across three counties in southeast Michigan from March 2 to April 11, 2020. Patients were excluded if younger than 18 years old. The study was approved by the Institutional Review Board, and consent was waived.
2.2. Echocardiography
Examinations were performed with dedicated full‐capacity machines. Measurements were performed in accordance with the 2015 American Society of Echocardiography (ASE) quantification guidelines 5 by two readers (JS and RB) blinded to each other and laboratory data. Inter‐observer and intra‐observer variations were analyzed with 25% of examinations over‐read. 6 Right ventricular (RV) enlargement was defined by the ratio of RV to left ventricular (LV) basal diameter classified as mild (0.67–0.9), moderate (1.0), and severe (>1.0) enlargement. RV dysfunction was assessed visually as Doppler evaluation was limited. LV dilation was determined by volumetric dimensions per ASE guidelines. 5
2.3. Laboratory studies
Nucleic acid testing for SARS‐CoV‐2 was obtained during the index hospitalization via nasal swab or respiratory culture. Serum assays were also obtained during the index hospitalization. Elevated troponin I level was defined as >0.03 ng/mL, and elevated D‐dimer level was defined as >499 ng/mL.
2.4. Statistical analysis
Categorical variables were presented as number and percentages, while continuous variables were presented as averages with standard deviation. Median values were also provided for continuous echocardiographic variables. A t test was used for assessment of average D‐dimer levels between RV dimension groups, but otherwise, a Mann‐Whitney U test was used to compare continuous variables. A Fisher exact test was used to compare categorical variables. Inter‐observer and intra‐observer variabilities were calculated by (A − B)/[(A + B)/2], with A representing the primary measurement and B representing the secondary measurement. 6
3. RESULTS
3.1. Population
Of 1780 patients admitted with SARS‐CoV‐2, 66 underwent a TTE. Patients were commonly African American, male, obese, with hypertension, and with diabetes (Table 1). Troponin and D‐dimer levels were frequently elevated. D‐dimer and troponin levels were not obtained in 16 and 7 patients, respectively. The majority were ICU‐admitted (n = 38, 57.6%) with 23 (34.8%) mechanically ventilated (MV), and 25 (37.9%) died. TTE indications included shock, cardiorespiratory failure, and concern for new wall‐motion abnormalities. Examinations were predominantly focused, and timing averaged 3 ± 4.5 days from admission.
TABLE 1.
Baseline characteristics and laboratory data in patients admitted with SARS‐CoV‐2 infection
| Baseline characteristic | N (%) | Mean ± SD | Normal range |
|---|---|---|---|
| Total | 66 (100) | ||
| Female | 28 (42.4) | ||
| Race | |||
| African American | 45 (68.2) | ||
| Caucasian | 21 (31.8) | ||
| Age, years | 60 ± 15.7 | ||
| BMI, kg/m2 | 43 ± 12.4 | ||
| Hypertension | 38 (57.6) | ||
| Diabetes mellitus | 23 (34.8) | ||
| Coronary artery disease | 10 (15.2) | ||
| Chronic obstructive lung disease | 8 (12.1) | ||
| End stage renal disease | 3 (4.5) | ||
| Venous thromboembolism | 6 (9.1) | ||
| Congestive heart failure | 7 (10.6) | ||
| Clinical characteristics | |||
| Peak creatinine, mg/dL | 2.4 ± 2.9 | 0.5–1.1 | |
| Peak ALT, U/L | 58 ± 68 | 9–47 | |
| Peak AST, U/L | 67 ± 58 | 0–34 | |
| D‐Dimer>499, ng/mL‡ | 47 (71.2) | 3823 ± 3122 | 0–499 |
| D‐Dimer>3000, ng/mL‡ | 26 (39.4) | ||
| TnI >0.03, ng/mL† | 41 (62.1) | 1.03 ± 2.04 | 0.0–0.3 |
Percent of non‐diagnostic or missing measurements denoted: *<10, †10–19, ‡20–29, §30–39, ǁ40–49, ¶50–59, #70–79, **80–89.
Abbreviations: ALT = Alanine aminotransferase; AST = Aspartate aminotransferase; BMI = Body mass index; SD = Standard Deviation; TnI = Troponin I.
3.2. Right ventricle and D‐dimer findings
RV dilation was common, but typically with intact systolic function (Table 2). RV size could not be graded in 6 patients with technically difficult studies due to poor acoustic windows, but function could be visually assessed in 5 of these patients using multiple alternative views. The incidence of elevated D‐dimer was similar among those with moderate/severe vs. mild/no RV dilation (16[69.6%] vs 25[67.6%], P = 1.0). Average D‐dimer levels in moderate/severe vs. mild/no RV dilation were 3736 ± 2986 vs 4141 ± 3351 ng/mL (P = .679). The mortality rate in patients with moderate/severe dilation was 50% vs. 26.3% among those with mild/no RV dilation (P = .09). The mortality was similar among patients with elevated and normal D‐dimer (20 [40.4%] and 1 [33.3%], P = 1) irrespective of RV size. Pulmonary hypertension was confirmed in 3 and excluded in 19 patients. A small pericardial effusion was present in 7(10.6%).
TABLE 2.
Echocardiographic right ventricular findings in patients admitted with SARS‐CoV‐2 infection
| Mean ± SD | Median | Normal range | |
|---|---|---|---|
| Right ventricle dimension | |||
| RVOT PLAX proximal (cm)† | 3.2 ± 0.5 | 3.2 | 2.0–3.0 |
| RVOT PSAX proximal (cm)§ | 3.0 ± 0.5 | 2.9 | 2.1–3.5 |
| RV basal diameter (cm)ǁ | 3.7 ± 0.8 | 3.7 | 2.5–4.1 |
| RV mid diameter (cm)¶ | 2.8 ± 0.8 | 2.6 | 1.9–3.5 |
| RV longitudinal length (cm)ǁ | 7.1 ± 1.2 | 6.9 | 5.9–8.3 |
| RV wall thickness (cm)ǁ | 0.6 ± 0.2 | 0.6 | 0.1–0.5 |
| RV/LV ratio* | 0.9 ± 0.3 | 0.8 | <0.67 |
| RV Enlargement (n = 60) | |||
| None | 11 (18.3) | ||
| Mild | 27 (45.0) | ||
| Moderate | 13 (21.7) | ||
| Severe | 9 (15.0) | ||
| RV Pulsed Doppler S' (cm/s)# | 12.8 ± 3.3 | 12.1 | ≥9.5 |
| TAPSE (mm)** | 20.9 ± 5.0 | 20 | ≥17 |
| RV dysfunction (n = 65) | |||
| None | 47 (72.3) | ||
| Mild | 10 (15.4) | ||
| Moderate | 5 (7.7) | ||
| Severe | 3 (4.6) | ||
| TR Vmax (cm/s)§ | 211 ± 86 | 236 | |
| PI Vmax (cm/s)# | 106 ± 58 | 100 | |
| RA pressure by IVC, mmHg (n = 54) | |||
| 3 | 23 (42.6) | ||
| 8 | 2 (3.7) | ||
| 15 | 6 (11.1) | ||
| Invasive mechanical ventilation | 23 (42.6) | ||
Percent of non‐diagnostic or missing measurements denoted: *<10, †10–19, ‡20–29, §30–39, ǁ40–49, ¶50–59, #70–79, **80–89.
IVC = Inferior vena cava; LV = Left ventricle; OT = Outflow tract; PI = Pulmonic insufficiency; PLAX = Parasternal long axis; PSAX = Parasternal short axis; RA = Right atrium; RV = Right ventricle; SD = Standard Deviation; TAPSE = Tricuspid annular plane systolic excursion; TR = Tricuspid regurgitation; Vmax = Maximum velocity.
3.3. Left ventricle and troponin findings
Most patients had increased LV wall thickness (WT) while dilatation was rare (Table 3). Troponin was elevated among tested patients with and without increased WT in 30 (66.7%) and 11 (52.4%), respectively (P = .231). Average troponin levels among those with vs. without increased WT were 0.88 ± 1.9 vs 1.36 ± 2.4 ng/mL (P = .772). Twelve patients had reduced ejection fraction (LVEF), seven had previously known, three without prior imaging, and two were newly diagnosed. Troponin was normal in patients with newly reduced LVEF, and no difference in average levels between preserved vs. low LVEF (0.96 ± 2 vs 1.8 ± 2.5 ng/mL, P = .11). Of those with elevated troponin, two patients were diagnosed with myopericarditis and one with supraventricular tachycardia to explain the troponin elevation. Mortality rates were no different between those with low LVEF vs. normal LVEF (4 [33.3%] vs 21 [38.9%], P = 1). Elevated troponin portended a significantly higher mortality when compared to patients with normal troponin levels (19 [46.3%] vs 3 [16.7%], P < .05) irrespective of LVEF.
TABLE 3.
Echocardiographic left ventricular findings in patients admitted with SARS‐CoV‐2 Infection
| Mean ± SD | Median | Normal Range | ||
|---|---|---|---|---|
| Male | Female | |||
| Left ventricle dimension | ||||
| LV septal wall thickness (cm)* | 1.1 ± 0.25 | 1.1 | 0.6–1.0 | 0.6–0.9 |
| LV posterior wall thickness (cm)* | 1.1 ± 0.26 | 1 | 0.6–1.0 | 0.6–0.9 |
| LV ESV (mL)‡ | 34 ± 17 | 32 | 21–61 | 14–42 |
| LV EDV (mL)‡ | 87 ± 24 | 88 | 62–150 | 46–106 |
| Simpson's Biplane LV EF (%)‡ | 60 ± 12 | 60 | 52–72 | 54–74 |
| Visual LV EF (%) | 59 ± 10 | 60 | 52–72 | 54–74 |
| Increased LV wall thickness (n=62) | ||||
| None | 16 (25.8) | |||
| Mild | 37 (59.7) | |||
| Moderate | 4 (6.4) | |||
| Severe | 5 (8.1) | |||
| LV Dilation (n=47) | ||||
| None | 40 (85.1) | |||
| Mild | 3 (6.4) | |||
| Moderate | 3 (6.4) | |||
| Severe | 1 (2.1) | |||
Percent of non–diagnostic or missing measurements denoted: *<10, †10–19, ‡20–29, §30–39, ǁ40–49, ¶50–59, #70–79, **80–89.
EDV = End diastolic volume; EF = Ejection fraction; ESV = End systolic volume; LV = Left ventricle; SD = Standard Deviation.
4. DISCUSSION
Our population represents a high‐risk group evident by higher D‐dimer and troponin levels than previously reported, 1 , 2 , 3 along with ICU, invasive MV, and mortality rates. The paucity of TTE studies is evident during this pandemic, which is consistent with recommendations by the American College of Cardiology/ASE and European Society of Cardiology (ESC). 7 , 8 Examinations should be focused to reduce sonographer exposure time and only performed if results change management. For example, ESC recommends TTE be performed when troponin levels are greater than five times the upper limit of normal and no other indication of type 1 myocardial infarction is present. 8
Not surprisingly, many had RV dilation (Figure 1), 9 which could result from pulmonary embolism or elevated pulmonary vascular resistance from hypoxia and high mean airway pressures from extraordinary MV settings. There was no specific correlation with D‐dimer levels and RV dilation. While average RV‐focused dimensions were normal, 81.7% of patients had RV enlargement by RV:LV > 0.67. This is attributable to many patients with nondiagnostic/missing RV‐focused views. To a lesser extent, the RV:LV may be impacted by decreased LV chamber sizes amidst a high inotropic state such as infection and sympathomimetic drug use. Interestingly, RV dilation was not associated with a higher mortality. Unlike previous reports, 1 , 3 D‐dimer elevation was also not associated with higher mortality. However, any conclusion is limited as only 3 patients in this study had a negative D‐dimer measured.
FIGURE 1.
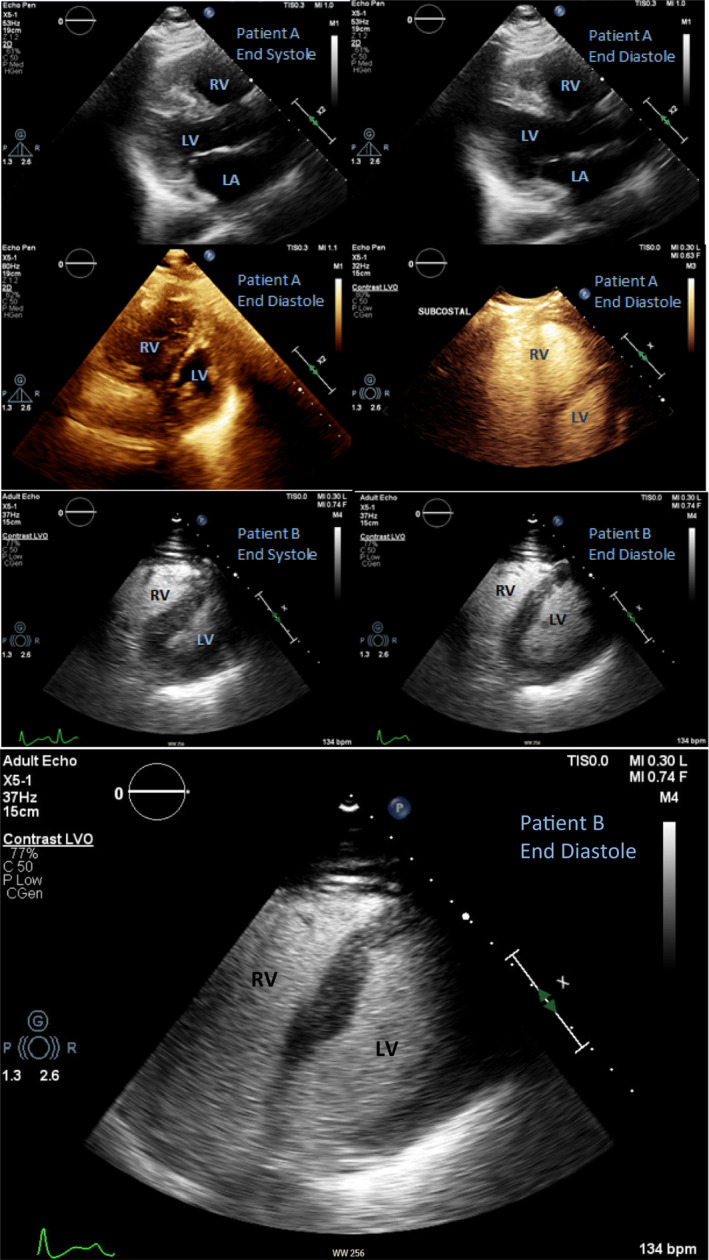
Echocardiographic images of two mechanically ventilated, SARS‐CoV‐2–infected patients exemplify intact LVEF, severe right ventricular enlargement, and “D”‐shaped septum. This pattern has been previously reported and postulated to correlate with the “H” pulmonary phenotype. 9
Similarly, there was no correlation with reduced LVEF and elevated troponin. Very few had reduced LVEFs, of which most were previously reduced. This is consistent with a limited case series previously reported, 4 and reports of nonobstructive coronaries amidst elevated troponin and ST elevation. 10 The degree of troponin elevation trended toward higher average levels among patients with low LVEF, which is consistent with preexisting cardiac disease. While a low LVEF was not associated with a higher mortality, this study did re‐demonstrate that elevated troponin is associated with a higher mortality rate. 2 , 3 , 11 Patients with increased WT were more likely to have elevated troponin, but degree of elevation was higher with normal WT; albeit neither of these findings reached statistical significance. Without definitive testing and renal function correlates, we can only speculate patients with increased WT have higher likelihood of detectable troponin due to larger LV mass or confounding comorbidities, but higher levels in those with normal WT are associated with direct cell injury, strain, or underlying coronary artery disease.
The study limitations include technically difficult images due to MV, patient positioning, airspace disease, and obesity. As such, the mid‐RV free wall was poorly visualized leading to the highest inter‐observer and intra‐observer variability of 0.36 and 0.27, respectively, when measuring RV wall thickness. The best inter‐observer variability was 0–0.01 with tricuspid annular plane systolic excursion, RV pulsed Doppler S', and right atrial pressure estimate. Intra‐observer variability was best in assessing pericardial effusion, RV pulsed Doppler S', and tricuspid regurgitation maximum velocity at 0–0.01. Selection bias occurred with our high‐risk patient sample including severity of clinical status and baseline comorbidities. Attempts to limit testing also resulted in a low study sample size. Finally, confirmatory testing for abnormal findings is lacking to avoid staff exposure.
5. CONCLUSION
In conclusion, echocardiographic utilization with SARS‐CoV‐2 is minimal, clinically directed, and usually reveals significant RV dilatation in the face of elevated D‐dimer. However, despite a troponin abnormality in over 60% of patients, LVEF is not commonly reduced but does coincide with increased WT.
CONFLICTS OF INTEREST
There are no conflicts of interest to disclose.
Schott JP, Mertens AN, Bloomingdale R, et al. Transthoracic echocardiographic findings in patients admitted with SARS‐CoV‐2 infection. Echocardiography. 2020;37:1551–1556. 10.1111/echo.14835
REFERENCES
- 1. Wang D, Hu BO, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan. China. JAMA. 2020;323(11):1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guo T, Fan Y, Lu Z, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020;5(7):811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou F, Yu T, Cao B, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid‐19 in critically ill patients in the Seattle Region ‐ case series. N Engl J Med. 2020;382(21):2012‐2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lang RM, Badano LP, Mor‐Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1‐39. [DOI] [PubMed] [Google Scholar]
- 6. Popović Z, Thomas J. Assessing observer variability: a user’s guide. Cardiovasc Diagn Ther. 2017;7(3):317‐324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kirkpatrick JN, Mitchell C, Taub C, et al. ASE statement on protection of patients and echocardiography service providers during the 2019 novel coronavirus outbreak. Am Coll Cardiol. 2020;75(24):3078‐3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Andreini D, Arbelo E, Barbato E, et al.ESC Guidance for the Diagnosis and Management of CV Disease during the COVID‐19 Pandemic. 2020. https://www.escardio.org/Education/COVID‐19‐and‐Cardiology/ESC‐COVID‐19‐Guidance.
- 9. Guarracino F, Vetrugno L, Forfori F, et al. Lung, heart, vascular, and diaphragm ultrasound examination of COVID‐19 patients: a comprehensive approach. J Cardiothorac Vasc Anesth. 2020. 10.1053/j.jvca.2020.06.013 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bangalore S, Sharma A, Slotwiner A, et al. ST‐segment elevation in patients with Covid‐19‐ a case series. N Engl J Med. 2020;382(25):2478‐2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802. [DOI] [PMC free article] [PubMed] [Google Scholar]


