Summary
Despite the ongoing coronavirus disease 2019 (COVID‐19) pandemic, elective paediatric surgery must continue safely through the first, second and subsequent waves of disease. This study presents outcome data from a children's hospital in north‐west England, the region with the highest prevalence of COVID‐19 in England. Children and young people undergoing elective surgery isolated within their household for 14 days, then presented for real‐time reverse transcriptase polymerase chain reaction testing for severe acute respiratory syndrome coronavirus disease‐2 (SARS‐CoV‐2) within 72 h of their procedure (or rapid testing within 24 h in high‐risk cases), and completed a screening questionnaire on admission. Planned surgery resumed on 26 May 2020; in the four subsequent weeks, there were 197 patients for emergency and 501 for elective procedures. A total of 488 out of 501 (97.4%) elective admissions proceeded, representing a 2.6% COVID‐19‐related cancellation rate. There was no difference in the incidence of SARS‐CoV‐2 among children and young people who had or had not isolated for 14 days (p > 0.99). One out of 685 (0.1%) children who had surgery re‐presented to the hospital with symptoms potentially consistent with SARS‐CoV‐2 within 14 days of surgery. Outcomes were similar to those in the same time period in 2019 for length of stay (p = 1.0); unplanned critical care admissions (p = 0.59); and 14‐day hospital re‐admission (p = 0.17). However, the current cohort were younger (p = 0.037); of increased complexity (p < 0.001) and underwent more complex surgery (p < 0.001). The combined use of household self‐isolation, testing and screening questionnaires has allowed the re‐initiation of elective paediatric surgery at high volume while maintaining pre‐COVID‐19 outcomes in children and young people undergoing surgery. This may provide a model for addressing the ongoing challenges posed by COVID‐19, as well as future pandemics.
Keywords: COVID‐19, elective surgery, paediatrics, testing
Introduction
The scale of the challenge facing the UK National Health Service (NHS) after the first wave of coronavirus disease 2019 (COVID‐19) is only just coming to light. Estimates suggest NHS waiting lists in England could reach 10 million patients, generating a backlog of work predicted to take up to 2 years to overcome [1, 2]. The considerations for re‐initiating and continuing elective surgery in paediatric practice are different from those faced within adult services [3]. The impact on the whole family must be considered, including the child's return to education; the parents 'return to work; as well as the potential for wider social and economic consequences. Many children present for straightforward day‐case procedures where there is limited exposure to the wider hospital environment and staff [4], and given what is now understood about the prevalence of severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) in the general paediatric population [5, 6, 7], hospital attendance for surgery may represent a child's highest risk of SARS‐CoV‐2 infection. There is, therefore, a need to demonstrate outcomes and develop the evidence‐base for guidance on the re‐initiation and continuation of elective surgery for children and young people through what is likely to be a protracted pandemic.
Paediatric anaesthesia in the context of an ongoing respiratory viral infection is associated with an increased incidence of peri‐operative complications which vary in severity from minor respiratory symptoms to unexplained death [8, 9, 10, 11]. It is therefore likely that the increased peri‐operative risks of SARS‐CoV‐2 in children outweigh the benefits of non‐essential surgery. Few data exist around the consequences of anaesthesia and surgery for children with SARS‐CoV‐2 infection. In the adult population, the COVIDSurg study demonstrated that postoperative pulmonary complications occurred in 51% of those with peri‐operative SARS‐CoV‐2 infection, and that these were associated with mortality as high as 24% [12]. Children and young people account for only 1–5% of patients diagnosed with COVID‐19, experience milder disease and have a better prognosis [13, 14, 15, 16, 17, 18, 19, 20]. However, a proportion require hospitalisation and a small number need ventilatory support for respiratory failure [13, 21, 22, 23]. There are also well‐described clusters of patients who have required critical care support due to paediatric inflammatory multisystem syndrome temporally associated with SARS‐CoV‐2 [13, 24, 25, 26, 27, 28, 29].
Consensus regarding the benefits of pre‐operatively testing asymptomatic children and the optimal duration of household isolation is evolving [3, 28, 29]. Heterogenous practice exists between paediatric specialist centres. Viral shedding is thought to be greater in adults [30, 31], so there are also potential questions regarding whether it may be more beneficial to screen parents and carers instead of their children, particularly in the context of regional disease surges [32]. The negative predictive value of real‐time reverse transcriptase‐polymerase chain reaction in SARS‐CoV‐2 is also unclear. We are yet to understand whether it differs in children compared with adults. Additionally, during periods of low disease prevalence (e.g. < 1% community prevalence), the positive predictive value of the test becomes poor as the pre‐test probability of actually having the virus is very low. Despite the high analytical sensitivity of reverse transcriptase‐polymerase chain reaction, studies demonstrate false negatives of 20–40% from upper airway swabs in adults with confirmed disease [33, 34, Yang et al., unpublished observations, https://doi.org/10.1101/2020.02.11.20021493]. This may be higher in children due to the technical challenges of obtaining upper respiratory tract swabs from patients who are unable to fully co‐operate with the procedure. Furthermore, given the incubation period and pathogenesis of SARS‐CoV‐2, a negative test on one day does not guarantee the absence of infection the next [35].
COVID‐19 has highlighted the impact of health inequality, with higher death rates seen in the most deprived communities [36]. It is therefore paramount that the delivery of surgery within children's specialist services during the endemic phase of COVID‐19 minimises the impact of these existing health inequalities. The north‐west of England has seen the highest infection rate for SARS‐CoV‐2 in England with continued surges in disease prevalence [32]. Catchments in the Merseyside area, where our institution is located, report some of the highest population cumulative incidences in the region. In addition, Liverpool and Knowsley, local communities served by our hospital, are two of the most deprived local authorities in the UK [37]. We therefore aimed to report our outcomes and experiences in re‐initiating and continuing elective paediatric surgical services at a specialist children's hospital at a time of global and national uncertainty.
Methods
This observational cohort study was conducted between 23 March and 5 July 2020 in accordance with the strengthening the reporting of observational studies in epidemiology (STROBE) statement utilising routinely collected institutional data to prospectively evaluate the impact of pre‐operative household isolation and SARS‐CoV‐2 screening on children presenting for surgery at a specialist paediatric centre. The UK Health Research Authority's research decision tool (hra‐decisiontools.org.uk/research) was consulted to determine that this project was considered to be a service evaluation by the NHS. Approval was therefore not sought from a research ethics committee, but this was registered prospectively with our institutional clinical governance board and the principles of good clinical practice were adhered to throughout the study.
Data were collected in two phases. Phase 1 was a retrospective analysis of urgent and emergency paediatric surgical cases presenting between 23 March 2020 (the start of the UK lockdown and cessation of all elective non‐cardiac surgery at our institution) and 25 May 2020. Following the re‐initiation of elective surgery, phase 2 commenced with prospective data analysis of all children and young people undergoing elective surgery between 26 May 2020 and 21 June 2020. Within phases 1 and 2 we evaluated patient, surgical and hospital demographic data alongside SARS‐CoV‐2 testing outcomes utilising electronic case notes. In phase 2, unplanned admissions to critical care, 14‐day re‐admission rates and length of stay were also evaluated.
Data were analysed with Microsoft Excel (Version 16.37; Microsoft, Inc., Redmond, WA, USA) and Prism (Version 8.4.3(471); GraphPad Software, Inc., San Diego, CA, USA). The D'Agostino‐Pearson test was used to determine Gaussian and non‐Gaussian distribution of data; the Mann–Whitney U, chi‐squared and Fisher's exact tests were used as appropriate for numerical and categorical data.
Elective surgical pathway
All patients scheduled for elective surgery at Alder Hey Children's Hospital after 26 May 2020 received instructions to self‐isolate with their families as a household for 14 days before surgery. Patients then underwent SARS‐CoV‐2 testing by trained staff using real‐time reverse transcriptase‐polymerase chain reaction from a combined nose and throat swab 72 h before surgery, either in clinic, at the hospital drive‐in testing facility or at a community pop‐up station (in the case of families residing at a considerable distance from our hospital). Parents and carers of patients who tested negative for SARS‐CoV‐2 were informed of the test result and asked to attend the hospital as planned. Patients who tested positive were offered appropriate medical treatment and advice and had their procedures postponed for 4 weeks, with the requirement for two negative tests before being allowed to proceed to surgery.
On both the day of swabbing and procedure, clinical screening paired with a three‐question screening questionnaire (online Supporting Information, Appendices S1 and S2) was conducted by nursing staff as part of the hospital admission procedure to identify whether patients or any member of their household had experienced symptoms of COVID‐19 or been in contact with anyone who had, within the preceding 14 days. Patients undergoing elective surgery who fell into the category of being high‐risk (undergoing cardiac surgery; requiring planned postoperative admission to intensive care; or at the specific consultant request for reasons otherwise undefined) also underwent a rapid (result available within 2 h) SARS‐CoV‐2 test, taken by a combined nose and throat swab on admission within 24 h of surgery. Patients who were deemed low risk on the screening questionnaire and who had a negative rapid test (if conducted) then proceeded to elective surgery.
In addition to appropriate medical care or advice, the households of patients deemed high risk for SARS‐CoV‐2 or who had a positive rapid test completed a survey to identify how well they had been able to self‐isolate as a household and any barriers faced.
Routine nose and throat samples collected into viral transport media were processed by real‐time reverse transcriptase‐polymerase chain reaction at nearby hospital virology laboratories (Public Health England Medical Microbiology Partnership, Manchester Royal Infirmary, Manchester from 23 March to 3 May and Liverpool Clinical Laboratory, Liverpool University Hospitals Foundation Trust from 4 May to present) using the Roche cobas® SARS‐CoV‐2 assay (F. Hoffmann‐La Roche AG, Basel, Switzerland) or the Cepheid Xpert® Xpress SARS‐CoV‐2 assay (Cepheid, Inc., Sunnyvale, CA, USA). The turnaround time for the routine samples was usually within 24 h. Samples selected for rapid testing were processed at the Alder Hey microbiology laboratory using the Cepheid assay with a turnaround time of within 2 h from receipt of the swab in the laboratory [38].
Emergency surgical pathway
Due to the unplanned nature of admission and presentation, children and young people presenting for emergency surgery at Alder Hey Children's Hospital received no instructions to self‐isolate with their families as a household before presentation to hospital. Patients underwent SARS‐CoV‐2 testing by trained staff utilising real‐time reverse transcriptase‐polymerase chain reaction from a combined nose and throat swab and clinical screening. Depending on the urgency of surgery, some patients proceeded to the operating theatre before SARS‐CoV‐2 swab results were available.
Results
As of 7 September 2020, the north‐west of England has 758.1 cases per 100,000 population, the highest in England [32]. Between 23 March 2020 and 21 June 2020, there were 5034 admissions to our institution (Fig. 1). In this time 2246/5034 (44.6%) admissions were tested for SARS‐CoV‐2, 359 (16.0%) of which used the rapid test. Eighteen out of 2246 (0.8%) of swabs resulted in positive tests in 18/1919 (0.9%) different patients.
Figure 1.
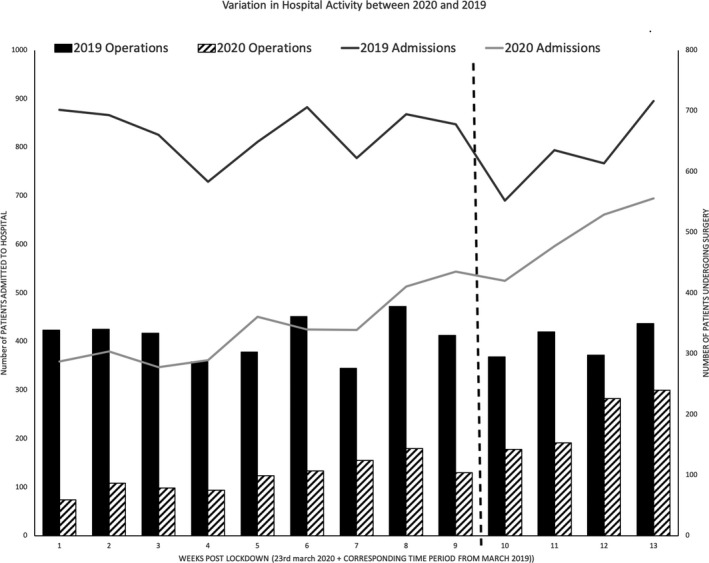
Variation in hospital activity at Alder Hey Children's NHS Foundation Trust between 2019 and 2020. Data are representative of actual number of children and young people presenting to hospital. The horizontal line before week 10 represents the start of elective services. Admissions (line graph) are 2019 admissions ( ) and 2020 admissions (
) and 2020 admissions ( ); operations (bar graph) are 2019 operations (
); operations (bar graph) are 2019 operations ( ) and 2020 operations (
) and 2020 operations ( ).
).
Phase 1: emergency surgery
Between 23 March 2020 and 25 May 2020, 669 children and young people aged 0–18 years with a median (IQR [range]) age of 4.1 (0.7–9.4 [0.0–18.4]) years, underwent 878 anaesthetics for urgent or emergency surgery at our institution. This compared with 2948 anaesthetics in the same time period in 2019; a reduction of 70.2% (Fig. 1). Peri‐operative testing for SARS‐CoV‐2 was performed before 436/878 (49.7%) operations, on 373/669 (55.8%) patients. Of patients who were tested, 5/373 (1.3%) were positive for SARS‐CoV‐2. At the time of anaesthesia, 4/5 patients were not known to be positive for SARS‐CoV‐2. Three of these children were asymptomatic and presented for urgent major cardiac, urology and plastic surgical procedures. The other SARS‐CoV‐2‐positive patients included one who was pyrexial with a suspected diagnosis of appendicitis and another with respiratory symptoms requiring an emergency gastrointestinal procedure.
Phase 2: elective surgery
Table 1 details the SARS‐CoV‐2‐related cancellations. The outcomes of children and young people in the re‐initiation phase of elective surgery are compared with a similar time period in 2019 in Table 2.
Table 1.
Features of the children and young people who had elective procedures cancelled during the first 4 weeks of elective surgery (26 May 2020–21 June 2020).
| Patient | Proposed procedure | Cancellation mode | Cancellation rationale |
|---|---|---|---|
| A | Dental surgery | Self: patient and family | Fear of swabbing process/hospital COVID‐19 risk |
| B | Dental surgery | Self: patient and family | Fear of swabbing process/hospital COVID‐19 risk |
| C | Plastic surgery | Admissions team | Swab outside 72‐h window |
| D | General surgery | Admissions team | Swab outside 72‐h window |
| E | Dental surgery | Self: patient and family | Fear of swabbing process/hospital COVID‐19 risk |
| F | Dental surgery | Admissions team | Failed 14‐day isolation of household |
| G | Radiology | Pre‐operative assessment | COVID‐19‐positive test – asymptomatic |
| H | ENT surgery | Admissions team | Symptomatic member of household |
| I | Major cardiac surgery | Pre‐operative assessment | Failed 14‐day isolation of household |
| J | ENT surgery | Admissions team | High patient temperature |
| K | Day case surgery | Admissions team | High patient temperature |
| L | Radiology | Self: patient and family | Symptomatic member of household report positive COVID‐19 test |
| M | Radiology | Self: patient and family | Symptomatic member of household report positive COVID‐19 test |
ENT, ear nose and throat.
Table 2.
Features and outcomes of children undergoing surgery during re‐initiation phase of elective surgery during the COVID‐19 pandemic (26 May 2020–21 June 2020) compared with the previous year before the pandemic (27 May 2019–23 June 2019). Values are median (IQR [range]) or number (proportion).
| Post‐COVID‐19 (n = 757) | Pre‐COVID‐19 (n = 1279) | p value | |
|---|---|---|---|
| Age (years) | 5.0 (1.7–10.8 [0.0–24.5]) | 5.8 (2.4–11.2 [0.0–20.6]) | 0.037 |
| Sex | 0.925 | ||
| Female | 301 (39.8%) | 512 (40.0%) | |
| Male | 456 (60.2%) | 767 (60.0%) | |
| ASA | 2 (2–3 [1–4]) | 1 (1–3 [1–5]) | <0.001 |
| 1 | 346 (45.7%) | 763 (59.7%) | |
| 2 | 204 (26.9%) | 249 (19.5%) | |
| 3 | 198 (26.2%) | 248 (19.4%) | |
| 4 | 9 (1.2%) | 17 (1.3%) | |
| 5 | 0 (0.0%) | 2 (0.2%) | |
| Urgency of surgery | <0.001 | ||
| Emergency | 240 (31.7%) | 325 (25.4%) | |
| Day case | 309 (40.8%) | 636 (49.7%) | |
| Other elective | 208 (27.5%) | 318 (24.8%) | |
| Speciality | <0.001 | ||
| Burns and plastics | 96 (12.7%) | 158 (12.4%) | |
| Cardiac | 76 (10.0%) | 92 (7.2%) | |
| Craniofacial and neurosurgical | 33 (4.4%) | 57 (4.5%) | |
| Dermatology and rheumatology | 10 (1.3%) | 9 (0.7%) | |
| Ear nose and throat | 58 (7.7%) | 142 (11.1%) | |
| Ophthalmology | 18 (2.4%) | 41 (3.2%) | |
| Gastro‐enterology | 34 (4.5%) | 51 (4.0%) | |
| Gynaecology | 0 (0%) | 5 (0.4%) | |
| Haematology and oncology | 55 (7.3%) | 48 (3.8%) | |
| Orthopaedics and spinal | 78 (10.3%) | 141 (11.0%) | |
| Maxillofacial and dental | 72 (9.5%) | 124 (9.7%) | |
| Other medical | 4 (0.5%) | 9 (0.7%) | |
| General surgery | 10 (13.2%) | 192 (15.0%) | |
| Radiology | 85 (11.2%) | 117 (9.1%) | |
| Respiratory | 6 (0.8%) | 13 (1.0%) | |
| Urology | 30 (4.0%) | 67 (5.2%) | |
| Miscellaneous: vascular access | 2 (0.3%) | 13 (1.0%) | |
| Postoperative care | 0.160 | ||
| Non‐critical care admissions (%) | 715 (95.5%) | 1226 (95.9%) | |
| Critical care admissions (%) | 42 (4.5%) | 53 (4.1%) | |
| Cardiac surgery | 32 (4.2%) | 28 (2.1%) | |
| Non‐cardiac neonates | 4 (0.5%) | 7 (0.5%) | |
| Neurosurgery | 1 (0.1%) | 2 (0.2%) | |
| Other inpatients | 5 (0.7%) | 15 (1.2%) | |
| Other surgery | 0 (0.0%) | 1 (0.1%) | |
| Unplanned critical care admissions | 0 (0.0%) | 1 (1.9%) | 1.0 |
| Elective surgery | 0 | 0 | |
| Emergency surgery | 0 | 1 | |
| Elective postoperative length of stay | 0 (0–2 [0–14]) | 0 (0–2 [0–14]) | 0.590 |
| 14‐day unplanned readmission rate | 15 (2.0%) | 15 (1.2%) | 0.180 |
| Elective surgery | 8 (1.1%) | 7 (0.5%) | |
| Emergency surgery | 7 (0.9%) | 8 (0.6%) |
Between 25 May 2020 and 21 June 2020, 488/501 (97.4%) children and young people with a median (IQR [range]) age of 5.4 (2.2–11.4 [0.0–24.5]) years underwent 517 anaesthetics for elective surgery. One hundred and ninety‐seven children and young people aged 4.6 (1.1–8.6 [0.0–17.2]) years underwent 240 anaesthetics for emergency surgery. This is compared with 1279 operations that took place in the same time period in 2019, accounting for a 41% reduction in total theatre throughput (Fig. 1). In the third and fourth weeks since resuming elective surgery, our centre was operating at 72% of historical capacity (Fig. 1). There was no difference in the incidence of SARS‐CoV‐2 among children and young people who had or had not isolated for 14 days: 1/496 (0.2%) in the planned elective surgery population compared with 0/197 (0.0%) in the unplanned emergency surgical population (p > 0.99).
Within the planned elective surgery group 13/501 (2.6%) children and young people had their surgery postponed as they were either confirmed or suspected high‐risk of SARS‐CoV‐2. This represents a 2.6% SARS‐CoV‐2‐related cancellation rate (Table 1). Five out of 501 (1.0%) children and young people had surgery postponed without testing for SARS‐CoV‐2. Three of the 501 (0.6%) patients did not get tested because their families refused to attend the hospital citing a fear of the hospital swabbing process and potentially acquiring nosocomial COVID‐19. One child had an autistic spectrum disorder and the family felt they would not tolerate swabbing and declined to trial the process. The other 2/501 (0.4%) patients did not attend hospital for swabbing due to confirmed SAR‐CoV‐2 within members of their household, though these patients were not known to be symptomatic for disease. Two out of 501 (0.4%) patients had procedures cancelled due to failure of members of their household to isolate for 14 days: one child, scheduled to undergo major cardiac surgery, was from a household where a parent cared for both the partner and siblings. Three out of 501 (0.6%) children and young people had procedures cancelled due to symptoms of SARS‐CoV‐2 or symptomatic members of the household, in all of these patients, test results were negative. Three out of 501 (0.6%) patients had their surgery cancelled due to test results outside the 72‐h window of safety.
Compared with those undergoing surgery in a similar time period in 2019, patients having elective surgery during the re‐initiation phase were younger (p = 0.037); had higher ASA physical statuses (p < 0.001); had a lower proportion of day‐case procedures and a greater proportion of emergency and other elective procedures (p < 0.001); and were from a wider spectrum of surgical specialities (p < 0.001; Table 2). There was no difference in length of stay; unplanned critical care admissions; and 14‐day re‐admissions to hospital. No patients were diagnosed with SARS‐CoV‐2 on repeat postoperative tests and none failed testing utilising a combined nose and throat swab administered by staff.
Discussion
This study presents peri‐operative outcomes from children and young people undergoing surgery during the endemic phase of COVID‐19. At present, few data exist regarding peri‐operative outcomes of SARS‐CoV‐2 in the paediatric population. It is, however, understood that the challenges of re‐initiating and continuing elective surgery in children during the COVID‐19 pandemic are different from those faced by adult services.
Our data suggest that in children and young people undergoing surgery during the endemic phase of COVID‐19, a combined approach of 14‐day household isolation, pre‐operative testing and clinical screening confers comparable levels of safety and peri‐operative outcomes to surgery undertaken before the COVID‐19 pandemic. Within our institution, a younger cohort of patients with a greater co‐morbid burden underwent more complex surgery but had similar outcomes to pre‐COVID‐19 patients.
We found the risk of peri‐operative infection of SARS‐CoV‐2 in children and young people presenting for elective surgery to be low, which is reassuring given that a visit to hospital for surgery has been hypothesised to represent a child's highest risk of contracting SARS‐CoV‐2 [3]. Though children with peri‐operative infection may not necessarily present back to the hospital where their surgery took place, the low peri‐operative infection rates observed within our institution likely reflect the high proportion of day‐case procedures where there is likely limited exposure to staff and the wider hospital environment. Day‐case surgery represents the bulk of paediatric operations that take place within the UK, particularly in non‐specialist children's hospitals [4].
This observational cohort study indicates that, now that the UK is past the first surge of disease during the endemic phase of COVID‐19, the risk of peri‐operative SARS‐CoV‐2 in the paediatric population is not significantly different among groups of children and young people that have isolated for 14 days compared with those who have not. We observed the same incidence of disease in children and young people undergoing elective surgery who had received instructions to isolate as household for 14 days compared with those presenting for emergency surgery who had not. This is despite the location of our institution in a region with the highest population cumulative incidence of SARS‐CoV‐2 in England [32]. The reasons behind this are likely multifactorial. At the time of this study, community prevalence of SARS‐CoV‐2 is low and the prevalence and disease burden of SARS‐CoV‐2 is lower still within the paediatric population when compared with adults [13, 14]. Anecdotal data also suggest that adherence to recommended isolation within populations is generally poor even for short periods of time (< 72 h), so it is possible that the data from defined isolated patient groups may not be from a truly isolated population [3]. To date, households admitting to failing to self‐isolate have had surgery at our institution postponed, but in light of our findings and new national guidance it would be beneficial for paediatric institutions to adopt a pragmatic approach involving risk assessment of patient comorbidities against surgical factors such as type and severity of surgery. Further studies and wider publication of national paediatric datasets are still required to determine the optimal duration of household isolation. Moving forward, our institution is routinely isolating households following swab testing for < 72 h before surgery.
The variability of symptomatology seen in the children and young people presenting for elective surgery at our institution supports the peri‐operative use of hybrid approaches that combine pre‐operative testing and clinical screening in the paediatric population. Given that the greater disease burden of SARS‐CoV‐2 is in the adult population [13, 14], unanswered questions still exist regarding the benefits of testing and clinically screening the parents and carers who accompany children and young people to hospital for surgery and the staff caring for them, particularly at times of high community prevalence. Well‐designed multicentre studies are required to produce more generalisable data.
Our results indicated a high degree of acceptability and sustainability of our approach of pre‐operative swab testing and 14‐day household isolation. However, local and national lockdown measures have since changed, and as children and young people return to school, these low failure rates may not be replicable. Children and young people cannot isolate independently, so the duration of household isolation requires careful consideration of the wider social and economic consequences, evaluating the implications of missed school (for both patient and their siblings) as well as missed work and potential loss of income to families. It is important to ensure that health inequalities already highlighted by the pandemic [36] do not increase further during the COVID‐19 recovery phase, particularly as the understanding of self‐isolation continues to evolve. Further evidence and outcome data are required around shorter periods of household isolation.
The generalisability of our data is limited by its observational nature, recording a unique and fluctuant disease phenomenon in the context of a rapidly evolving evidence base. It also has the additional limitation of being from a single UK centre. We also acknowledge that patients in standalone children's hospitals may be subject to different risks of peri‐operative infection compared with children's services co‐located within adult trusts. Alder Hey Children's NHS Foundation trust is a busy specialist centre delivering a range of quaternary and tertiary surgical procedures to children and young people from a wide geographical region encompassing north‐west England, Ireland and Wales. However, given our location within the region of highest SARS‐CoV‐2 disease prevalence in England, with associated high levels of social deprivation, which is acknowledged as a risk factor for high disease prevalence and poor disease outcomes in SARS‐CoV‐2 [32, 36], our data are relevant to both specialist and non‐specialist centres providing paediatric surgery throughout the UK.
During a pandemic, there will always be a proportion of children requiring urgent, complex, time‐critical operations. Similarly, some elective surgery cannot be postponed indefinitely as there are potentially serious adverse consequences of delay in some children and young people, including irreversible impairment of neurodevelopment and other avoidable morbidity. It is important to develop a strategy to maintain elective work safely while minimising the existing backlog of work and avoiding damaging effects to families and the wider community. The COVID‐19 pandemic presents continued uncertainty due to the ongoing fluctuations in disease prevalence [32]. These institutional data add to the limited existing evidence around the incidence of peri‐operative infection of SARS‐CoV‐2 in children and young people and presents outcomes from a model from which care can further be improved. Moving forward, guidance and institutional practice will evolve, informed by a growing knowledge base. The COVID‐19 pandemic highlights the need for NHS theatre services to benefit from big data through the creation of national platforms that facilitate real‐time information sharing of paediatric anaesthetic guidelines and local paediatric anaesthesia practice through electronic and app‐based systems, as in critical care.
In the decades preceding COVID‐19, the UK was fortunate to avoid the worst effects of other respiratory epidemics and pandemics including severe acute respiratory syndrome (2002–2004); H5N1 influenza (2008); H1N1 influenza (2009–2010); and Middle East respiratory syndrome (2012–2015). Given this recent historical context, another pandemic within our lifetimes does not seem unlikely. It is therefore important for healthcare organisations to become resilient through developing strategies for dealing with outbreaks of high‐impact respiratory pathogens. Though this study outlines the outcomes from a safe solution for reinitiating elective paediatric surgery in the context of COVID‐19 in a single centre, we believe that this may offer a basis for others to utilise in their own practice and adds to the evidence base upon which future guidelines will be built. Furthermore, there is potential to utilise similar principles paired with ongoing study to maintain elective paediatric surgical services during any future outbreaks of communicable disease.
Supporting information
Appendix S1. Alder Hey Children's NHS Foundation Trust pre‐operative assessment team guidance, in place between 26 May 2020 and 14 July 2020. COVID‐19 testing patients for elective surgery to ensure staff and teams are aware of the process to follow when referring elective surgical patients for COVID‐19 testing in addition to pre‐operative screening.
Appendix S2. Alder Hey Children's NHS Foundation Trust surgical admissions COVID‐19 screening tool. In place between 26 May 2020 and 3 August 2020.
Acknowledgements
Service evaluation registration ID 6093. The authors would like to thank J. Jensen; K. Edwardson; F. Potter; the Anaesthetic Pre‐assessment Service; the Surgical Admissions Lounge Team; the Day Case Surgery Team; the Information Department; the Microbiology Department; and the Theatre Team at Alder Hey Children's NHS Foundation Trust; the Virology Departments at Liverpool University Hospital Foundation NHS Trust, Royal Liverpool University Hospital and the Public Health England, Medical Microbiology Partnership, Manchester Royal Infirmary, for their co‐operation during this study. No external funding or competing interests declared.
Contributor Information
I. N. C. Okonkwo, Email: ijeoma.okonkwo@alderhey.nhs.uk, @alderheytheatre.
C. L. Shelton, @DrCliffShelton.
S. H. El‐Sheikha, @sarah_sheikha.
N. Herbert, @neil_herbert.
References
- 1. Campbell D. NHS hospital waiting lists could hit 10 million in England this year. Guardian 2020. https://www.theguardian.com/society/2020/jun/10/nhs‐hospital‐waiting‐lists‐could‐hit‐10‐million‐in‐england‐this‐year (accessed 07/09/2020). [Google Scholar]
- 2. The Royal College of Physicians . Returning the NHS to an even keel. 2020. https://www.rcplondon.ac.uk/guidelines‐policy/returning‐nhs‐even‐keel (accessed 07/09/2020).
- 3. The Royal College of Paediatrics and Child Health . National guidance for the recovery of elective surgery in children 2020. https://www.rcpch.ac.uk/resources/national‐guidance‐recovery‐elective‐surgery‐children (accessed 29/08/2020).
- 4. Department of Health . The NHS Plan: a plan for investment, a plan for reform. Report of a joint working party. 2000. https://webarchive.nationalarchives.gov.uk/20130124064356/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/@ps/documents/digitalasset/dh_118522.pdf (accessed 07/09/2020).
- 5. Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention . The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases. Zhonghua Liu Xing Bing Xue Za Zhi 2020; 41: 145–51. [DOI] [PubMed] [Google Scholar]
- 6. Bialek S, Boundy E, Bowen V, et al. Severe outcomes among patients with coronavirus disease 2019 (COVID‐19) – United States, February 12–March 16, 2020. Morbidity and Mortality Weekly Report 2020; 69: 343–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Livingston E, Bucher K. Coronavirus disease 2019 (COVID‐19) in Italy. Journal of the American Medical Association 2020; 323: 1335. [DOI] [PubMed] [Google Scholar]
- 8. Cohen M. Should you cancel the operation when a child has an upper respiratory tract infection? Anesthesia and Analgesia 1991; 72: 282–8. [DOI] [PubMed] [Google Scholar]
- 9. Tait AR, Malviya S. Anesthesia for the child with an upper respiratory tract infection: still a dilemma? Anesthesia and Analgesia 2005; 100: 59–65. [DOI] [PubMed] [Google Scholar]
- 10. Williams OA, Hills R, Goddard JM. Pulmonary collapse during anaesthesia in children with respiratory tract symptoms. Anaesthesia 1992; 47: 411–3. [DOI] [PubMed] [Google Scholar]
- 11. Tait AR, Malviya S, Voepel‐Lewis T, Munro H, Siewert M, Pandit U. Risk factors for perioperative adverse respiratory events in children with upper respiratory tract infections. Anesthesiology 2001; 95: 299–306. [DOI] [PubMed] [Google Scholar]
- 12. Nepogodiev D, Glasbey JC, Li E, et al. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS‐CoV‐2 infection: an international cohort study. Lancet 2020; 396: 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Götzinger F, Santiago‐García B, Noguera‐Julián A, et al. COVID‐19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child and Adolescent Health 2020; 4: 653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ludvigsson JF. Systematic review of COVID‐19 in children shows milder cases and a better prognosis than adults. Acta Paediatrica 2020; 109: 1088–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cai J, Xu J, Lin D, et al. A Case Series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clinical Infectious Diseases 2020; 28: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xia W, Shao J, Guo Y, et al. Clinical and CT features in pediatric patients with COVID‐19 infection: different points from adults. Pediatric Pulmonology 2020; 55: 1169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wei M, Yuan J, Liu Y, Fu T, Yu X, Zhang Z. Novel coronavirus infection in hospitalized infants under 1 year of age in China. Journal of the American Medical Association 2020; 323: 1313–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lu X, Zhang L, Du H, et al. SARS‐CoV‐2 Infection in Children. New England Journal of Medicine 2020; 382: 1663–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhu L, Wang J, Huang R, Liu L, Zhao H, Wu C, Zhu C. Clinical characteristics of a case series of children with coronavirus disease 2019. Pediatric Pulmonology 2020; 55: 1430–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ibrahim LF, Tosif S, McNab S, et al. SARS‐CoV‐2 testing and outcomes in the first 30 days after the first case of covid‐19 at an Australian children's hospital. Emergency Medicine Australasia 2020. [Epub 10 May]: 32; 801–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zeng L, Xia S, Yuan W, Yan K, Xiao F, Shao J, Zhou W. Neonatal early‐onset infection with SARS‐CoV‐2 in 33 neonates born to mothers with COVID‐19 in Wuhan, China. Journal of the American Medical Association Pediatrics 2020; 174: 722–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Qiu L, Jiao R, Zhang A, et al. A typical case of critically ill infant of coronavirus disease 2019 with persistent reduction of T lymphocytes. Pediatric Infectious Disease Journal 2020; 39: e87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kamali Aghdam M, Jafari N, Eftekhari K. Novel coronavirus in a 15‐day‐old neonate with clinical signs of sepsis, a case report. Infectious Diseases 2020; 52: 427–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS‐CoV‐2. Journal of the American Medical Association 2020; 324: 259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Riphagen S, Gomez X, Gonzalez‐Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID‐19 pandemic. Lancet 2020; 395: 1607–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. DeBiasi RL, Song X, Delaney M, et al. Severe COVID‐19 in children and young adults in the Washington, DC metropolitan region. Journal of Pediatrics 2020; 223: 199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jones VG, Mills M, Suarez D, et al. COVID‐19 and Kawasaki disease: novel virus and novel case. Hospital Pediatrics 2020; 10: 537–40. [DOI] [PubMed] [Google Scholar]
- 28. National Institute for Health and Care Excellence . COVID‐19 rapid guideline: arranging planned care in hospitals and diagnostic services. [NG179]. 2020. https://www.nice.org.uk/guidance/NG179 (accessed 29/08/2020). [PubMed]
- 29. Public Health England . COVID‐19: Guidance for the remobilisation of services within health and care settings. 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/910885/COVID‐19_Infection_prevention_and_control_guidance_FINAL_PDF_20082020.pdf (accessed 30/08/2020).
- 30. He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID‐19. Nature Medicine 2020; 26: 672–5. [DOI] [PubMed] [Google Scholar]
- 31. Danis K, Epaulard O, Bénet T, et al. Cluster of coronavirus disease 2019 (Covid‐19) in the French Alps, 2020. Clinical Infectious Diseases 2020; 71: 825–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. UK Government . Coronavirus (COVID‐19) cases in the UK. 2020. https://coronavirus.data.gov.uk/#category=regions&map=rate (accessed 07/09/2020).
- 33. Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W. Detection of SARS‐CoV‐2 in different types of clinical specimens. Journal of the American Medical Association 2020; 323: 1843–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu R, Han H, Liu F. Positive rate of RT‐PCR detection of SARS‐CoV‐2 infection in 4880 cases from one hospital in Wuhan, China, from Jan to Feb 2020. Clinica Chimica Acta 2020; 505: 172–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huff HV, Singh A. Asymptomatic transmission during the COVID‐19 pandemic and implications for public health strategies. Clinical Infectious Diseases 2020. Epub 28 May. 10.1093/cid/ciaa654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Public Health England . Disparities in the risk and outcomes of COVID‐19. 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/892085/disparities_review.pdf (accessed 15/06/2020).
- 37. Ministry of Housing, Communities and Local Government . The English Indices of Deprivation 2019: research report. 2019. https://www.gov.uk/government/publications/english‐indices‐of‐deprivation‐2019‐research‐report (accessed 15/06/2020).
- 38. Smithgall MC, Scherberkova I, Whittier S, Green DA. Comparison of Cepheid Xpert Xprss and Abbott ID Now to Roche cobas for the rapid detection of SARS‐CoV‐2. Journal of Clinical Virology 2020; 128: 104428. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Alder Hey Children's NHS Foundation Trust pre‐operative assessment team guidance, in place between 26 May 2020 and 14 July 2020. COVID‐19 testing patients for elective surgery to ensure staff and teams are aware of the process to follow when referring elective surgical patients for COVID‐19 testing in addition to pre‐operative screening.
Appendix S2. Alder Hey Children's NHS Foundation Trust surgical admissions COVID‐19 screening tool. In place between 26 May 2020 and 3 August 2020.


