Abstract
Background
Obstetrical complications affect more than a third of women globally, but are underrepresented in clinical research. Little is known about the comprehensive obstetrical clinical trial landscape, how it compares with other fields, or factors associated with the successful completion of obstetrical trials.
Objective
This study aimed to characterize obstetrical clinical trials registered on ClinicalTrials.gov with the primary objective of identifying features associated with early discontinuation and results reporting.
Study Design
This is a cross-sectional study with descriptive, logistic regression and Cox regression analyses of clinical trials registered on ClinicalTrials.gov. Our primary exposure variables were trial focus (obstetrical or nonobstetrical) and trial funding (industry, United States government, or academic). We conducted additional exploratory analyses of other trial features including design, enrollment, and therapeutic focus. We examined the associations of exposure variables and other trial features with 2 primary outcomes: early discontinuation and results reporting.
Results
We downloaded data for all studies (N=332,417) registered on ClinicalTrials.gov from October 1, 2007, to March 9, 2020, from the Aggregate Analysis of ClinicalTrials.gov database. We excluded studies with a noninterventional design (n=63,697) and those registered before October 1, 2007 (n=45,209). A total of 4276 obstetrical trials (1.9%) (ie, interventional studies) and 219,235 nonobstetric trials (98.1%) were compared. Among all trials, 2.8% of academic-funded trials, 1.9% of United States government–funded trials, and 0.4% of industry-funded trials focused on obstetrics. The quantity of obstetrical trials increased over time (10.8% annual growth rate). Compared with nonobstetrical trials, obstetrical trials had a greater risk of early discontinuation (adjusted hazard ratio, 1.40; 95% confidence interval, 1.21–1.62; P<.0001) and similar odds of results reporting (adjusted odds ratio, 0.89; 95% confidence interval, 0.72–1.10; P=.19). Among obstetrical trials funders after controlling for confounding variables, United States government–funded trials were at the lowest risk of early discontinuation (United States government, adjusted hazard ratio, 0.23; 95% confidence interval, 0.07–0.69; P=.009; industry reference; academic, adjusted hazard ratio, 1.04; 95% confidence interval, 0.62–1.74; P=.88). Academic-funded trials had the lowest odds of results reporting after controlling for confounding variables (academic institutions, adjusted odds ratio, 0.39; 95% confidence interval, 0.22–0.68; P=.0009; industry reference; United States government, adjusted odds ratio, 1.06; 95% confidence interval, 0.53–2.09; P=.87).
Conclusion
Obstetrical trials represent only 1.9% of all clinical trials in ClinicalTrials.gov and have comparatively poor completion. All stakeholders should commit to increasing the number of obstetrical trials and improving their completion and dissemination to ensure clinical research reflects the obstetrical burden of disease and advances maternal health.
Key words: ClinicalTrials.gov, industry, maternal-fetal medicine, maternal health, National Institutes of Health, obstetrical complications, obstetrical investigations, obstetrical morbidity, obstetrical studies, research funding
AJOG MFM at a Glance.
Why was this study conducted?
This study aimed to characterize obstetrical clinical trials and determine which trial features are associated with trial completion and results reporting.
Key findings
Less than 2% of clinical trials registered to ClinicalTrials.gov between 2007 and 2020 focused on obstetrics. Obstetrical trials were at a greater risk of early discontinuation than nonobstetrical trials. Among obstetrical trials, United States government–funded trials were least likely to be discontinued early and academic institution–funded trials had the lowest odds of results reporting compared with other funders.
What does this add to what is known?
This study presents a comparison of obstetrical and nonobstetrical trials that includes funding sources and trial characteristics, novel assessments of temporal trends only possible with a large sample size and greater time period, and a unique examination of associations between obstetrical trial funding and the likelihood of early discontinuation and results reporting, while controlling for confounding variables.
Introduction
Clinical trials represent the gold standard for advancing evidence-based medicine and clinical care.1 ClinicalTrials.gov is one of the largest international databases of clinical trials, containing nearly half of all registered trials.2 Since 2007, the United States federal law has required that most phase 2 to 4 intervention studies register in ClinicalTrials.gov.3
Obstetrical complications affect nearly a third of women in high-income countries and a greater proportion of women in low-income countries.4 Despite the perinatal burden of disease, proportionally few clinical trials focus on complications of pregnancy, and pregnant women are often excluded from clinical trials that evaluate therapies used to treat chronic conditions in pregnant women.5 To address the paucity of obstetrical studies, the National Institutes of Health put forth guidelines to safely include pregnant women in clinical trials and recommended trial breadth capture the spectrum of pharmaceuticals commonly taken during pregnancy.6 In addition, the World Health Organization,7 the Institute of Medicine,8 the Centers for Disease Control and Prevention,9 and the American College of Obstetricians and Gynecologists10 called for increased obstetrical clinical trials and for the quantity of obstetrical trials to better match the obstetrical burden of disease.
Despite these initiatives, the landscape and key drivers of obstetrical clinical trial success remain poorly understood. Previous studies describing obstetrical clinical trials examined only small sample sizes or short time frames, and none included multivariable associative analyses.5 , 11, 12, 13 A comprehensive understanding of obstetrical clinical trials to date presents a first step toward determining how to improve and expand the evidence base. In view of the importance of clinical trials informing evidence-based care, we sought to characterize and analyze key features of obstetrical studies registered on ClinicalTrials.gov and to identify factors associated with early trial discontinuation and results reporting.
Materials and Methods
Data source
We downloaded data for all studies submitted to ClinicalTrials.gov before March 9, 2020, from the Aggregate Analysis of ClinicalTrials.gov (AACT) database.14 We selected studies after October 1, 2007, to align with the Food and Drug Administration Amendments Act, which mandated the registration of most United States phase 2 to 4 intervention studies in ClinicalTrials.gov.15 After the implementation of the Food and Drug Administration Amendments Act, ClinicalTrials.gov captured a more representative sample of the entire clinical trial landscape, and for many years after, it was the largest database of clinical trials globally.16 We included all registered studies with an interventional study design (ie, clinical trials). We excluded studies registered before October 1, 2007, and those with an observational design (ie, not clinical trials). The University Institutional Review Board exempted our study from review because it only involved publicly available data without personal health information or human subjects.
Definitions
Obstetrical and nonobstetrical trials
To identify potential obstetrical trials, we followed a published protocol to identify Medical Subject Heading and disease terms relevant to obstetrical trials.17 , 18 The protocol for extraction and categorization was set a priori (detailed definitions and labeling methods described in the Appendix). These terms were then used to extract data on all trials relevant to obstetrics from the AACT database.14 Obstetrical trials were manually reviewed, verified, and categorized to one or multiple therapeutic focus categories as follows: cesarean delivery; nutritional status in pregnancy; labor anesthesia and analgesia; infections in pregnancy, labor, and the puerperium; fetal focused; threatened early delivery; mental and behavioral disorder; diabetes mellitus in pregnancy; bleeding and hemorrhage; labor augmentation and induction; hypertensive disorders of pregnancy; breastfeeding; pregnancy loss; obstetrical trauma; thromboembolic disease of pregnancy; vomiting in pregnancy; and other. Nonobstetrical trials were defined as all trials in the AACT database within the same time frame and without content relevant to obstetrics. The 2 first authors and the 7 trained researchers who labeled all trials demonstrated more than 90% agreement on a training set of more than 200 trials. To verify data, the first author randomly selected more than 10% of all labeled trials for concordance with the protocol. The first authors adjudicated all discrepancies in labeling.
For analysis, each therapeutic focus was treated as a binary variable with 2 categories: (1) trials labeled with the therapeutic focus and (2) all other trials that were not labeled with the therapeutic focus.
Exposures and outcomes
Our primary exposure variables were trial focus (obstetrical or nonobstetrical) and trial funding (industry, United States government, or academic). We conducted additional exploratory analyses of other trial features including characteristics, design, enrollment, and therapeutic focus. Obstetrical trials were compared with nonobstetrical trials in terms of funding source and other trial features. Obstetrical trial features were also compared by trial funder.
We examined the associations of the exposure variables and other trial features with 2 primary outcomes: early discontinuation and results reporting within 3 years of trial completion. “Early discontinuation” was defined as a trial stopped early with the status “Terminated” or “Suspended.” In analysis of early discontinuation, we excluded trials documented as having a duration of <1 day (2.1%), trials with the status “Withdrawn” (defined as those terminating before the enrollment of participants [2.8%]), and trials without a verified status (13.2%).19 Only trials completed by March 9, 2017, were included in the analysis of results reporting to align with federal mandates for delayed submission of results within 3 years of trial completion.20
Trial features
We categorized 12 trial features for comparison and exposure analysis: (1) funding, (2) primary purpose, (3) phase, (4) number of arms, (5) enrollment, (6) year of trial registration, (7) blinding, (8) randomization, (9) oversight by a data safety monitoring committee, (10) location, (11) number of sites, and (12) study status.17
“Enrollment” included both actual enrollment (for completed trials) and anticipated enrollment (for ongoing trials with continued enrollment).
We classified “funding” consistently with previous studies based on the trial sponsor and collaborating agencies.17 , 18 If industry was a trial’s sponsor or a collaborator, the trial was classified as “industry.” Among remaining trials, if the United States government (ie, National Institutes of Health or another United States government agency) was the sponsor or a collaborator, the trial was classified as “United States government.” The remaining trials were categorized “academic” after a random sample analysis of 2500 remaining funders identified that 90.1% (99% confidence interval [CI], 88.56–91.64) of these funding sources were academic institutions as defined with United States legal code.21 , 22
“Location” was classified based on the World Bank definitions of high-, middle-, and low-income countries.23 Trials with at least 1 site in a high-income country were considered high income, and those in low- and middle-income countries were considered low and middle income.
Analysis
We summarized data with descriptive statistics including frequencies, percentages, and 2-sided Pearson chi-square tests. We analyzed change over time—independence and significance with annual growth rates and post hoc Mann-Kendall tests, respectively.24 Only years with a completed 12-month cycle were included in growth analyses (2008–2019).
We conducted univariable and multivariable analyses with Cox proportional hazard models for early discontinuation and logistic regression for results reporting resulting in adjusted hazard ratios (aHRs) and adjusted odds ratios (aORs). Although we created Kaplan-Meier curves for both outcomes, the analysis of results reporting used only a logistic model. Cox models for early discontinuation use a time variable consistent with the duration of each trial from initiation to either discontinuation (event) or if ongoing the data download date. Log-rank tests were used to verify the independence of the Kaplan-Meier curves. All 12 trial features listed earlier, except for the trial feature under investigation, were treated as confounding variables in multivariable analysis.
All analyses were 2 sided with statistical significance at the α=.05 level. Analyses were performed using the R statistical programming language, version 3.5.0 (The R Foundation, https://www.r-project.org/).
Missing data
Multiple imputation analysis and multiple imputation by chained equations were conducted for all missing data in multivariable analysis.25 , 26 We generated 20 imputed datasets and modeled continuous data using Bayesian linear regression, binary data with Bayesian logistic regression, and categorical data with Bayesian polytomous regression, all with analytical variables as covariables. Parameters of interest were estimated separately in each imputed dataset and subsequently pooled using Rubin’s rules.27 We present the imputed outcomes in results.
Results
Trial population
A total of 332,417 studies were registered in ClinicalTrials.gov on March 9, 2020; 108,906 studies were excluded because they were registered before 2007 (n=45,209) or had a noninterventional study design (n=63,697) (Figure 1 ). Of the trials included, 1.9% (n=4276) were obstetrical trials, with more than 3.4 million estimated participants, and 98.1% (n=219,235) were nonobstetrical comparison trials.
Figure 1.
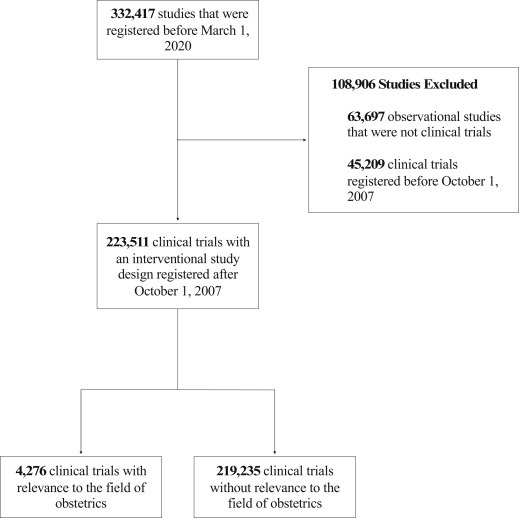
CONSORT diagram of clinical trials included in the analysis
CONSORT, Consolidated Standards of Reporting Trials.
Steinberg et al. The obstetrical clinical trial landscape. AJOG MFM 2021.
Obstetrical trials compared with nonobstetrical trials
Funding
The funding sources of obstetrical trials differed from nonobstetrical trials, with the vast majority of obstetrical clinical trials funded by academic institutions (85.5%) followed by the United States government (7.7%) and industry (6.8%) (Figure 2 ; Supplemental Table 1). In contrast, a large proportion of nonobstetrical trials were funded by both academic institutions (57.1%) and industry (35.0%), with a smaller proportion funded by the United States government (7.9%). Among all trials, obstetrics was the focus in 2.8% of academic-funded trials, 1.9% of United States government–funded trials, and 0.4% of industry-funded trials.
Figure 2.
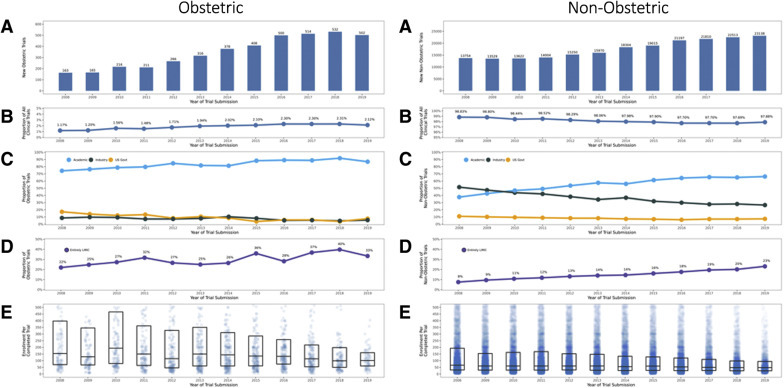
Characteristics of obstetrical and nonobstetrical trials over time
A, Number of trials. B, Percentage of total trials. C, Percentage of trials within obstetrical and nonobstetrical trials funded by industry, the United States government, or academics. Funding categories were determined with data on the sponsor and collaborators. Industry funding includes trials with an industry sponsor or collaborating agency. United States government trials include remaining trials with a United States government sponsor or collaborating agency. Academic trials include all other trials. D, Percentage of trials within obstetrical trials and nonobstetrical trials in only low- and middle-income countries. E, Density plot of trial enrollment; each dot represents 1 trial. Enrollment included both actual enrollment (for completed trials) and anticipated enrollment (for ongoing trials with continued enrollment).
Steinberg et al. The obstetrical clinical trial landscape. AJOG MFM 2021.
Other trial characteristics
Obstetrical trials focused on prevention more frequently (31.5% vs 10.2%) and on treatment less frequently than nonobstetrical trials (39.3% vs 64.0%) (Supplemental Table 1). Obstetrical trials had more participants per trial (enrollment of >100, 61.5% vs 35.4%) and had more frequent blinding, randomization, and reporting to a data safety monitoring committee. A greater proportion of obstetrical trials occurred exclusively in low- and middle-income countries (27.5%) than nonobstetrical trials (13.9%). The quantity of obstetrical trials increased at a greater annual growth rate than nonobstetrical trials (obstetrical 10.8% vs nonobstetrical 4.8%) (Supplemental Table 2).
Obstetrical trial characteristics
Funding
Within obstetrical trials, compared with other sources of funding, those with United States government funding had more randomization, increased enrollment (>100), and more oversight by a data safety monitoring committee (Table 1 ). In contrast, a greater proportion of obstetrical trials with industry funding had lower enrollment (<100), no blinding, and no randomization.
Table 1.
| Trial feature | Industry | Academic | United States government | Chi2P value |
|---|---|---|---|---|
| Total | 290 (6.8) | 3655 (85.6) | 331 (7.7) | |
| Primary purpose | ||||
| Treatment | 116 (40.0) | 1467 (40.1) | 98 (29.6) | .0002 |
| Basic science | 5 (1.7) | 84 (2.3) | 17 (5.1) | |
| Prevention | 92 (31.7) | 1119 (30.6) | 134 (40.5) | |
| Otherc | 67 (23.1) | 894 (24.5) | 76 (23.0) | |
| Missing | 10 (3.4) | 91 (2.5) | 6 (1.8) | |
| Phase | ||||
| Not Applicabled | 127 (43.8) | 2455 (67.2) | 223 (67.4) | <.0001 |
| Phase 1 | 13 (4.5) | 107 (2.9) | 16 (4.8) | |
| Phase 1/2–2 | 46 (15.9) | 221 (6.0) | 34 (10.3) | |
| Phase 2/3–3 | 58 (20.0) | 414 (11.3) | 35 (10.6) | |
| Phase 4 | 46 (15.9) | 458 (12.5) | 23 (6.9) | |
| Enrollmente | ||||
| 0–9 | 25 (8.6) | 169 (4.6) | 10 (3.0) | <.0001 |
| 10–49 | 57 (19.7) | 545 (14.9) | 39 (11.8) | |
| 50–99 | 38 (13.1) | 698 (19.1) | 53 (16.0) | |
| 100–499 | 115 (39.7) | 1655 (45.3) | 140 (42.3) | |
| 500–999 | 24 (8.3) | 237 (6.5) | 34 (10.3) | |
| >999 | 30 (10.3) | 337 (9.2) | 55 (16.6) | |
| Missing | 1 (0.3) | 14 (0.4) | 0 | |
| Blinding | ||||
| None | 166 (57.2) | 1926 (52.7) | 174 (52.6) | <.0001 |
| Single | 31 (10.7) | 947 (25.9) | 100 (30.2) | |
| Double | 93 (32.1) | 773 (21.1) | 56 (16.9) | |
| Missing | 0 | 9 (0.2) | 1 (0.3) | |
| Randomization | ||||
| Nonrandomized | 80 (27.6) | 706 (19.3) | 36 (10.9) | <.0001 |
| Randomized | 207 (71.4) | 2918 (79.8) | 295 (89.1) | |
| Missing | 3 (1.0) | 31 (0.8) | 0 | |
| Oversight by a data safety monitoring committee | ||||
| No | 155 (53.4) | 1976 (54.1) | 138 (41.7) | <.0001 |
| Yes | 120 (41.4) | 1341 (36.7) | 174 (52.6) | |
| Missing | 15 (5.2) | 338 (9.2) | 19 (5.7) | |
| Location | ||||
| High-income countries | 210 (72.4) | 2125 (58.1) | 222 (67.1) | <.0001 |
| Low- and middle-income countries only | 47 (16.2) | 1048 (28.7) | 79 (23.9) | |
| Missing | 33 (11.4) | 482 (13.2) | 30 (9.1) | |
| Therapeutic focusf | ||||
| Infection | 55 (19.0) | 283 (7.7) | 100 (30.2) | <.0001 |
| Nutrition | 40 (13.8) | 458 (12.5) | 55 (16.6) | .10 |
| Fetal | 27 (9.3) | 378 (10.3) | 17 (5.1) | .009 |
| Early delivery | 25 (8.6) | 292 (8.0) | 11 (3.3) | .002 |
| Diabetes mellitus | 22 (7.6) | 266 (7.3) | 19 (5.7) | .56 |
| Mental health | 21 (7.2) | 267 (7.3) | 66 (19.9) | <.0001 |
| Cesarean delivery | 19 (6.6) | 659 (18.0) | 8 (2.4) | <.0001 |
| Labor Augmentation | 19 (6.6) | 235 (6.4) | 3 (0.9) | .0002 |
| Hemorrhage | 19 (6.6) | 281 (7.7) | 5 (1.5) | .0001 |
| Hypertension | 13 (4.5) | 229 (6.3) | 12 (3.6) | .08 |
| Breastfeeding | 10 (3.4) | 158 (4.3) | 29 (8.8) | .0006 |
| Anesthesia | 10 (3.4) | 501 (13.7) | 4 (1.2) | <.0001 |
| Trauma | 7 (2.4) | 85 (2.3) | 3 (0.9) | .24 |
| Pregnancy loss | 6 (2.1) | 139 (3.8) | 4 (1.2) | .02 |
| Thromboembolic | 3 (1.0) | 21 (0.6) | 0 | .22 |
| Vomiting | 2 (0.7) | 17 (0.5) | 2 (0.6) | .83 |
| Other | 37 (12.8) | 441 (12.1) | 44 (13.3) | .77 |
Values are number (percentage) unless indicated otherwise.
Steinberg et al. The obstetrical clinical trial landscape. AJOG MFM 2021.
Percentages may not sum up to 100 because of rounding
Funding categories were determined with data on the sponsor and collaborators. Industry funding includes trials with an industry sponsor or collaborating agency. United States government trials include remaining trials with a United States government sponsor or collaborating agency. Academic trials include all other trials
Other primary purposes include diagnostic, screening, supportive care, health services research, and other
On ClinicalTrials.gov, “Not Applicable” is used to describe trials without Food and Drug Administration–defined phases, including trials of devices or behavioral interventions
Enrollment included both actual enrollment (for completed trials) and anticipated enrollment (for ongoing trials with continued enrollment)
Trials could have >1 therapeutic focus. For analysis, each therapeutic focus was treated as a binary variable.
Change over time
The total number of obstetrical trials doubled from 2007 to 2013 (n=1366) to 2014 to 2020 (n=2910) (Figure 2). The increase in obstetrical trials varied by funding source: academic-funded trials increased at nearly double the rate of industry-funded trials, and United States government–funded trials had no meaningful change (Supplemental Table 2).
Therapeutic focus
The plurality of obstetrical trials focused on cesarean delivery (16.0% of all obstetrical trials) (Supplemental Table 3) with most of these funded by academic institutions (Table 1). Infection was the most frequent therapeutic focus among both industry- (19.0%) and United States government–funded trials (30.2%). Obstetrical trauma, thrombosis, and vomiting were the least frequent therapeutic foci across all funders with the lowest enrollment (Supplemental Figure; Supplemental Table 3).
Supplemental Figure.
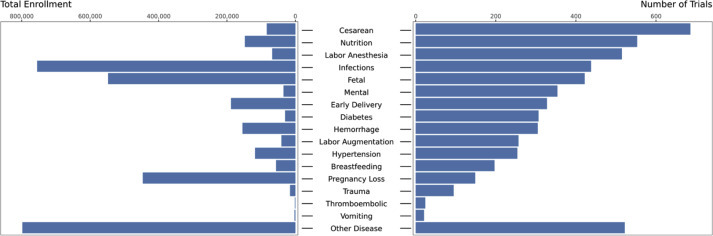
Trial quantity and enrollment by therapeutic area of focus
Trials could have multiple therapeutic foci. Enrollment included both actual enrollment (for completed trials) and anticipated enrollment (for ongoing trials with continued enrollment).
Steinberg et al. The obstetrical clinical trial landscape. AJOG MFM 2021.
Early trial discontinuation
Obstetrical trials compared with nonobstetrical trials
Between 2007 and 2020, 47.5% of all obstetrical trials reached completion, representing more than 2.8 million participants (Supplemental Table 4). A total of 7.2% trials were discontinued early and 13.2% had missing or unknown study status. In contrast, 51.3% of nonobstetrical trials were completed, 8.8% were discontinued early, and 10.2% had missing or unknown study status. Compared with nonobstetrical trials, obstetrical trials had a greater adjusted risk of early discontinuation (aHR, 1.40; 95% CI, 1.21–1.62; P<.0001) (Table 2 ; unadjusted analysis results in Supplemental Table 5).
Table 2.
Associations between trail features and early discontinuation in obstetrical clinical trials
| Trial feature | Hazard ratio (95% confidence interval) | P value |
|---|---|---|
| All trials | ||
| Nonobstetrical | Reference | |
| Obstetrical | 1.40 (1.21–1.62) | <.0001 |
| Obstetrical trials only | ||
| Fundinga | ||
| Industry | Reference | |
| Academic | 1.04 (0.62–1.74) | .88 |
| United States government | 0.23 (0.07–0.69) | .009 |
| Primary purpose | ||
| Treatment | Reference | |
| Basic science | 0.58 (0.17–1.99) | .38 |
| Prevention | 0.95 (0.64–1.42) | .82 |
| Otherb | 1.33 (0.87–2.03) | .19 |
| Phase | ||
| Phase 2/3–3 | Reference | |
| Not Applicablec | 0.54 (0.34–0.86) | .009 |
| Phase 1 | 0.52 (0.25–1.09) | .08 |
| Phase 1/2–2 | 0.64 (0.33–1.23) | .18 |
| Phase 4 | 1.17 (0.67–2.04) | .15 |
| Enrollmentd | ||
| 100–499 | Reference | |
| 0–9 | 49.82 (30.10–82.47) | <.0001 |
| 10–49 | 6.29 (4.10–9.64) | <.0001 |
| 50–99 | 2.01 (1.20–3.36) | .007 |
| 500–999 | 0.72 (0.25–2.02) | .53 |
| >999 | 0.79 (0.37–1.65) | .53 |
| Blinding | ||
| None | Reference | |
| Single | 0.62 (0.40–0.96) | .03 |
| Double | 0.83 (0.54–1.26) | .37 |
| Randomization | ||
| Nonrandomized | Reference | |
| Randomized | 2.00 (1.28–3.13) | .002 |
| Oversight by a data safety monitoring committee | ||
| No | Reference | |
| Yes | 1.12 (0.82–1.54) | .47 |
| Location | ||
| Low- and middle-income country only | Reference | |
| High-income country | 1.83 (1.05–3.21) | .03 |
| Number of facilities | ||
| 1 | Reference | |
| >2 | 1.35 (0.92–1.98) | .13 |
| Therapeutic focuse | ||
| All other trials | Reference | |
| Cesarean delivery | 1.72 (1.03–2.87) | .04 |
| Nutrition | 1.04 (0.63–1.73) | .87 |
| Anesthesia | 1.86 (1.12–3.10) | .02 |
| Infection | 1.57 (0.95–2.62) | .08 |
| Fetal | 0.95 (0.59–1.51) | .81 |
| Mental health | 0.94 (0.52–1.70) | .84 |
| Early delivery | 1.08 (0.67–1.74) | .75 |
| Diabetes mellitus | 0.59 (0.33–1.06) | .08 |
Steinberg et al. The obstetrical clinical trial landscape. AJOG MFM 2021.
Funding categories were determined with data on the sponsor and collaborators. Industry funding includes trials with an industry sponsor or collaborating agency. United States government trials include remaining trials with a United States government sponsor or collaborating agency. Academic trials include all other trials
Other primary purposes include diagnostic, screening, supportive care, health services research, and other
On ClinicalTrials.gov, “Not Applicable” is used to describe trials without Food and Drug Administration–defined phases, including trials of devices or behavioral interventions
Enrollment included both actual enrollment (for completed trials) and anticipated enrollment (for ongoing trials with continued enrollment)
Trials could have >1 therapeutic focus. For analysis, each therapeutic focus was treated as a binary variable.
Obstetrical trials only
Among obstetrical trials, United States government–funded trials had the lowest adjusted risk of early discontinuation of any funding source (United States government, aHR, 0.23; 95% CI, 0.07–0.69; P=.009, industry reference; academic, aHR, 1.04; 95% CI, 0.62–1.74; P=.88, Figure 3 ). Randomized trials and trials in high-income countries had a greater adjusted hazard of early discontinuation than nonrandomized trials and trials exclusively in low- and middle-income countries, respectively (randomization, aHR, 2.00; 95% CI, 1.28–3.13; P=.002; location, aHR, 1.83; 95% CI, 1.05–3.21; P=.03). Trials with the therapeutic foci of either anesthesia or cesarean delivery showed a greater adjusted hazard of early discontinuation than any other therapeutic foci.
Figure 3.
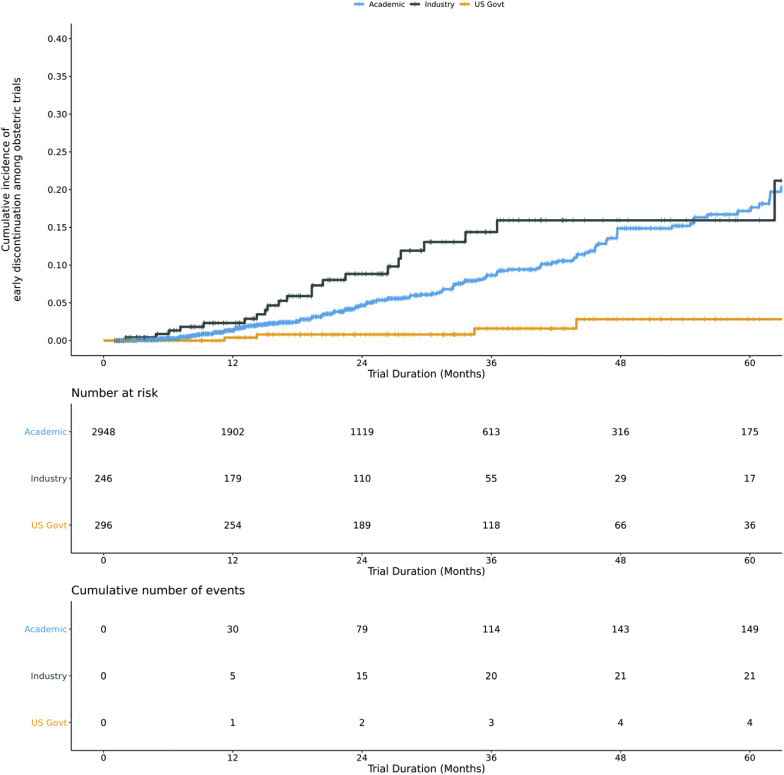
Kaplan-Meier curves of early discontinuation by trial funder
Funding categories were determined with data on the sponsor and collaborators. Industry funding includes trials with an industry sponsor or collaborating agency. United States government trials include remaining trials with a United States government sponsor or collaborating agency. Academic trials include all other trials. The log-rank P value for Kaplan-Meier curve was <.0001.
Steinberg et al. The obstetrical clinical trial landscape. AJOG MFM 2021.
Results reporting
Obstetrical trials compared with nonobstetrical trials
Obstetrical trials had similar adjusted odds of reporting results as nonobstetrical trials (aOR, 0.89; 95% CI, 0.72–1.10; P=.19) (unadjusted analysis results in Supplemental Table 6, adjusted analysis results in Supplemental Table 7).
Obstetrical trials only
By March 8, 2017, a total of 1411 obstetrical trials (102 industry, 138 United States government, and 1171 academic) had reached completion, but only 216 (15.3%) reported results to ClinicalTrials.gov by March 9, 2020 (Supplemental Tables 6 and 8).
Among obstetrical trials, academic-funded trials had the lowest adjusted odds of reporting results compared with other funders (academic, aOR, 0.39; 95% CI, 0.22–0.68; P=.0009; industry reference; United States government, aOR, 1.06; 95% CI, 0.53–2.09; P=.87) (Figure 4 ; Supplemental Table 5). Trials that focused on cesarean deliveries had the greatest adjusted odds of reporting results (aOR, 2.07; 95% CI, 1.29–3.34; P=.003) compared with other trial foci. Trials in high-income counties had greater results reporting than those exclusively in low- and middle-income countries (aOR, 2.13; 95% CI, 1.35–3.36; P=.001).
Figure 4.
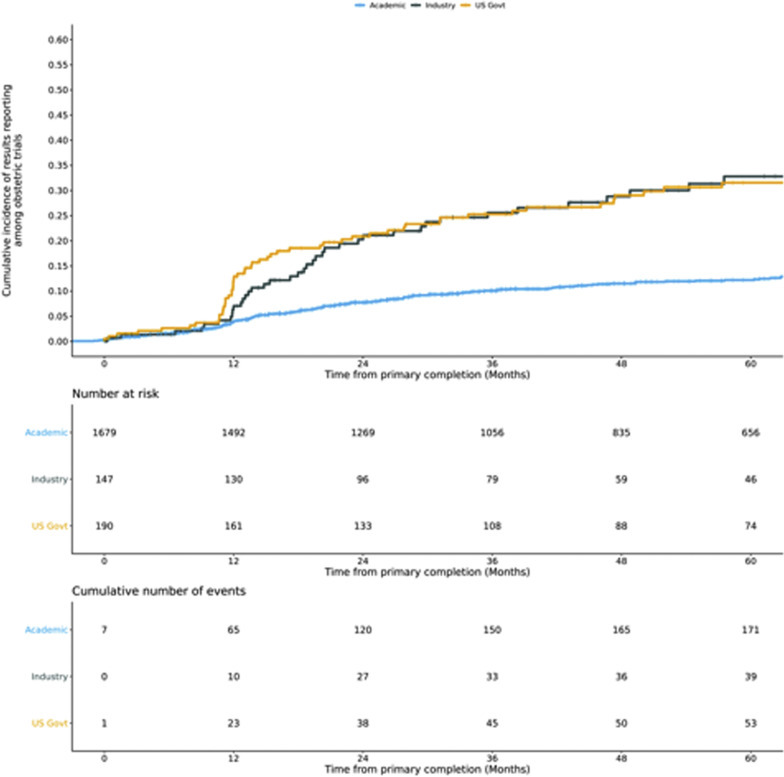
Kaplan-Meier curves of results reporting by trial funder
Funding categories were determined with data on the sponsor and collaborators. Industry funding includes trials with an industry sponsor or collaborating agency. United States government trials include remaining trials with a United States government sponsor or collaborating agency. Academic trials include all other trials.
Steinberg et al. The obstetrical clinical trial landscape. AJOG MFM 2021.
Comment
Principal findings
This study demonstrates that obstetrical clinical trials represent <2% of all clinical trials and, when initiated, are at a greater risk of early discontinuation than nonobstetrical trials. Although obstetrical trials report results at a similar rate to nonobstetrical, only 15.3% of completed obstetrical trials report results. Our temporal analysis shows limited improvement in obstetrical trial quantity, almost entirely driven by academic institutions.
Our study adds to previous investigations of obstetrical trials by suggesting the link among funding, metrics of trial quality, trial completion, and results reporting. We found that, among obstetrical trials, industry funds proportionally fewer large-scale trials, blinded trials, and randomized trials. In contrast, United States government–funded obstetrical trials are the most likely to meet the Consolidated Standards of Reporting Trials recommendations for trial quality28 and to reach completion. When examining results reporting by funder, even after controlling for other variables, we identified that academic-funded trials are least likely to report results compared with other funders.
Results in context
Notably, 4 previous investigations have analyzed obstetrical trials in the ClinicalTrials.gov registry, all using a smaller sample of trials (n=325 trials13 and n=5 trials5) or covering a shorter time span (2013–201411 and 2007–201212). Although previous studies suggest that obstetrics is the focus of 6% of all trials12 and 1%5 to 16%12 of industry-funded trials in ClinicalTrials.gov, our findings paint a bleaker picture; we found that only 1.9% of trials and 0.4% of industry-funded trials focus on obstetrics. Even with the inclusion of observational studies, the 6% estimate in Stockmann et al’s12 investigation still likely overrepresents obstetrical clinical trials, perhaps because their search query included neonatal terms and the study did not describe a manual review to remove trials unrelated to obstetrics after the query. Moreover, 3 of the previous obstetrical studies were limited to Federal and Drug Administration–approved trials,13 pharmaceutical trials,11 and phase 4 trials, respectively.5 We included all obstetrical clinical trials between 2007 and 2020, but similarly found the number of obstetrical studies pales in comparison with that of other fields.
More studies were dedicated to oncology (8992), cardiology (3437), and mental health between 2007 and 2010 (3695)29 than those dedicated to obstetrics in the decade after 2007 (3166). The paucity of obstetrical studies carries implications in the changing international health ecosystem. One recent study found that only 1.7% of coronavirus disease 2019 research pertains to pregnancy.30 Our findings suggest the limited number of obstetrical trials may correlate to the dearth of industry funding in obstetrics. Previous investigations have indicated that industry may forgo obstetrical trials owing to inadequate pipelines for obstetrical drug and device development,31 regulatory and financial prioritization of participant homogeneity,32 and avoidance of potential maternal and fetal liability.33 This is an important delineation, because industry is one of the most significant contributors to production, marketing, and distribution of new therapies.34 Furthermore, this deficit may have untoward implications for the development of pharmaceuticals and treatments specific for obstetrical conditions.
Clinical implications
Despite calls by leading experts and international organizations to increase obstetrical clinical trials,7, 10 , 35 the relative shortage in obstetrical clinical trials remains an urgent issue. Other studies have explored the multifaceted etiology of obstetrical trial scarcity including historical precedents, ethical quandaries, participant, funder and institutional review board reluctance, and risk assessment.6 , 8 , 36 , 37 A recent report recommended increased funding from the National Institutes of Health specifically as a means to expand obstetrical and gynecologic research and improve women’s health outcomes.38 Obstetrical clinical research represents a key component of the multifaceted efforts needed to decrease the maternal burden of disease and advance treatments for obstetrical conditions.4 , 35 The progress of the field warrants and partially depends on a greater commitment of resources and funding by all clinical trial sponsors.
In addition to increasing the number of obstetrical clinical trials, improvement in obstetrical trial completion and dissemination is important. We found that United States government–funded trials more consistently meet quality metrics (eg, sample size, randomization, blinding, and oversight by a data safety monitoring committee)28 and more frequently reach completion than academic or industry trials. The quality and success of United States government–funded obstetrical trials may speak to the potential role of increased regulation and present a model for increasing obstetrical trials completion.
The low results reporting, particularly in academic-funded trials, may be a reflection of relatively limited resources in academia. Although federal statues require many clinical trials to report their results within one year with the option to delay for 2 years,20 the parameters of the statutes have evolved over the past decade and barriers exist to consistent results reporting.15 , 39, 40, 41 Stockmann et al12 identified that for obstetrical trials, ClinicalTrials.gov provides the most complete repository for trial results, which is more comprehensive than peer-reviewed publications. Trial results and their implications take on a greater nuance in pregnancy trials where interventions may affect both maternal and fetal health.42 A lack of results can bias the literature, squander limited resources, and hinder medical innovation. Greater results reporting within obstetrical trials continues to be a relevant goal with implications for all expecting parents.
Strengths and limitations
Our analysis uniquely compares obstetrical and nonobstetrical trials and trial features among different funders. This comparison allows for the extrapolation of factors that may contribute to the low number of obstetrical trials and the increased risk of early discontinuation among obstetrical trials. We present novel assessments of temporal trends only possible with a large sample size and greater time period. This study examines the associations between obstetrical trial funding and early discontinuation and results reporting while controlling for confounding variables. Our multivariable approach clarifies which trial features are most important when considering mechanisms to improve obstetrical clinical trial completion and dissemination.
Our study is not without limitations. First, the ClinicalTrials.gov registry represents only a sample of all global clinical trials.15 However, 1 study found that ClinicalTrials.gov contains the greatest number of obstetrical trials compared with other databases.11 Second, because this analysis involves multiple testing, it is possible that the strength of association seen for some trial features may be the result of chance. Finally, the limitations of ClinicalTrials.gov have been described in other studies and apply to this analysis. These include changes to the database over time,40 nuances of recruitment description and progress,12 the inability to verify the validity of all trial data,18 and the limited trial features and descriptions available for analysis.
Research implications
We characterize the obstetrical clinical trial landscape and trial features associated with early discontinuation and results reporting. A more granular view of the research by disease category and analyses comparing trial quantity with burden of disease could highlight areas for increased clinical trial focus. In addition, manual reviews of trial discontinuation reasons could identify why trials in obstetrics discontinue early.
Conclusions
The obstetrical clinical trial portfolio remains sparse but has shown growth over time. Increased industry investment in obstetrical trials may present an important step toward expanding available obstetrical therapies. All stakeholders must commit to improving obstetrical clinical trial completion rates and results dissemination to ensure trial findings advance the maternal health evidence base. Clinical trial research—in conjunction with health system strengthening and effective national policies—could decrease maternal morbidity and mortality.4 Improvement in obstetrical outcomes depends, in part, on research investments that bridge the gap between the breadth of obstetrical disease and the quantity, quality, and dissemination of obstetrical clinical trials.
Footnotes
The authors report no conflict of interest.
Drs Steinberg and Weeks are co-first authors. Drs Lyell and Turner are co-senior authors.
Cite this article as: Steinberg JR, Weeks BT, Reyes GA, et al. The obstetrical research landscape: a cross-sectional analysis of clinical trials from 2007-2020. Am J Obstet Gynecol MFM 2021;3:100253.
Appendix
Obstetrical therapeutic focus and disease category definitions and subgroups
A total of 8 researchers were trained with respect to the obstetrical therapeutic focus categorization system. Collectively, they manually reviewed all trials including titles, abstracts, and detailed descriptions to verify and categorize obstetrical trials. Disagreements and inconsistencies were adjudicated by the lead authors (J.R.S. and B.T.W.).
For analysis, each therapeutic focus was treated as a binary variable with 2 categories: (1) trials labeled with the therapeutic focus and (2) all other trials that were not labeled with the therapeutic focus (eg, [1] trials with the label “pregnancy loss,” [2] trials without the label “pregnancy loss”). The reference for multivariate analysis of associations of therapeutic foci with trial outcomes was (2) all other trials that were not labeled with the therapeutic focus. Trials in the reference consequently varied among therapeutic foci.
The labels listed below were used to categorize the therapeutic foci of different obstetrical trials:
-
1.
Cesarean delivery: All trials focused on cesarean delivery interventions or conducted only in patients receiving cesarean deliveries
-
2.
Nutritional status in pregnancy: Trials that focused on obesity, malnutrition, mineral deficiencies, and/or amino acid deficiencies
-
3.
Labor anesthesia and analgesia: Trials that focused on complications of anesthesia during pregnancy or after delivery, including pulmonary, cardiac, and central nervous system complications; toxic reaction to local anesthesia; spinal and epidural anesthesia–induced complications, including headache; and/or failed or difficult intubation
-
4.
Infections in pregnancy, labor, and the puerperium: Trials that focused on chorioamnionitis; infections of genital and urinary tracts; sexually transmitted infections and pregnancy; viral hepatitis, HIV, and other viral infection; protozoal diseases; breast infections; pneumonia; tuberculosis; infection of the obstetrical surgical wound; fever of unknown origin; and/or sepsis
-
5.
Fetal focused: Trials that focused on prenatal genetic screening, preimplantation genetic diagnosis, nonstress testing, intrapartum fetal heart rate monitoring, fetal (not neonatal) abnormalities, multiple gestation, polyhydramnios, oligohydramnios, hydrops, macrosomia, intrauterine growth restriction, and/or malpresentation
-
6.
Threatened early delivery: Trials that focused on preterm labor, false labor, cervical insufficiency, and/or premature rupture of membranes
-
7.
Mental and behavioral disorders: Trials that focused on postpartum mood disturbance or blues and/or postpartum depression or psychosis
-
8.
Diabetes mellitus in pregnancy: Trials that focused on preexisting diabetes mellitus complicating pregnancy; and/or gestational diabetes
-
9.
Bleeding and hemorrhage: Trials that focused on hemorrhage and spotting in early pregnancy; antepartum, intrapartum, and postpartum hemorrhage; placenta previa; placental abruption; placenta accreta, increta, and percreta; and/or postpartum uterine atony
-
10.
Labor augmentation and induction: Trials that focused on postdates pregnancy, cervical ripening, induction of labor, and/or prolonged labor
-
11.
Hypertensive disorders of pregnancy: Trials that focused on preexisting hypertension complicating the pregnancy, edema and proteinuria, pregnancy-induced hypertension, and/or preeclampsia and eclampsia
-
12.
Breastfeeding: Trials that focused on breastfeeding, nipple pain/trauma, nipple infection, duct pathology, mastitis, breast abscess, and galactocele and hypogalactia
-
13.
Pregnancy loss: Trials that focused on early pregnancy loss, ectopic pregnancy, spontaneous abortion, and/or fetal reduction
-
14.
Obstetrical trauma: Trials that focused on perineal, high vaginal, or cervical laceration; uterine rupture; postpartum uterine inversion; obstetrical damage from instruments; obstetrical injury to pelvic organs; and/or pelvic hematoma
-
15.
Thromboembolic disease of pregnancy: Trials that focused on deep vein thrombosis, amniotic fluid embolism, pyemic and septic embolism, and/or other obstetrical embolism (excluding cerebrovascular disease)
-
16.
Vomiting in pregnancy: Trials that focused on excessive vomiting, hyperemesis gravidarum, late vomiting of pregnancy, and/or morning sickness
-
17.
Other: Trials that focused on cerebrovascular disease (cerebral venous thrombosis, subarachnoid hemorrhage, and/or stroke), noninfectious liver disease in pregnancy (liver and biliary tract disorders, cholestasis of pregnancy, and/or acute fatty liver of pregnancy), and noninfectious renal disease in pregnancy (postpartum acute kidney failure, hepatorenal syndrome, kidney stones)
Supplemental Table 1.
Characteristics of obstetrical and nonobstetrical clinical trialsa
| Trial feature | Nonobstetrical | Obstetrical | Chi2P value |
|---|---|---|---|
| Total | 219,235 (98.1) | 4276 (1.9) | |
| Fundingb | |||
| Industry | 76,768 (35.0) | 290 (6.8) | <.0001 |
| United States government | 17,350 (7.9) | 331 (7.7) | |
| Academic | 125,117 (57.1) | 3655 (85.5) | |
| Primary purpose | |||
| Treatment | 140,234 (64.0) | 1681 (39.3) | <.0001 |
| Basic science | 12,154 (5.5) | 106 (2.5) | |
| Prevention | 22,394 (10.2) | 1345 (31.5) | |
| Otherc | 37,961 (17.3) | 1037 (24.3) | |
| Missing | 6492 (3.0) | 107 (2.5) | |
| Phase | |||
| Not Applicabled | 99,542 (45.4) | 2805 (65.6) | <.0001 |
| Phase 1 | 30,146 (13.8) | 136 (3.2) | |
| Phase 1/2–2 | 42,144 (19.2) | 301 (7.0) | |
| Phase 2/3–3 | 26,058 (11.9) | 507 (11.9) | |
| Phase 4 | 21,345 (9.7) | 527 (12.3) | |
| Enrollmente | |||
| 0–9 | 16,056 (7.3) | 204 (4.8) | <.0001 |
| 10–49 | 78,930 (36.0) | 641 (15.0) | |
| 50–99 | 45,792 (20.9) | 789 (18.5) | |
| 100–499 | 61,257 (27.9) | 1910 (44.7) | |
| 500–999 | 8863 (4.0) | 295 (6.9) | |
| >999 | 7628 (3.5) | 422 (9.9) | |
| Missing | 709 (0.3) | 709 (0.3) | |
| Blinding | |||
| None | 124,461 (56.8) | 2266 (53.0) | <.0001 |
| Double | 50,675 (23.1) | 922 (21.6) | |
| Single | 43,125 (19.7) | 1078 (25.2) | |
| Missing | 974 (0.4) | 10 (0.2) | |
| Randomization | |||
| Nonrandomized | 74,065 (33.8) | 822 (19.2) | <.0001 |
| Randomized | 142,302 (64.9) | 3420 (80.0) | |
| Missing | 2868 (1.3) | 34 (0.8) | |
| Oversight by a data safety monitoring committee | |||
| No | 115,552 (52.7) | 2269 (53.1) | <.0001 |
| Yes | 78,713 (35.9) | 1635 (38.2) | |
| Missing | 24,970 (11.4) | 372 (8.7) | |
| Location | |||
| High-income countries | 158,210 (72.2) | 2557 (59.8) | <.0001 |
| Low- and middle-income countries only | 30,428 (13.9) | 1174 (27.5) | |
| Missing | 30,597 (14.0) | 545 (12.7) |
Values are number (percentage) unless indicated otherwise.
Steinberg et al. The obstetrical clinical trial landscape. AJOG MFM 2021.
Percentages may not sum up to 100 because of rounding
Funding categories were determined with data on the sponsor and collaborators. Industry funding includes trials with an industry sponsor or collaborating agency. United States government trials include remaining trials with a United States government sponsor or collaborating agency. Academic trials include all other trials
Other primary purposes include diagnostic, screening, supportive care, health services research, and other
On ClinicalTrials.gov, “Not Applicable” is used to describe trials without Food and Drug Administration–defined phases, including trials of devices or behavioral interventions
Enrollment included both actual enrollment (for completed trials) and anticipated enrollment (for ongoing trials with continued enrollment).
Supplemental Table 2.
Yearly growth statistics for obstetrical trials
| Variable | Annual growth rate | Annual growth rate Mann-Kendall P value |
Relative annual growth ratea | Relative annual growth rate Mann-Kendall P value |
|---|---|---|---|---|
| Obstetrical trials | 10.8% | <.0001 | ||
| Nonobstetrical Trials | 4.8% | <.0001 | ||
| Fundingb | ||||
| United States government | 2.8% | .49 | −7.2% | .003 |
| Industry | 6.5% | .02 | −3.8% | .05 |
| Academic | 12.4% | <.0001 | 1.4% | .0007 |
| Study location | ||||
| High-income countries | 7.9% | .0005 | −1.4% | .007 |
Steinberg et al. The obstetrical clinical trial landscape. AJOG MFM 2021.
The relative annual growth rate (the change in percent) compares the relative growth of subcategories within a single category. It measures the change in the proportion of trials within that subcategory as a function of all trials in the category
Funding categories were determined with data on the sponsor and collaborators. Industry funding includes trials with an industry sponsor or collaborating agency. United States government trials include remaining trials with a United States government sponsor or collaborating agency. Academic trials include all other trials.
Supplemental Table 3.
Trial quantity and enrollment by therapeutic area of focus
| Therapeutic area of focusa | Trials | Enrollmentb | ||
|---|---|---|---|---|
| Cesarean delivery | 686 | (16.0) | 83,394 | (2.4) |
| Nutrition | 553 | (12.9) | 147,584 | (4.2) |
| Other disease | 522 | (12.2) | 798,361 | (22.9) |
| Labor anesthesia | 515 | (12.0) | 67,789 | (1.9) |
| Infections | 438 | (10.2) | 755,241 | (21.6) |
| Fetal indications | 422 | (9.9) | 547,647 | (15.7) |
| Mental health | 354 | (8.3) | 34,820 | (1.0) |
| Early delivery | 328 | (7.7) | 188,101 | (5.4) |
| Diabetes mellitus | 307 | (7.2) | 29,970 | (0.9) |
| Hemorrhage | 305 | (7.1) | 154,760 | (4.4) |
| Labor Augmentation | 257 | (6.0) | 40,946 | (1.2) |
| Hypertension | 254 | (5.9) | 118,076 | (3.4) |
| Breastfeeding | 197 | (4.6) | 56,389 | (1.6) |
| Pregnancy loss | 149 | (3.5) | 446,114 | (12.8) |
| Trauma | 95 | (2.2) | 15,585 | (0.4) |
| Thromboembolic | 24 | (0.6) | 1881 | (0.1) |
| Vomiting | 21 | (0.5) | 2658 | (0.1) |
Values are number (percentage) unless indicated otherwise.
Steinberg et al. The obstetrical clinical trial landscape. AJOG MFM 2021.
Trials could have multiple therapeutic areas of foci
Enrollment included both actual enrollment (for completed trials) and anticipated enrollment (for ongoing trials with continued enrollment).
Supplemental Table 4.
Obstetrical trial study status and enrollment on March 9, 2020
| Study status | Total trials | Total enrollmenta |
|---|---|---|
| Completed | ||
| Completed | 2032 | 2,870,525 |
| Ongoing | ||
| Active, not recruiting | 192 | 218,518 |
| Enrolling by invitation | 31 | 33,787 |
| Not yet recruiting | 249 | 234,939 |
| Recruiting | 899 | 693,661 |
| Discontinued Early | ||
| Suspended | 10 | 1401 |
| Terminated | 181 | 30,369 |
| Withdrawn | 118 | 918 |
| Unknown | ||
| Unknown status | 564 | 376,470 |
All definitions and terms as defined by the ClinicalTrials.gov glossary.1
Steinberg et al. The obstetrical clinical trial landscape. AJOG MFM 2021.
Enrollment included both actual enrollment (for completed trials) and anticipated enrollment (for ongoing trials with continued enrollment).
Supplemental Table 5.
Univariable analyses of association between trial between trail features and early discontinuation
| Trail feature | Hazard ratio (95% confidence interval) | P value |
|---|---|---|
| Fundinga | ||
| Industry | Reference | |
| Academic | 0.73 (0.45–1.18) | .20 |
| United States government | 0.12 (0.04–0.35) | <.001 |
| Primary purpose | ||
| Treatment | Reference | |
| Basic science | 0.39 (0.12–1.30) | .12 |
| Prevention | 0.43 (0.29–0.62) | <.001 |
| Otherb | 0.76 (0.51–1.13) | .17 |
| Phase | ||
| Phase 2/3–3 | Reference | |
| Not Applicablec | 0.95 (0.61–1.50) | .84 |
| Phase 1 | 1.79 (0.83–3.88) | .14 |
| Phase 1/2–2 | 1.13 (0.56–2.27) | .73 |
| Phase 4 | 1.04 (0.58–1.88) | .90 |
| Enrollmentd | ||
| 100–499 | Reference | |
| 0–9 | 32.45 (20.75–50.75) | <.001 |
| 10–49 | 5.20 (3.39–7.99) | <.001 |
| 50–99 | 1.68 (0.97–2.93) | .07 |
| 500–999 | 0.72 (0.26–2.04) | .54 |
| >999 | 0.81 (0.39–1.68) | .56 |
| Year | ||
| 2007–2013 | Reference | |
| 2014–2020 | 1.17 (0.86–1.59) | .33 |
| Blinding | ||
| None | Reference | |
| Double | 1.17 (0.83–1.66) | .37 |
| Single | 0.66 (0.43–1.00) | .053 |
| Randomization | ||
| Nonrandomized | Reference | |
| Randomized | 0.87 (0.59–1.28) | .48 |
| Oversight by a data safety monitoring committee | ||
| No | Reference | |
| Yes | 0.83 (0.61–1.13) | .24 |
| Location | ||
| Low- and middle-income countries only | Reference | |
| High-income countries | 2.91 (1.68–5.04) | <.001 |
| Number of facilities | ||
| 1 | Reference | |
| >2 | 0.92 (0.64–1.30) | .63 |
| Therapeutic focuse | ||
| All other therapeutic foci | Reference | |
| Cesarean delivery | 1.51 (0.95–2.38) | .08 |
| Nutrition | 0.61 (0.37–0.99) | .046 |
| Anesthesia | 2.16 (1.42–3.29) | <.001 |
| Infection | 0.85 (0.52–1.41) | .53 |
| Fetal | 1.12 (0.72–1.75) | .61 |
| Mental health | 0.77 (0.43–1.38) | .38 |
| Early delivery | 1.47 (0.94–2.30) | .09 |
| Diabetes mellitus | 1.00 (0.59–1.71) | .99 |
Steinberg et al. The obstetrical clinical trial landscape. AJOG MFM 2021.
Funding categories were determined with data on the sponsor and collaborators. Industry funding includes trials with an industry sponsor or collaborating agency United States government trials include remaining trials with a United States government sponsor or collaborating agency
Other primary purposes include diagnostic, screening, supportive care, health services research, and other
On ClinicalTrials.gov, “Not Applicable” is used to describe trials without Food and Drug Administration–defined phases, including trials of devices or behavioral interventions
Enrollment included both actual enrollment (for completed trials) and anticipated enrollment (for ongoing trials with continued enrollment)
Trials could have >1 therapeutic focus. For analysis, each therapeutic focus was treated as a binary variable.
Supplemental Table 6.
Univariable analyses of association between trial features and results reporting within 3 years of completion
| Trail feature | Odds ratio (95% confidence interval) | P value |
|---|---|---|
| Fundinga | ||
| Industry | Reference | |
| Academic | 0.48 (0.27–0.82) | .008 |
| United States government | 0.93 (0.47–1.83) | .83 |
| Primary purpose | ||
| Treatment | Reference | |
| Basic science | 0.86 (0.33–2.26) | .76 |
| Prevention | 0.71 (0.48–1.07) | .10 |
| Otherb | 0.71 (0.44–1.14) | .15 |
| Phase | ||
| Phase 2/3–3 | Reference | |
| Not Applicablec | 0.68 (0.40–1.16) | .16 |
| Phase 1 | 0.81 (0.29–2.30) | .70 |
| Phase 1/2–2 | 1.42 (0.70–2.86) | .33 |
| Phase 4 | 1.23 (0.65–2.32) | .52 |
| Enrollmentd | ||
| 100–499 | Reference | |
| 10–49 | 1.13 (0.71–1.78) | .61 |
| 50–99 | 0.87 (0.54–1.38) | .55 |
| 500–999 | 0.70 (0.31–1.59) | .40 |
| >999 | 0.44 (0.21–0.95) | .035 |
| Year | ||
| 2007–2013 | Reference | |
| 2014–2020 | 1.22 (0.85–1.73) | .28 |
| Blinding | ||
| None | Reference | |
| Double | 1.30 (0.85–2.00) | .22 |
| Single | 1.22 (0.80–1.86) | .36 |
| Randomization | ||
| Nonrandomized | Reference | |
| Randomized | 1.44 (0.87–2.38) | .16 |
| Oversight by a data safety monitoring committee | ||
| No | Reference | |
| Yes | 1.23 (0.87–1.74) | .25 |
| Location | ||
| Low- and middle-income countries only | Reference | |
| High-income countries | 2.04 (1.34–3.13) | <.001 |
| Number of facilities | ||
| 1 | Reference | |
| >2 | 1.74 (1.18–2.56) | .005 |
| Therapeutic focuse | ||
| All other therapeutic foci | Reference | |
| Cesarean delivery | 1.55 (1.01–2.37) | .043 |
| Nutrition | 0.56 (0.31–0.99) | .046 |
| Anesthesia | 1.52 (0.95–2.42) | .08 |
| Infection | 1.12 (0.67–1.87) | .67 |
| Fetal | 1.16 (0.63–2.13) | .63 |
| Mental health | 1.01 (0.53–1.95) | .97 |
| Early delivery | 0.87 (0.39–1.94) | .74 |
| Diabetes mellitus | 0.75 (0.35–1.58) | .45 |
Steinberg et al. The obstetrical clinical trial landscape. AJOG MFM 2021.
Funding categories were determined with data on the sponsor and collaborators. Industry funding includes trials with an industry sponsor or collaborating agency United States government trials include remaining trials with a United States government sponsor or collaborating agency
Other primary purposes include diagnostic, screening, supportive care, health services research, and other
On ClinicalTrials.gov, “Not Applicable” is used to describe trials without Food and Drug Administration–defined phases, including trials of devices or behavioral interventions
Enrollment included both actual enrollment (for completed trials) and anticipated enrollment (for ongoing trials with continued enrollment)
Trials could have >1 therapeutic focus. For analysis, each therapeutic focus was treated as a binary variable.
Supplemental Table 7.
Association between trial features and results reporting within 3 years of completion
| Trial feature | Odds ratio (95% confidence interval) | P value |
|---|---|---|
| All trials | ||
| Nonobstetrical | Reference | |
| Obstetrical | 0.89 (0.72–1.10) | .19 |
| Obstetrical trials only | ||
| Fundinga | ||
| Industry | Reference | |
| Academic | 0.39 (0.22–0.68) | .0009 |
| United States government | 1.06 (0.53–2.09) | .87 |
| Primary purpose | ||
| Treatment | Reference | |
| Basic science | 1.49 (0.62–3.56) | .37 |
| Prevention | 0.98 (0.65–1.47) | .92 |
| Otherb | 0.85 (0.51–1.43) | .54 |
| Phase | ||
| Phase 2/3–3 | Reference | |
| Not Applicablec | 0.82 (0.47–1.43) | .49 |
| Phase 1 | 0.89 (0.34–2.39) | .82 |
| Phase 1/2–2 | 1.91 (0.99–3.70) | .05 |
| Phase 4 | 0.92 (0.47–1.79) | 80 |
| Enrollmentd | ||
| 100–499 | Reference | |
| 0–9 | 0.44 (0.05–3.56) | .44 |
| 10–49 | 1.08 (0.66–1.76) | .77 |
| 50–99 | 0.86 (0.55–1.35) | .53 |
| 500–999 | 0.62 (0.28–1.37) | .24 |
| >999 | 0.61 (0.31–1.21) | .16 |
| Blinding | ||
| None | Reference | |
| Double | 0.82 (0.52–1.31) | .41 |
| Single | 0.90 (0.58–1.39) | .63 |
| Randomization | ||
| Nonrandomized | Reference | |
| Randomized | 1.36 (0.81–2.28) | .25 |
| Oversight by a data safety monitoring committee | ||
| No | Reference | |
| Yes | 1.41 (0.98–2.04) | .07 |
| Location | ||
| Low- and middle-income countries only | Reference | |
| High-income countries | 2.13 (1.35–3.36) | .001 |
| Number of facilities | ||
| 1 | Reference | |
| ≥2 | 1.22 (0.80–1.87) | .35 |
| Therapeutic focuse | ||
| All other therapeutic foci | Reference | |
| Cesarean delivery | 2.07 (1.29–3.34) | .003 |
| Nutrition | 0.75 (0.44–1.29) | .30 |
| Anesthesia | 1.07 (0.62–1.87) | .80 |
| Infection | 1.25 (0.75–2.09) | .39 |
| Fetal | 0.75 (0.37–1.53) | .43 |
| Mental health | 0.51 (0.23–1.12) | .09 |
| Early delivery | 0.51 (0.23–1.12) | .88 |
| Diabetes mellitus | 0.83 (0.39–1.77) | .63 |
Steinberg et al. The obstetrical clinical trial landscape. AJOG MFM 2021.
Funding categories were determined with data on the sponsor and collaborators. Industry funding includes trials with an industry sponsor or collaborating agency United States government trials include remaining trials with a United States government sponsor or collaborating agency
Other primary purposes include diagnostic, screening, supportive care, health services research, and other
On ClinicalTrials.gov, “Not Applicable” is used to describe trials without Food and Drug Administration–defined phases, including trials of devices or behavioral interventions
Enrollment included both actual enrollment (for completed trials) and anticipated enrollment (for ongoing trials with continued enrollment)
Trials could have >1 therapeutic focus. For analysis, each therapeutic focus was treated as a binary variable.
Supplemental Table 8.
Results reporting among trials completed by March 9, 2017a
| Completed trials | Results reported by March 9, 2020 | |
|---|---|---|
| Total | 1411 | 216 (15.3) |
| fundingb | ||
| Industry | 102 | 29 (28.4) |
| Academic | 1171 | 149 (12.7) |
| United States government | 138 | 38 (27.5) |
Values are number (percentage) unless indicated otherwise.
Steinberg et al. The obstetrical clinical trial landscape. AJOG MFM 2021.
Only trials completed by March 8, 2017, were included in the analysis of results reporting to align with federal mandates for delayed submission of results information within 3 years of trial completion
Funding categories were determined with data on the sponsor and collaborators. Industry funding includes trials with an industry sponsor or collaborating agency. United States government trials include remaining trials with a United States government sponsor or collaborating agency. Academic trials include all other trials.
References
- 1.Zarin D.A., Tse T., Williams R.J., Califf R.M., Ide N.C. The ClinicalTrials.gov results database--update and key issues. N Engl J Med. 2011;364:852–860. doi: 10.1056/NEJMsa1012065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banno M., Tsujimoto Y., Kataoka Y. Studies registered in non-ClinicalTrials.gov accounted for an increasing proportion of protocol registrations in medical research. J Clin Epidemiol. 2019;116:106–113. doi: 10.1016/j.jclinepi.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Tse T., Williams R.J., Zarin D.A. Reporting “basic results” in ClinicalTrials.gov. Chest. 2009;136:295–303. doi: 10.1378/chest.08-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kassebaum N.J., Bertozzi-Villa A., Coggeshall M.S. Global, regional, and national levels and causes of maternal mortality during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:980–1004. doi: 10.1016/S0140-6736(14)60696-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shields K.E., Lyerly A.D. Exclusion of pregnant women from industry-sponsored clinical trials. Obstet Gynecol. 2013;122:1077–1081. doi: 10.1097/AOG.0b013e3182a9ca67. [DOI] [PubMed] [Google Scholar]
- 6.Sheffield J.S., Siegel D., Mirochnick M. Designing drug trials: considerations for pregnant women. Clin Infect Dis. 2014;59(Suppl7):S437–S444. doi: 10.1093/cid/ciu709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vallotton M.B. Council for international organizations of medical sciences perspectives: protecting persons through international ethics guidelines. Int J Integr Care. 2010;10(Suppl):e008. doi: 10.5334/ijic.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomson A. Women and health research, ethical and legal issues of including women in clinical studies. Midwifery. 1995;11:52. [Google Scholar]
- 9.Broussard C.S., Frey M.T., Hernandez-Diaz S. Developing a systematic approach to safer medication use during pregnancy: summary of a Centers for Disease Control and Prevention--convened meeting. Am J Obstet Gynecol. 2014;211:208–214.e1. doi: 10.1016/j.ajog.2014.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Committee on Ethics, American College of Obstetricians and Gynecologists ACOG Committee Opinion no. 377: Research involving women. Obstet Gynecol. 2007;110:731–736. doi: 10.1097/01.AOG.0000263926.75016.db. [DOI] [PubMed] [Google Scholar]
- 11.Scaffidi J., Mol B.W., Keelan J.A. The pregnant women as a drug orphan: a global survey of registered clinical trials of pharmacological interventions in pregnancy. BJOG. 2017;124:132–140. doi: 10.1111/1471-0528.14151. [DOI] [PubMed] [Google Scholar]
- 12.Stockmann C., Sherwin C.M.T., Koren G. Characteristics and publication patterns of obstetric studies registered in Clin Trials.gov. J Clin Pharmacol. 2014;54:432–437. doi: 10.1002/jcph.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Checketts J.X., Evans M.B., Athale A.H. Compliance on mandatory data reporting in registered obstetrics trials. Am J Perinatol. 2018;35:1192–1196. doi: 10.1055/s-0038-1642620. [DOI] [PubMed] [Google Scholar]
- 14.NMC Clinical trials transformation initiative. 2018. https://www.ctti-clinicaltrials.org/ Available at:
- 15.Tse T., Fain K.M., Zarin D.A. How to avoid common problems when using ClinicalTrials.gov in research: 10 issues to consider. BMJ. 2018;361:k1452. doi: 10.1136/bmj.k1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viergever R.F., Ghersi D. The quality of registration of clinical trials. PLoS One. 2011;6:e14701. doi: 10.1371/journal.pone.0014701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner B., Rajeshuni N., Tran E.M. Characteristic of ophthalmology trials registered in ClinicalTrials.gov, 2007-2018. Am J Ophthalmol. 2020;211:132–141. doi: 10.1016/j.ajo.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Liu X., Zhang Y., Tang L.L. Characteristics of radiotherapy trials compared with other oncological clinical trials in the past 10 years. JAMA Oncol. 2018;4:1073–1079. doi: 10.1001/jamaoncol.2018.0887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ClinicalTrials.gov protocol registration data element definitions for interventional and observational studies. ClinicalTrials.gov. 2019 https://prsinfo.clinicaltrials.gov/definitions.html Available at: [Google Scholar]
- 20.FDAAA 801 and the final rule. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/manage-recs/fdaaa#WhenDoINeedToSubmitResults Available at:
- 21.Discrimination based on sex or blindness, 20 USC ch 38, §1681 (2020) https://uscode.house.gov/view.xhtml?req=granuleid:USC-prelim-title20-section1681&num=0&edition=prelim Available at: Accessed October 30, 2019.
- 22.General definition of institution of higher education, 20 USC ch 28, §1001 (2012) https://www.law.cornell.edu/uscode/text/20/1001 Available at:
- 23.Countries and economies. The World Bank Group. 2020. https://data.worldbank.org/country Available at:
- 24.McLeod A.I. Kendall rank correlation and Mann-Kendall trend test. 2012. http://btr0x2.rz.uni-bayreuth.de/math/statlib/R/CRAN/doc/packages/Kendall.pdf Available at:
- 25.Ware J.H., Harrington D., Hunter D.J., D’Agostino R.B. Missing Data. N Engl J Med. 2012;367:1353–1354. [Google Scholar]
- 26.Buuren S.V., Groothuis-Oudshoorn K. MICE: multivariate imputation by chained equations in R. J Stat Soft. 2010;45:1–68. [Google Scholar]
- 27.Campion W.M. Multiple imputation for nonresponse in surveys by Donald B. Rubin. J Mark Res. 1989;26:485–486. [Google Scholar]
- 28.Moher D., Schulz K.F., Altman D.G., CONSORT The CONSORT statement: revised recommendations for improving the quality of reports of parallel group randomized trials. BMC Med Res Methodol. 2001;1:2. doi: 10.1186/1471-2288-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Califf R.M., Zarin D.A., Kramer J.M., Sherman R.E., Aberle L.H., Tasneem A. Characteristics of clinical trials registered in ClinicalTrials.gov, 2007-2010. JAMA. 2012;307:1838–1847. doi: 10.1001/jama.2012.3424. [DOI] [PubMed] [Google Scholar]
- 30.Smith D.D., Pippen J.L., Adesomo A.A., Rood K.M., Landon M.B., Costantine M.M. Exclusion of pregnant women from clinical trials during the coronavirus disease 2019 pandemic: a review of international registries. Am J Perinatol. 2020;37:792–799. doi: 10.1055/s-0040-1712103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fisk N.M., Atun R. Market failure and the poverty of new drugs in maternal health. PLoS Med. 2008;5:e22. doi: 10.1371/journal.pmed.0050022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nazha B., Mishra M., Pentz R., Owonikoko T.K. Enrollment of racial minorities in clinical trials: old problem assumes new urgency in the age of immunotherapy. Am Soc Clin Oncol Educ Book. 2019;39:3–10. doi: 10.1200/EDBK_100021. [DOI] [PubMed] [Google Scholar]
- 33.van der Graaf R., van der Zande I.S.E., den Ruijter H.M. Fair inclusion of pregnant women in clinical trials: an integrated scientific and ethical approach. Trials. 2018;19:78. doi: 10.1186/s13063-017-2402-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moses H., 3rd, Matheson D.H., Cairns-Smith S., George B.P., Palisch C., Dorsey E.R. The anatomy of medical research: US and international comparisons. JAMA. 2015;313:174–189. doi: 10.1001/jama.2014.15939. [DOI] [PubMed] [Google Scholar]
- 35.D’Alton M.E., Friedman A.M., Bernstein P.S. Putting the “M” back in maternal-fetal medicine: a 5-year report card on a collaborative effort to address maternal morbidity and mortality in the United States. Am J Obstet Gynecol. 2019;221:311–317.e1. doi: 10.1016/j.ajog.2019.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baylis F. Pregnant women deserve better. Nature. 2010;465:689–690. doi: 10.1038/465689a. [DOI] [PubMed] [Google Scholar]
- 37.Palmer S., Pudwell J., Smith G.N., Reid R.L. Optimizing participation of pregnant women in clinical trials: factors influencing decisions about participation in medication and vaccine trials. J Obstet Gynaecol Can. 2016;38:945–954. doi: 10.1016/j.jogc.2016.04.100. [DOI] [PubMed] [Google Scholar]
- 38.Rice L.W., Cedars M.I., Sadovsky Y. Increasing NIH funding for academic departments of obstetrics and gynecology: a call to action. Am J Obstet Gynecol. 2020;223:79.e1–79.e8. doi: 10.1016/j.ajog.2020.03.022. [DOI] [PubMed] [Google Scholar]
- 39.Zarin D.A., Tse T., Williams R.J., Carr S. Trial reporting in ClinicalTrials.gov - the final rule. N Engl J Med. 2016;375:1998–2004. doi: 10.1056/NEJMsr1611785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson M.L., Chiswell K., Peterson E.D., Tasneem A., Topping J., Califf R.M. Compliance with results reporting at ClinicalTrials.gov. N Engl J Med. 2015;372:1031–1039. doi: 10.1056/NEJMsa1409364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DeVito N.J., Bacon S., Goldacre B. Compliance with legal requirement to report clinical trial results on ClinicalTrials.gov: a cohort study. Lancet. 2020;395:361–369. doi: 10.1016/S0140-6736(19)33220-9. [DOI] [PubMed] [Google Scholar]
- 42.Jean S., Hurtado K. FDA Regulations and Definitions, FDA & HHS Similarities and Differences, Applicability of Each. https://oprs.usc.edu/files/2017/05/Week_3.pdf Available at:


