Abstract
Sodium‐glucose co‐transporter‐2 (SGLT2) inhibitors are widely prescribed in people with type 2 diabetes. We aimed to investigate whether SGLT2 inhibitor prescription is associated with COVID‐19, when compared with an active comparator. We performed a propensity‐score‐matched cohort study with active comparators and a negative control outcome in a large UK‐based primary care dataset. Participants prescribed SGLT2 inhibitors (n = 9948) and a comparator group prescribed dipeptidyl peptidase‐4 (DPP‐4) inhibitors (n = 14 917) were followed up from January 30 to July 27, 2020. The primary outcome was confirmed or clinically suspected COVID‐19. The incidence rate of COVID‐19 was 19.7/1000 person‐years among users of SGLT2 inhibitors and 24.7/1000 person‐years among propensity‐score‐matched users of DPP‐4 inhibitors. The adjusted hazard ratio was 0.92 (95% confidence interval 0.66 to 1.29), and there was no evidence of residual confounding in the negative control analysis. We did not observe an increased risk of COVID‐19 in primary care amongst those prescribed SGLT2 inhibitors compared to DPP‐4 inhibitors, suggesting that clinicians may safely use these agents in the everyday care of people with type 2 diabetes during the COVID‐19 pandemic.
Keywords: antidiabetic drug, DPP‐4 inhibitor, pharmaco‐epidemiology, SGLT2 inhibitor, type 2 diabetes
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) was first reported in December 2019 in the district of Wuhan, China. The consequent multi‐system illness, COVID‐19, was particularly severe in older adults and those with comorbidities, 1 including diabetes. 2 The potential impact of drugs commonly used in diabetes on susceptibility to SARS‐CoV‐2 infection is of significant clinical and public health interest. 3 , 4
Sodium‐glucose co‐transporter‐2 (SGLT2) inhibitors have an established beneficial impact on cardiovascular risk, blood pressure, chronic kidney disease progression and body weight. 5 They are associated with promotion of ACE2 activity, which may facilitate viral entry into cells, thereby increasing the susceptibility to clinically evident disease. They may also however provide a degree of protection via favourable impact on glycaemic control and inflammation. 6 Given the adverse outcomes associated with COVID‐19 in the context of diabetes, and the uncertainty surrounding underlying mechanisms, an investigation of the potential for these commonly prescribed medications to influence presentation and course of COVID‐19 is of importance.
Current practice guidelines and expert opinion recommend that SGLT2 inhibitor treatment is discontinued in the community during acute illness and on hospital admission due to the increased risk of diabetic ketoacidosis. 7 , 8 However, there is currently no empirical evidence that use of SGLT2 inhibitors increases the risk of developing COVID‐19 among people with type 2 diabetes in the primary care setting.
We aimed to investigate the risk of a composite of confirmed or suspected COVID‐19 in people with type 2 diabetes prescribed SGLT2 inhibitors compared to a propensity‐score‐matched cohort of individuals prescribed a dipeptidyl peptidase‐4 (DPP‐4) inhibitor as an active comparator in primary care.
2. MATERIALS AND METHODS
Further methodological detail can be found in Appendix S1.
2.1 Study design
We conducted a population‐based retrospective cohort study in people with type 2 diabetes, comparing users of SGLT2 inhibitors to propensity score‐matched users of DPP‐4 inhibitors. Data for this cohort study were derived from The Health Improvement Network (THIN), a database of routinely collected primary care records which is generalizable to the UK population. In 2020, it included data from 365 practices with approximately 2.1 million active adult patients. To improve data quality, general practices were eligible to be included in this study 12 months after the installation of Vision software and 12 months after reporting acceptable mortality rates. 9
2.2 Study population
Individuals aged ≥18 years with a diagnosis of type 2 diabetes and registered with an eligible general practice for ≥1 year on the index date (January 30, 2020) were included. Patients diagnosed with diabetes before the age of 12 years, with a record of type 1 diabetes, pancreatitis, adverse reaction to antidiabetic agents at any time point, or pregnancy in the preceding year, were excluded.
2.3 Exposure
Current users of SGLT2 or DPP‐4 inhibitors were defined as individuals with a record of prescription lasting beyond the index date.
People with type 2 diabetes with a current prescription of SGLT2 inhibitors (exposed group) were compared to individuals with a current prescription of DPP‐4 inhibitors (comparator group). Neither cohort had a current prescription of the comparator medication. DPP‐4 inhibitors were chosen as they are safe in the long‐term management of people with diabetes, 10 and are actively recommended as a first intensification step. 11 Exposed and comparator groups were mutually exclusive.
2.4 Matching
On the index date, participants in the exposed group were propensity‐score‐matched to participants in the comparator group. Propensity score for use of SGLT2 inhibitors was estimated using a logistic regression model including a set of covariates described in the covariates section below.
2.5 Follow‐up period
Participants were followed up from January 30, 2020 (index date) until the earliest of the following dates: date of the outcome, death, patient left practice, practice ceased contributing to the database, or study end date (July 22, 2020).
2.6 Outcomes
The primary outcome was a composite of confirmed or suspected diagnosis of COVID‐19. Secondary outcomes were confirmed diagnosis of COVID‐19 and COVID‐19‐related death. COVID‐19‐related death was defined as a death date not later than 28 days after occurrence of the primary outcome. We used a negative control outcome, incident back pain, during follow‐up to assess the possibility of surveillance bias and unobserved confounding. 12 All outcomes were defined using the relevant clinical (Read) codes (Table S1).
2.7 Covariates
Covariates included: sociodemographic characteristics; lifestyle and metabolic profile; presence of comorbid conditions; comorbid conditions identified as risk factors for COVID‐19; diabetes‐specific conditions or measures indicating diabetes severity; diabetes duration; and history of prescriptions of relevant drugs. For a full list of covariates, please see Appendix S1.
2.8 Statistical analysis
Crude incidence rates per 1000 person‐years of COVID‐19 and the negative control outcome, back pain, were estimated for the exposed and the comparator cohort. A Cox proportional hazards regression model was used to determine crude and adjusted hazard ratios (HRs) for SGLT2 inhibitor compared to DPP‐4 inhibitor treatment for the COVID‐19 outcome and the negative control outcome. Survival curves for the exposed and the unexposed groups were generated for the unmatched and the propensity‐score‐matched cohorts.
3. RESULTS
A total of 9948 eligible people with type 2 diabetes with a current prescription for SGLT2 inhibitors, and 14 917 people with a current prescription for DPP‐4 inhibitors were identified.
3.1.1. Baseline characteristics
In the unmatched cohort, the SGLT2 inhibitor cohort was younger, comprised a greater proportion of men, had a higher mean body mass index, a higher proportion of current smokers and a higher prevalence of excess alcohol use than the DPP‐4 inhibitor cohort (Table S4).
The SGLT2 inhibitor cohort had a lower prevalence of comorbidities compared to the DPP‐4 inhibitor cohort (Table S4). The cohorts had a similar mean duration of diabetes and prevalence of diabetes complications (Table S4).
The proportion of the SGLT2 inhibitor cohort that had previously used a DPP‐4 inhibitor was 36.8%, and 9.1% of the DPP‐4 inhibitor cohort had previously used an SGLT2 inhibitor, prior to the washout period (Table S4)
Following 1:1 propensity‐score matching, 7676 eligible individuals prescribed SGLT2 inhibitors were compared to 7676 matched individuals prescribed DPP‐4 inhibitors. After matching, the characteristics of the DPP‐4 inhibitor and SGLT2 inhibitor cohorts, including demographic and behavioural risk factors, diabetes duration, diabetes complications, comorbidities, metabolic profile, and prior prescriptions, were similar (Table 1).
TABLE 1.
Baseline demographic characteristics and behavioural risk factors, diabetes complications and comorbidities, metabolic characteristics, and medications used prior to index date
| Characteristic | Propensity‐score‐matched | P | |
|---|---|---|---|
| DPP‐4 inhibitor users (n = 7676) | SGLT2 inhibitor users (n = 7676) | ||
| Mean (SD) age, years | 62.6 (10.4) | 60.6 (10.8) | <0.001 |
| Men, n (%) | 4777 (62.2) | 4812 (62.7) | 0.57 |
| Mean (SD) BMI, kg/m2 | 32.2 (6.5) | 33.0(6.7) | <0.001 |
| Smoking status, n (%) | 0.58 | ||
| Non‐smokers | 3776 (49.2) | 3816 (50.3) | |
| Ex‐smokers | 2724 (35.5) | 2676 (34.9) | |
| Current smokers | 1158 (15.1) | 1121 (14.6) | |
| Missing | 18 (0.2) | 18 (0.2) | |
| Excessive alcohol use, n (%) | 433 (5.6) | 472 (6.1) | 0.19 |
| Mean (SD) diabetes duration,years | 10.6 (6.2) | 10.3 (6.3) | 0.001 |
| Diabetes complications, n (%) | |||
| Peripheral neuropathy | 456 (5.9) | 476 (6.2) | 0.52 |
| Diabetic foot disease | 272 (3.5) | 272 (3.5) | 1.00 |
| Sight threating retinopathy | 773 (10.1) | 864 (11.3) | 0.02 |
| Baseline diabetes complications and comorbidities | |||
| Comorbidities, n (%) | |||
| Ischaemic heart disease | 1242 (16.2) | 1225 (16.0) | 0.73 |
| Stroke/TIA | 438 (5.7) | 429 (5.6) | 0.78 |
| Heart failure | 291 (3.8) | 276 (3.6) | 0.55 |
| Peripheral vascular disease | 227 (3.0) | 219 (2.9) | 0.74 |
| Atrial fibrillation | 407 (5.3) | 375 (4.9) | 0.26 |
| Rheumatoid arthritis | 112 (1.5) | 101 (1.3) | 0.49 |
| Hypertension | 4267 (55.6) | 4171 (54.3) | 0.12 |
| Liver disease | 452 (5.8) | 511 (6.7) | 0.01 |
| Chronic kidney disease | 660 (8.6) | 590 (7.7) | 0.04 |
| Cancers a | 671 (8.7) | 612 (8.0) | 0.09 |
| Blood and bone marrow cancer | 90 (1.2) | 80 (1.0) | 0.49 |
| Chronic respiratory disease | 553 (7.2) | 505 (6.6) | 0.13 |
| Baseline metabolic characteristics | |||
| Diastolic BP, n (%) | 0.28 | ||
| <90 mmHg | 7087 (92.3) | 7035 (91.6) | |
| ≥90 mmHg | 583 (7.6) | 633 (8.2) | |
| Missing | 6 (0.1) | 8 (0.1) | |
| Systolic BP, n (%) | 0.03 | ||
| <140 mmHg | 5613 (73.1) | 5754 (75.0) | |
| ≥140 mmHg | 2057 (26.8) | 1914 (24.9) | |
| Missing, n (%) | 6 (0.1) | 8 (0.1) | |
| Total cholesterol, n (%) | <0.001 | ||
| <5.2 mmol/L | 6431 (83.8) | 6208 (80.9) | |
| 5.2–6.2 mmol/L | 848 (11.0) | 964 (12.6) | |
| ≥6.2 mmol/L | 362 (4.7) | 470 (6.1) | |
| Missing | 35 (0.5) | 34 (0.4) | |
| eGFR, n (%) | 0.11 | ||
| <30 mL/min/1.73m2 | 22 (0.3) | 9 (0.1) | |
| 30 to <60 mL/min/1.73m2 | 518 (6.7) | 538 (7.0) | |
| ≥60 mL/min/1.73m2 | 7109 (92.6) | 7098 (92.5) | |
| Missing, n (%) | 27 (0.4) | 31 (0.4) | |
| HbA1c, n (%) | <0.001 | ||
| <48 mmol/mol | 598 (7.8) | 530 (6.9) | |
| 48–57.9 mmol/mol | 2121 (27.6) | 1909 (24.9) | |
| ≥58 mmol/mol | 4851 (63.2) | 5144 (67.0) | |
| Missing | 106 (1.4) | 93 (1.2) | |
| Baseline medications used prior to index date, n (%) | |||
| DPP‐4 inhibitors | 7676 (100) | 2443 (31.8) b | <0.001 |
| SGLT2 inhibitors | 959 (12.5) b | 7676 (100) | <0.001 |
| Metformin | 7517 (98.0) | 7519 (98.0) | 1.00 |
| Glucagon‐like peptide‐1 | 762 (9.9) | 970 (12.6) | <0.001 |
| Insulin | 1058 (13.8) | 1328 (17.3) | <0.001 |
| Thiazolidinediones | 1150 (15.0) | 1188 (15.5) | 0.41 |
| Sulphonyureas | 4236 (55.2) | 4241 (55.3) | 0.95 |
| ACE inhibitors/ARBs | 5281 (68.8) | 5270 (68.7) | 0.86 |
| Other antihypertensives | 4849 (63.2) | 4754 (61.9) | 0.12 |
| Anticoagulants | 753(9.8) | 713 (9.3) | 0.28 |
| Antiplatelets | 3427 (44.6) | 3331 (43.4) | 0.12 |
| Lipid‐lowering drugs | 6641 (86.5) | 6574 (85.6) | 0.12 |
| Immunosuppressive drugs | 289 (3.8) | 269 (3.5) | 0.41 |
| Systemic corticosteroids | 749 (9.8) | 687 (8.9) | 0.09 |
Abbreviations: ACE, angiotensin‐converting enzyme; ACR, albumin–creatinine ratio; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; DPP‐4, dipeptidyl peptidase‐4; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; SGLT2, sodium‐glucose co‐transporter‐2; TIA, transient ischaemic attack.
Excluding melanoma, and blood and bone marrow cancers.
Last used ≥90 days before the index date.
3.1.2. Main analysis
In the unmatched cohort, after adjusting for potential confounders, no statistically significant difference in the risk of primary care consultations for a composite of confirmed or suspected COVID‐19 (adjusted hazard ratio [HR] 0.86, 95% confidence interval [CI] 0.64–1.16) or confirmed COVID‐19 (adjusted HR 0.66, 95% CI 0.35– 1.23; Figure 1) was observed in the SGLT2 inhibitor compared to the DPP‐4 inhibitor cohort. During the same period, in the unmatched group the crude incidence rate for developing back pain (negative control) was 10.7 per 1000 person‐years in the exposed group compared to 8.8 per 1000 person‐years in the comparator group. This translated to an adjusted HR of 1.11 (95% CI 0.72–1.72; Table S5).
FIGURE 1.
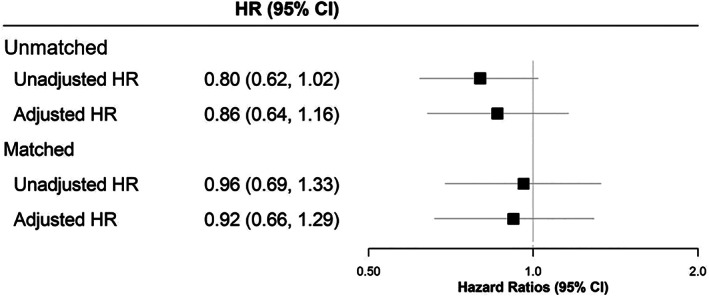
Forest plot showing hazard ratios (HRs) for suspected/confirmed COVID‐19 in individuals prescribed sodium‐glucose co‐transporter‐2 (SGLT2) inhibitors compared to those prescirbed dipeptidyl peptidase‐4 (DPP‐4) inhibitors. CI, confidence interval
Following propensity‐score matching, 69 individuals in the exposed group (current SGLT2 inhibitor users only) and 72 individuals in the comparator group (current DPP‐4 inhibitor users only) were diagnosed with a composite of confirmed or suspected COVID‐19, corresponding to crude incidence rates of 19.1 and 19.9 per 1000 person‐years, respectively (Table S5; Figure S1). There were 15 individuals with a record of confirmed COVID‐19 and one COVID‐19‐related death in the exposed group and 18 individuals with a record of confirmed COVID‐19 and four COVID‐19‐related deaths in the comparator group (current DPP‐4 inhibitor users only).
No statistically significant difference in risk of primary care consultation for a composite of confirmed or suspected COVID‐19 (adjusted HR 0.92, 95% CI 0.66– 1.29) or confirmed COVID‐19 (adjusted HR 0.78, 95% CI 0.39–1.56; Figure 1) was observed in the SGLT2 inhibitor compared to the DPP‐4 inhibitor cohort. When examining the rate of developing back pain in this cohort, the crude incidence rates were 11.4 and 8.6 per 1000 person‐years, respectively, for the exposed and comparator groups (Table S5). This related to a non‐statistically significant difference in risk between the groups (adjusted HR 1.22, 95% CI 0.76–1.96).
Given the limited number of events for COVID‐19‐related death, the HRs were not calculated.
4. DISCUSSION
This is the first study to explore the relationship between SGLT2 inhibitor use and the risk of developing COVID‐19. After adjusting for a number of potential confounders, and accounting for confounding by indication bias, we found no significant association between use of SGLT2 inhibitors and primary care consultation for confirmed or suspected COVID‐19 infection, when compared to use of DPP‐4 inhibitors in a large primary care cohort of people with type 2 diabetes.
Type 2 diabetes has been identified as a predictor of severe COVID‐19, and poor glycaemic control is associated with increased risk of severe COVID‐19 and mortality. 2 , 13 However, information regarding which specific glucose‐lowering agents can be used to safely maintain or improve glycaemic control in the midst of the COVID‐19 pandemic is currently limited. 14 The present study found no evidence that SGLT2 inhibitors alter the risk of developing COVID‐19. This helps to provide reassurance that these drugs can be used safely in primary care.
There have been case reports of people with diabetes and COVID‐19 presenting with euglycaemic ketoacidosis. SGLT2 inhibitors selectively reduce interstitial volume and may exert anti‐inflammatory effects, 15 which could further influence outcomes from COVID‐19. The current recommendation of stopping SGLT2 inhibitor treatment when admitted to hospital should therefore still be followed in people with type 2 diabetes and COVID‐19.
The present study has a number of limitations. Data quality is reliant on the coding practices of primary care clinicians and administrators. Individuals who present to primary care are more likely to be symptomatic rather than asymptomatic, and this latter group is likely therefore to be underrepresented. We did not have access to data on hospitalization, inpatient management, ethnicity or socio‐economic status. In addition, there was a relatively small number of outcome events.
The study also has some important strengths. We attempted to minimize the effect of the differences in baseline characteristics between groups, as well as confounding by indication bias, by using a propensity‐score‐matched design. Levels of missingness were low. This resulted in robust comparisons of similar individuals in the primary analysis. We adjusted for a large number of potential confounders, with analyses repeated using a negative control. This study adds to the understanding of COVID‐19 management in the primary care setting.
Our findings show no evidence that SGLT2 inhibitor use influences susceptibility to developing COVID‐19 among people with type 2 diabetes in the primary care setting. These results are reassuring and suggest that clinicians can use these agents to improve glycaemic control in people with type 2 diabetes in the primary care setting during the COVID‐19 pandemic, particularly given that hyperglycaemia is a poor prognostic outcome of COVID‐19.
CONFLICTS OF INTEREST
A.S., G.N.T., and K.N. have received funding from AstraZeneca (RSBD20464) unrelated to this manuscript. W.H. reports personal fees and non‐financial support from Novo Nordisk, Eli Lilly, Astra‐ Zeneca, Boehringer Ingelheim and NAPP. A.A.T. reports personal fees and non‐financial support from Novo Nordisk, Eli Lilly, AstraZeneca and Boehringer Ingelheim, personal fees from Janssen, and non‐financial support from Impeto Medical, ResMed and Aptiva. K.N. reports fees from Sanofi, MSD and Boehringer Ingelheim outside the submitted work. K.T reports fees from Sanofi and AstraZeneca. P.N reports fees from Eli Lilly. The other authors (C.S., J.W., K.G., D.A., S.D., N.B., J.S.C., A.A., J.C., K.O., M.B., T.T., S.G., K.K.C., T.M., G.G., N.J.A. and S.H.) report no conflict of interest.
AUTHOR CONTRIBUTIONS
C.S., J.W. and K.G. contributed equally to the concept, analysis and writing of this work. S.H. and K.N. contributed equally as senior authors, overseeing research design and execution. All other authors contributed to the concept, data acquisition and analysis and writing of the text.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14203.
Supporting information
Appendix S1. Supporting information.
Sainsbury C, Wang J, Gokhale K, et al. Sodium‐glucose co‐transporter‐2 inhibitors and susceptibility to COVID‐19: A population‐based retrospective cohort study. Diabetes Obes Metab. 2021;23:263–269. 10.1111/dom.14203
Contributor Information
Christopher Sainsbury, Email: c.sainsbury.1@bham.ac.uk.
Nicola J. Adderley, Email: N.J.Adderley@bham.ac.uk.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from The Health Improvement Network (THIN). Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the authors with the permission of The Health Improvement Network (THIN), .
REFERENCES
- 1. The OpenSAFELY Collaborative . OpenSAFELY: factors associated with COVID‐19‐related hospital death in the linked electronic health records of 17 million adult NHS patients. medRxiv preprint 2020.
- 2. Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID‐19‐related mortality in England: a whole‐population study. The Lancet Diabetes & Endocrinology. 2020;8(10):813–822. 10.1016/s2213-8587(20)30272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shi Q, Zhang X, Jiang F, et al. Clinical characteristics and risk factors for mortality of COVID‐19 patients with diabetes in Wuhan, China: a two‐center, retrospective study. Diabetes Care. 2020;43:1382‐1391. [DOI] [PubMed] [Google Scholar]
- 4. Chen Y, Yang D, Cheng B, et al. Clinical Characteristics and Outcomes of Patients With Diabetes and COVID‐19 in Association With Glucose‐Lowering Medication. Diabetes Care. 2020;43(7):1399–1407. 10.2337/dc20-0660. [DOI] [PubMed] [Google Scholar]
- 5. Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state‐of‐the‐art review. Diabetologia. 2018;61(10):2108‐2117. [DOI] [PubMed] [Google Scholar]
- 6. Ceriello A, Standl E, Catrinoiu D, et al. Issues of cardiovascular risk Management in People with Diabetes in the COVID‐19 era. Diabetes Care. 2020;43:1427‐1432. 10.2337/dc20-0941. [DOI] [PubMed] [Google Scholar]
- 7. Bornstein SR, Rubino F, Khunti K, et al. Practical recommendations for the management of diabetes in patients with COVID‐19. Lancet Diabetes Endocrinol. 2020;8(6):546‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kramer CK, Zinman B. Sodium‐glucose Cotransporter‐2 (SGLT‐2) inhibitors and the treatment of type 2 diabetes. Annu Rev Med. 2019;70:323‐334. [DOI] [PubMed] [Google Scholar]
- 9. Maguire A, Blak BT, Thompson M. The importance of defining periods of complete mortality reporting for research using automated data from primary care. Pharmacoepidemiol Drug Saf. 2009;18(1):76‐83. [DOI] [PubMed] [Google Scholar]
- 10. Dalan R. Is DPP4 inhibition a comrade or adversary in COVID‐19 infection. Diabetes Res Clin Pract. 2020;164:108216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. National Institute for Health and Care Excellence . Type 2 diabetes in adults: management NICE guideline [NG28] 2019.
- 12. Sanderson E, Macdonald‐Wallis C, Davey Smith G. Negative control exposure studies in the presence of measurement error: implications for attempted effect estimate calibration. Int J Epidemiol. 2018;47(2):587‐596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holman N, Knighton P, Kar P, et al. Risk factors for COVID‐19‐related mortality in people with type 1 and type 2 diabetes in England: a population‐based cohort study. The Lancet Diabetes & Endocrinology. 2020;8(10):823–833. 10.1016/s2213-8587(20)30271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aronson JK, Ferner RE. Drugs and the renin‐angiotensin system in covid‐19. BMJ. 2020;369:m1313. [DOI] [PubMed] [Google Scholar]
- 15. Bossi AC, Forloni F, Colombelli PL. Lack of efficacy of SGLT2‐i in severe pneumonia related to novel coronavirus (nCoV) infection: no little help from our friends. Diabetes Ther. 2020;11:1605‐1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting information.
Data Availability Statement
The data that support the findings of this study are available from The Health Improvement Network (THIN). Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the authors with the permission of The Health Improvement Network (THIN), .


