Abstract
Background
Angiotensin-converting enzyme 2 (ACE2) is a novel regulator of the renin–angiotensin system that counteracts the adverse effects of angiotensin II. In heart failure patients, elevated plasma ACE2 activity predicted adverse events and greater myocardial dysfunction. We aimed to describe plasma ACE2 activity and its clinical associations in patients with kidney disease.
Methods
Patients recruited from a single centre comprised of chronic kidney disease Stage III/IV (CKD), haemodialysis patients and kidney transplant recipients (KTRs). Plasma ACE2 enzyme activity was measured using a fluorescent substrate assay in plasma, collected at baseline and stored at −80°C. Linear regression was performed in both males and females separately to determine the covariates associated with log-transformed ACE2.
Results
The median (interquartile range) plasma ACE2 activity in pmol/mL/min was 15.9 (8.4–26.1) in CKD (n = 59), 9.2 (3.9–18.2) in haemodialysis (n = 100) and 13.1 (5.7–21.9) in KTR (n = 80; P < 0.01). In male haemodialysis patients, ACE2 activity was 12.1 (6.8–19.6) compared with 4.4 (2.5–10.3) in females (P < 0.01). Log-transformed ACE2 plasma activity was associated with post-haemodialysis systolic blood pressure in females [β-coefficient 0.04, 95% confidence interval (95% CI) 0.01–0.06, P = 0.006]. In males, log-transformed ACE2 plasma activity was associated with B-type natriuretic peptide (β-coefficient 0.39, 95% CI 0.19–0.60, P < 0.001). Plasma ACE2 activity was not associated with mortality.
Conclusions
Plasma ACE2 activity is reduced in haemodialysis patients compared with CKD patients, and in female haemodialysis patients compared with male. The different associations of plasma ACE2 activity between male and female haemodialysis patients indicate that the role of ACE2 in cardiovascular disease may differ by gender.
Keywords: angiotensin-converting enzyme 2, cardiovascular disease, chronic dialysis, chronic kidney disease, end-stage kidney disease
INTRODUCTION
Cardiovascular disease (CVD) is a major comorbidity associated with chronic kidney disease (CKD) and end-stage kidney disease (ESKD), and confers an increased age-specific mortality compared with the general population [1, 2]. The mechanisms that contribute to the pathogenesis of CVD in CKD are complex and multiple, and include both traditional and non-traditional cardiovascular risk factors. These processes can be measured either by different biochemical tests or by biomarkers and include chronic sodium and water retention, sympathetic nervous system overactivity, enhanced activation of the renin–angiotensin system (RAS), vascular calcification, inflammation and oxidative stress [3].
Enhanced activation of the RAS plays a major role in the progression of cardiac and kidney injury [4]. The blockade of the RAS slows, but does not prevent renal and cardiac injury, suggesting that factors beyond the traditional RAS are involved in the progression of disease [5, 6]. The main effector pathway of the RAS involves angiotensin-converting enzyme (ACE), which converts angiotensin (Ang) I to Ang II, which in turn mediates its effects via the angiotensin Type 1 receptor and is responsible for the pathophysiological effects of the RAS. In 2000, a counter-balancing arm of the RAS was described [7] in which a new enzyme, ACE2, degrades Ang II, and generates Ang 1–7. Ang 1–7 acts via the Mas receptor [8] to counteract the adverse effects of angiotensin, Ang II. ACE2 was originally reported in the highest abundance in the organs of cardiovascular relevance, namely the heart and kidney [7], and it is expressed in both human cardiac [9] and renal tissues [10, 11]. Experimental studies suggest that changes in tissue ACE2 expression may be an important determinant of cardiac and renal injury [12, 13]. In the heart, lack of ACE2 is associated with impaired cardiac function [14] and, in the kidney, deletion or inhibition of ACE2 results in worsening kidney injury, indicating that ACE2 has renoprotective effects presumably owing to Ang II degradation [15–17].
As ACE2 is an integral cell membrane protein, it can undergo ‘shedding’ to release the catalytically active ectodomain into the circulation [18]. Currently, there are limited data on the measurement of ACE2 activity in the circulation in pathophysiological conditions. We have shown that plasma ACE2 levels are low in normal healthy volunteers [19], but significantly increased in the presence of cardiac disease [20]. There is one report that circulating ACE2 levels are increased in patients with Type 1 diabetes and vascular complications [21], but no reports, to date, on ACE2 levels in patients with CKD.
The aim of this study was to determine whether the level of plasma ACE2 activity differed between patients with CKD not yet requiring dialysis, patients undergoing maintenance haemodialysis and kidney transplant recipients (KTRs) and to determine what factors were associated with ACE2 plasma activity in this population.
SUBJECTS AND METHODS
This is a cross-sectional analysis of patients with CKD not requiring dialysis, patients receiving haemodialysis and KTRs recruited from August 2003 to August 2004. We have previously reported associations of the B-type natriuretic peptides (BNPs) and cardiac troponins in this cohort [22–24]. The Austin Health Human Research Ethics Committee approved the study.
Subjects
Eligible participants had CKD defined as a glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 by the Modified Diet in Renal Disease Equation 7 [25], were receiving haemodialysis or had received a kidney transplant. The only exclusion criterion was symptomatic CVD in the previous 3 months. A history of CVD was defined as a history of myocardial infarction, coronary revascularization, heart failure, stroke, carotid endarterectomy, lower limb revascularization or lower limb amputation. In patients undergoing haemodialysis, pre- and post-dialysis blood pressures were measured over the six dialysis sessions prior to the collection of blood.
Serum and plasma were collected from patients at baseline, transported on ice, centrifuged at 3400 g for 10 min and then frozen at –80°C until analysis. All samples collected from patients on haemodialysis were collected after the insertion of the dialysis needle prior to commencing dialysis, and on the middle dialysis day of the week.
Laboratory procedures
Plasma ACE2 activity was measured using a sensitive quenched fluorescent substrate-based assay, as previously described [19, 20]. Two hundred and fifty microlitres of plasma underwent an anion exchange extraction process using low-ionic-strength buffer (20 mmol/L Tris–HCl, pH 6.5) and ANXSepharose 4 Fast-Flowresin (Amersham Biosciences, GE Healthcare, Uppsala, Sweden) [19]. Following the extraction process, 100 μL of the eluate was incubated in duplicate with an ACE2-specific quenched fluorescent substrate (QFS), (7-methoxycoumarin-4-yl)-acetyl-Ala-Pro-Lys (2,4-dintirophenyl) (Auspep, Parkville, Victoria, Australia), with or without 100 μM ethylenediaminetetraacetic acid in a total volume of 200 μL. The rate of substrate cleavage was determined by comparison with a standard curve of the free fluorophore, 4-amino-methoxycoumarin (MCA; Sigma, MO, USA), and expressed as pmol of substrate cleaved/mL of plasma/min. In a subset of samples (pre-dialysis CKD n = 22; dialysis n = 17 and transplant n = 22), ACE2 was measured in plasma without performing the extraction process to remove the endogenous inhibitor of ACE2. This was to determine whether the endogenous inhibitor was affected by reduced kidney function. The intra-assay coefficient of variation (CV) in our laboratory was 5.6% (n = 20), and the inter-assay CV was 11.8% (n = 14). The plasma for ACE2 analyses was stored at −80°C for a median time of 62.6 months.
B-type natriuretic peptide 32 (BNP-32) and its N-terminal component (NT-BNP-76) were previously measured [24] using a paramagnetic particle chemiluminescent immunoenzymatic assay (Biosite Triage BNP) on a Beckman Coulter Access analyser (Beckman Instruments, Inc., Chaska, MN, USA) and an electrochemiluminescence assay (proBNP, Roche Diagnostics, Indianapolis, IN, USA) on an E170 analyser (Roche Diagnostics), respectively. The total CV as reported by the manufacturer was <7% for BNP-32 values between 40 and 4000 ng/L and <6% for values of NT-BNP-76 between 494 and 13 143 ng/L.
Statistical analysis
The values of plasma ACE2 activity and BNP were natural logarithm transformed for analysis because of their skewed distribution. This rendered a more normal distribution by visual inspection of the distribution of the variables and Q–Q plots against a normal distribution, but the Shapiro–Wilk test only confirmed a normal distribution for log-transformed NT-BNP-76. Analysis of variance (ANOVA) was used to compare levels between groups, and the Bonferroni method used to adjust for multiple comparisons. In addition, a non-parametric Kruskal–Wallis test with adjustment for multiple comparisons was performed, and a Wilcoxon signed-rank test used to compare ACE2 plasma activity before and after extraction of the endogenous inhibitor.
Associations of plasma ACE2 activity were initially examined by the comparison of levels between groups for dichotomous variables and linear regression for continuous variables. As ACE2 is located on the X-chromosome, a multivariate analysis was performed for males and females separately, and age, diabetes and history of CVD were included in all models. In addition, variables associated with plasma ACE2 activity with P < 0.1 were included, and a final model determined by backward stepwise elimination and confirmed by the analysis of likelihood ratios. Regression assumptions were assessed by inspection of the plots of the log of plasma ACE2 activity versus the variables and by analysis of standardized residuals. Influential points were examined by inspection of the leverage-versus-residual-squared plot for each model. A sensitivity analysis with influential observations omitted was performed. Because plasma ACE2 activity was log-transformed, the interpretation of the β-coefficient for systolic blood pressure is the % change in plasma ACE2 activity per 1 mmHg in systolic blood pressure. Because BNP is also log-transformed, the interpretation in this model is the % change in plasma ACE2 activity per 1% change in BNP [26].
Patients were followed for all-cause mortality and cardiovascular events. The latter were defined as a combined outcome of cardiac death, myocardial infarction, coronary revascularization procedure, stroke, peripheral vascular disease surgery or revascularization, and gut ischaemia and determined by an independent adjudication committee. Censoring occurred at the time of kidney transplantation, withdrawal from the study on 30 June 2006 for cardiovascular events and 30 June 2010 for all-cause mortality. Cox regression was performed to determine the association of plasma ACE2 activity with the outcome.
Statistical analysis was performed using STATA version 11.0 (Statacorp., College Station, TX, USA).
RESULTS
We recruited and measured ACE2 activity in 59 patients with CKD, 100 patients receiving haemodialysis and 80 KTRs. Patients with CKD not yet requiring dialysis were older with a higher prevalence of diabetes and CVD (Table 1). Haemodialysis patients had lower serum albumin and higher levels of C-reactive protein, BNP-32 and NT-BNP-76 (Table 1).
Table 1.
Baseline characteristics of the three patient groups
| Pre-dialysis (n = 59) | Dialysis (n = 100) | Transplant (n = 80) | P-values | |
|---|---|---|---|---|
| Age (years) | 66.1 ± 12.9 | 61.8 ± 14.7 | 52.6 ± 10.6 | <0.001 |
| Sex (males) | 43 (73%) | 58 (58%) | 53 (66%) | 0.15 |
| Diabetes | 26 (44%) | 21 (21%) | 11 (14%) | <0.001 |
| CVD | 24 (41%) | 33 (33%) | 10 (13%) | <0.001 |
| BP medication | 57 (95%) | 66 (66%) | 77 (96%) | <0.001 |
| RAS blockade | 43 (73%) | 41 (41%) | 44 (55%) | <0.001 |
| eGFR (mL/min) | 24.5 ± 10.8 | – | 35.1 ± 15.1 | <0.001 |
| Hgb (g/L) | 122.2 ± 16.8 | 121.4 ± 11.3 | 129.7 ± 16.0 | 0.001 |
| CRP (mg/L) | 4.0 (2.3–8.6) | 5.1 (3.1–13.2) | 2.1 (0.6–4.6) | <0.001 |
| Albumin (g/L) | 40.0 ± 4.1 | 37.8 ± 4.1 | 40.8 ± 4.0 | <0.001 |
| BNP-32 (ng/L) | 102 (46–248) | 233 (99–621) | 65 (38–164) | <0.001 |
| NT-BNP-76 (ng/L) | 698 (216–2829) | 4075 (1968–16 840) | 371 (141–879) | <0.001 |
| ACE2 activity full data (pmol/mL/min) | 15.9 (8.4–26.1) | 9.2 (3.9–18.2) | 13.1 (5.7–21.9) | 0.002 |
| ACE2 activity subset: inhibitor removed (pmol/mL/min) | 15.5 (5.9–26.1) | 6.0 (4.4–11.8) | 9.5 (6.1–14.2) | 0.16 |
| ACE2 activity subset: inhibitor not removeda (pmol/mL/min) | 3.5 (2.9–7.2)* | 2.8 (1.9–6.1)* | 3.5 (2.0–5.0)* | 0.34 |
The P-value is for comparison across all three groups (except eGFR).
CVD: cardiovascular disease; BP: blood pressure; RAS: renin–angiotensin system; Hgb: haemoglobin; CRP: C-reactive protein; eGFR: estimated glomerular filtration rate.
aMeasured without extraction of the endogenous inhibitor (see Subjects and methods).
*P < 0.001 for comparison with plasma from patients in the same patient group, inhibitor removed (see above).
ACE2 plasma activity between group comparisons
The median level of ACE2 plasma activity was lower in patients receiving dialysis than those with CKD and KTRs (Table 1). After adjustment for multiple comparisons, the difference in median ACE2 plasma activity was significant between patients with CKD and those requiring dialysis (P < 0.001). A similar result occurred with an ANOVA of natural log-transformed ACE2 activity.
The influence of gender on the level of ACE2 plasma activity was analysed using ANOVA, including terms for gender, modality and the interaction between the two. The mean levels of natural log-transformed ACE2 plasma activity were significantly different overall (P < 0.001), but there was no interaction between the difference in levels of ACE2 plasma activity by gender across the three patient groups (P = 0.16). Only in patients undergoing dialysis was the difference in mean natural log-transformed ACE2 plasma activity between males and females significant: 2.3 ± 1.2 in males and 1.4 ± 1.8 in females (P = 0.003). This held true when adjusted for multiple comparisons using a non-parametric test, and the median (interquartile range) was 12.1 (6.8–19.6) pmol/mL/min in males undergoing dialysis compared with 4.4 (2.5–10.3) pmol/mL/min in females undergoing dialysis (P < 0.01, Figure 1). The median ACE2 plasma activity differed by diabetes status in female patients on haemodialysis, but not in male patients receiving haemodialysis. The median (interquartile range) plasma ACE2 activity was 3.3 (2.2–8.3) pmol/mL/min in females without diabetes compared with 13.4 (4.6–25.0) pmol/mL/min in females with diabetes (P = 0.003), and 12.4 (6.8–20.9) pmol/mL/min in males without diabetes compared with 9.3 (3.6–16.6) pmol/mL/min in males with diabetes (P = 0.2).
FIGURE 1:

Levels of ACE2 plasma activity in patients with CKD, patients receiving dialysis and KTRs (A), and in the above subgroups by sex (B).
For comparison, the mean (±standard deviation) ACE2 plasma activity in 18 healthy subjects reported previously was 4.44 ± 2.38 pmol/mL/min [19]. However, these healthy control samples were not contemporaneous with the CKD samples analysed in this report. In the subset of samples used to compare ACE2 plasma activity assayed with and without extraction of the endogenous inhibitor, ACE2 plasma activity was substantially lower in the plasma assayed without extraction of the endogenous inhibitor (Table 1). There was no difference in ACE2 plasma activity between the treatment groups in this subset.
Predictors of ACE2 plasma activity
Predictors of plasma ACE2 activity were analysed in the patients undergoing dialysis only (n = 100), as the other groups were smaller and significant predictors not identified. ACE2 levels differed between males and females receiving dialysis, and sex-specific analyses were subsequently performed. In females undergoing dialysis, age, history of CVD, diabetes and average post-dialysis systolic blood pressure over the preceding six haemodialysis sessions were associated with natural log-transformed plasma ACE2 activity at the P < 0.10 level (Table 2, Model A). In the multivariate model including these variables, only the post-dialysis systolic blood pressure remained a significant predictor of natural log-transformed plasma ACE2 activity. Each 1 mmHg increase in systolic blood pressure was associated with a 4% increase in plasma ACE2 activity. A similar result was obtained with the pre-dialysis systolic blood pressure (data not shown). However, addition to this model of the use of antihypertensive medication, which was also significantly associated with ACE2 plasma activity, attenuated this association (Table 2, Model B). The number of antihypertensive medications included as a categorical variable similarly attenuated the association with the post-dialysis systolic blood pressure (data not shown). Neither form of BNP, nor time from beginning renal replacement therapy, was significantly associated with natural log-transformed plasma ACE2 activity in females undergoing dialysis.
Table 2.
Associations of natural logarithm-transformed plasma ACE2 activity in female patients receiving dialysis
| Variable | Univariate β-coefficient | P-values | Multivariate β-coefficient | P-values |
|---|---|---|---|---|
| Model A | ||||
| Age (per year) | 0.04 (0.0–0.07) | 0.074 | ||
| Diabetes | 1.40 (0.16–2.64) | 0.028 | ||
| CVD | 1.13 (−0.01–2.26) | 0.053 | ||
| Post-dialysis SBP (per 1 mmHg) | 0.04 (0.01–0.06) | 0.006 | 0.04 (0.01–0.06) | 0.006 |
| Model B | ||||
| Post-dialysis SBP (per 1 mmHg) | 0.04 (0.01–0.06) | 0.006 | 0.028 (0.023–0.053) | 0.029 |
| Antihypertensive use | 1.59 (0.57–2.61) | 0.003 | 1.06 (0.12–2.02) | 0.029 |
CVD: cardiovascular disease; SBP: systolic blood pressure.
In males undergoing dialysis, average post-dialysis systolic blood pressure over the preceding six haemodialysis sessions, serum albumin, BNP-32 and NT-BNP-76 (both natural log-transformed) and time since commencing renal replacement therapy were all significantly associated with natural log-transformed plasma ACE2 activity at the P < 0.10 level. Age, history of CVD and diabetes were also included in the multivariate model (Table 3). After elimination of the non-significant factors, natural log-transformed BNP-32 demonstrated the strongest association, followed by diabetes and time since commencing renal replacement therapy. Results were similar when natural log-transformed NT-BNP-76 was used instead of BNP-32 (Table 3). Both the use of an antihypertensive medication and the number of antihypertensive medications were not significant predictors of plasma ACE2 activity in males. Addition to multivariate models did not alter the association of plasma ACE2 activity with BNP (data not shown).
Table 3.
Associations of natural logarithm-transformed plasma ACE2 activity in male patients receiving dialysis
| Variables | Univariate β-coefficient | P-values | Multivariate β-coefficient | P-values |
|---|---|---|---|---|
| Model with BNP-32 | ||||
| Age (per year) | 0.01 (−0.01 to 0.03) | 0.26 | ||
| Diabetes | −0.67 (−1.47 to 0.13) | 0.10 | −1.01 (−1.73 to −0.28) | 0.007 |
| CVD | −0.11 (−0.80 to 0.57) | 0.75 | ||
| Post-dialysis SBP | 0.02 (0.0 to 0.04) | 0.016 | ||
| Dialysis vintage | −0.22 (−0.47 to 0.04) | 0.091 | −0.29 (−0.53 to −0.05) | 0.018 |
| Albumin (g/L) | −0.09 (−0.17 to −0.01) | 0.028 | ||
| BNP-32 (log change) | 0.34 (0.13 to 0.56) | 0.002 | 0.39 (0.19 to 0.60) | <0.001 |
| Model with NT-BNP-76 | ||||
| Diabetes | As above | −0.89 (−1.62 to −0.16) | 0.018 | |
| Dialysis vintage | As above | −0.34 (−0.59 to −0.09) | 0.08 | |
| NT-BNP-76 (log change) | 0.30 (0.10–0.50) | 0.004 | 0.34 (0.15 to 1.60) | 0.001 |
BNP-32 and NT-BNP-76 were natural logarithm transformed.
CVD: cardiovascular disease.
The use of drugs that inhibit the RAS did not affect plasma ACE2 activity in either of these groups.
Outcome associations of ACE2 plasma activity
After 433 person years of follow-up, there were 45 deaths. The level of plasma ACE2 activity was not associated with all-cause mortality (Figure 2). Cox regression analysis demonstrated a hazard ratio for death of 1.19 per log unit of plasma ACE2 activity [95% confidence interval (95% CI) 0.94–1.50, P = 0.14]. A similar hazard ratio was demonstrated for males and females separately. After 175 person years of follow-up, 22 cardiovascular events occurred, and the hazard ratio for such events was 0.97 (95% CI 0.74–1.26, P = 0.82).
FIGURE 2:
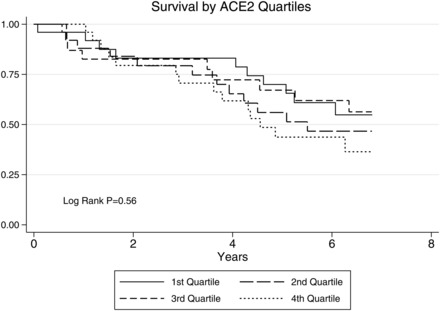
Survival by quartiles of plasma ACE2 activity in patients receiving haemodialysis.
DISCUSSION
The present study demonstrates that plasma ACE2 activity is lower in patients undergoing haemodialysis than in pre-dialysis patients with CKD and, in patients undergoing haemodialysis, males have significantly higher levels of plasma ACE2 activity than females. In these patients, the variable most strongly associated with plasma ACE2 activity differs between males and females. Plasma ACE2 activity did not predict patient outcome.
Although the cause and effect of these findings remain to be determined, the finding of lower plasma ACE2 activity in patients undergoing dialysis, who mostly have had progressive kidney disease over time, suggests a reduction in the generation of plasma ACE2 from either the heart or the kidney with chronicity of disease. Further studies are needed to determine whether lower levels of ACE2 in dialysis patients increase the risk of CVD by lowering the level of protection from the pathogenic effects of circulating Ang II. Although we do not have serial measures of ACE2 activity with disease progression in man, data from our rat models of CKD (subtotal nephrectomy) demonstrate that, in the short-term, kidney disease is associated with increased plasma ACE2 activity and reduced ACE2 activity within the remnant kidney [27], and with cardiac hypertrophy and increased cardiac ACE2 activity [28]. These results suggest that the increase in plasma ACE2 activity reflects increased cardiac rather than renal ACE2. However, in rats given a more chronic kidney injury, plasma ACE2 activity was unchanged, cardiac ACE2 activity did not increase, but ACE2 activity in the remnant kidney was reduced [29]. Thus, plasma ACE2 activity may increase early in the course of CKD, and be followed by a relative fall as CKD becomes established, with a further fall with an ESKD. The patients with renal transplants have plasma ACE2 levels that are more similar to CKD than to ESKD, which supports this hypothesis, although the limitation of patient numbers does not allow a definitive conclusion. Future prospective studies that are underway may provide important time-dependent changes in plasma ACE2 activity and the implications for CVD.
Within patients undergoing dialysis, a significant difference in the level of ACE2 plasma activity was demonstrated between males and females. Although the ACE2 gene is located on the X-chromosome, the level of ACE2 activity was higher in males, which confirms the work of others who showed that serum ACE2 activity is sex-dependent, with higher levels in males compared with females [21].
The analysis of predictors of plasma ACE2 activity in patients undergoing dialysis suggests that there may be different mechanisms contributing to the ACE2 levels for males and females. In males, the strongest association with plasma ACE2 activity was with BNP, which is associated with both left ventricular hypertrophy and systolic dysfunction in patients receiving haemodialysis [30]. The beta-coefficients in these models (Table 3) can be interpreted as a 1% change in the BNP-32 being associated with a 0.39% change in plasma ACE2 activity (or a 0.34% change per 1% increase in NT-BNP-76). This suggests that a myocardial source of plasma ACE2 activity is likely and is in agreement with studies in heart failure [31] that reported higher serum ACE2 activity was correlated with an increasing severity of heart failure and increasing BNP levels. Although this study did not analyse data separately for males and females, over 75% of the cohort was males.
In contrast to the data in males, higher blood pressure in females was significantly associated with plasma ACE2 levels. Whether the positive association with higher blood pressure reflects an increase in plasma ACE2 activity to enhance degradation of Ang II cannot be determined from this study. The association of plasma ACE2 activity and diabetes was in a different direction for males receiving haemodialysis than females, and there was a marked difference in plasma ACE2 activity by diabetes status in females, but not in males. The actual mechanisms behind these differential findings cannot be explained by these data. In future studies, measurement of other components of the ACE/ACE2 axis, including ACE, Ang II and Ang 1–7 would provide important data on the relative balance of the RAS in CKD and contribute to better understanding the mechanisms behind these findings.
The other main limitation of this study is the sample size, particularly as there are sex-dependent effects on plasma ACE2 activity. Future studies will need the power to allow separate analyses of males and females. A longitudinal study with serial measures of ACE2 over time as renal function declines is required to confirm the hypothesized changes over time, and ideally, tissue ACE2 activity would be measured. However, the latter may not be ethically possible so we are reliant on the results from the rodent models.
In conclusion, this early study shows that plasma ACE2 activity is lowest in patients undergoing haemodialysis compared with patients with CKD. There are significant differences in levels between males and females receiving haemodialysis and marked differences in clinical variables that are associated with higher levels of plasma ACE2 activity between the sexes. These important findings may eventually have both pathogenic and therapeutic implications for CVD and progression of renal disease in patients with CKD and suggest that future studies of the ACE/ACE2 axis in this patient group require sex-specific analyses and measurement of the other components.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to disclose. The results presented in this paper have not been published previously in whole or part, except in abstract format.
(See related article by Wysocki and Batlle. Reduced plasma ACE2 activity in dialysis patients: another piece in the conundrum of factors involved in hypertension and cardiovascular morbidity? Nephrol Dial Transplant 2013; 28: 2200–2202.)
ACKNOWLEDGEMENTS
Dr Roberts is supported by a National Health and Medical Research Council of Australia Health Professional Training Fellowship (628902). Dr Velkoska received support from the Austin Medical Research Foundation and the National Health and Medical Research Council (1048285) to enable the ACE2 assays to be performed.
REFERENCES
- 1.Grace B, Excell L, Dent H, et al. New patients, in ANZDATA Registry Report 2010. In: Mcdonald S, Excell L, Livingston B, editors. Australia and New Zealand Dialysis and Transplant Registry. South Australia: Adelaide; 2010. pp. 2-1–2-12. [Google Scholar]
- 2.Roberts MA, Polkinghorne KR, McDonald SP, et al. Secular trends in cardiovascular mortality rates of patients receiving dialysis compared with the general population. Am J Kidney Dis. 2011;58:64–72. doi: 10.1053/j.ajkd.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 3.Roberts MA, Hare DL, Ratnaike S, et al. Cardiovascular biomarkers in CKD: pathophysiology and implications for clinical management of cardiac disease. Am J Kidney Dis. 2006;48:341–360. doi: 10.1053/j.ajkd.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Velez JC. The importance of the intrarenal renin-angiotensin system. Nat Clin Pract Nephrol. 2009;5:89–100. doi: 10.1038/ncpneph1015. [DOI] [PubMed] [Google Scholar]
- 5.Parving HH, Lehnert H, Brochner-Mortensen J, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345:870–878. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- 6.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 7.Donoghue M, Hsieh F, Baronas E, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87:E1–9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 8.Santos RA, Simoes e Silva AC, Maric C, et al. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci USA. 2003;100:8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burrell LM, Risvanis J, Kubota E, et al. Myocardial infarction increases ACE2 expression in rat and humans. Eur Heart J. 2005;26:369–375. doi: 10.1093/eurheartj/ehi114. [DOI] [PubMed] [Google Scholar]
- 10.Reich HN, Oudit GY, Penninger JM, et al. Decreased glomerular and tubular expression of ACE2 in patients with type 2 diabetes and kidney disease. Kidney Int. 2008;74:1610–1616. doi: 10.1038/ki.2008.497. [DOI] [PubMed] [Google Scholar]
- 11.Mizuiri S, Hemmi H, Arita M, et al. Expression of ACE and ACE2 in individuals with diabetic kidney disease and healthy controls. Am J Kidney Dis. 2008;51:613–623. doi: 10.1053/j.ajkd.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 12.Tikellis C, Bialkowski K, Pete J, et al. ACE2 deficiency modifies renoprotection afforded by ACE inhibition in experimental diabetes. Diabetes. 2008;57:1018–1025. doi: 10.2337/db07-1212. [DOI] [PubMed] [Google Scholar]
- 13.Burrell LM, Johnston CI, Tikellis C, et al. ACE2, a new regulator of the renin-angiotensin system. Trends Endocrinol Metab. 2004;15:166–169. doi: 10.1016/j.tem.2004.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crackower MA, Sarao R, Oudit GY, et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 15.Ye M, Wysocki J, William J, et al. Glomerular localization and expression of angiotensin-converting enzyme 2 and angiotensin-converting enzyme: implications for albuminuria in diabetes. J Am Soc Nephrol. 2006;17:3067–3075. doi: 10.1681/ASN.2006050423. [DOI] [PubMed] [Google Scholar]
- 16.Soler MJ, Wysocki J, Ye M, et al. ACE2 inhibition worsens glomerular injury in association with increased ACE expression in streptozotocin-induced diabetic mice. Kidney Int. 2007;72:614–623. doi: 10.1038/sj.ki.5002373. [DOI] [PubMed] [Google Scholar]
- 17.Oudit GY, Herzenberg AM, Kassiri Z, et al. Loss of angiotensin-converting enzyme-2 leads to the late development of angiotensin II-dependent glomerulosclerosis. Am J Pathol. 2006;168:1808–1820. doi: 10.2353/ajpath.2006.051091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambert DW, Yarski M, Warner FJ, et al. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2) J Biol Chem. 2005;280:30113–30119. doi: 10.1074/jbc.M505111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lew RA, Warner FJ, Hanchapola I, et al. Angiotensin-converting enzyme 2 catalytic activity in human plasma is masked by an endogenous inhibitor. Exp Physiol. 2008;93:685–693. doi: 10.1113/expphysiol.2007.040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chong CP, Lim WK, Velkoska E, et al. N-terminal pro-brain natriuretic peptide and angiotensin-converting enzyme-2 levels and their association with postoperative cardiac complications after emergency orthopedic surgery. Am J Cardiol. 2012;109:1365–1373. doi: 10.1016/j.amjcard.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 21.Soro-Paavonen A, Gordin D, Forsblom C, et al. Circulating ACE2 activity is increased in patients with type 1 diabetes and vascular complications. J Hypertens. 2012;30:375–383. doi: 10.1097/HJH.0b013e32834f04b6. [DOI] [PubMed] [Google Scholar]
- 22.Roberts MA, Hare DL, Macmillan N, et al. Serial increased cardiac troponin T predicts mortality in asymptomatic patients treated with chronic haemodialysis. Ann Clin Biochem. 2009;46:291–295. doi: 10.1258/acb.2009.008213. [DOI] [PubMed] [Google Scholar]
- 23.Roberts MA, Macmillan N, Hare DL, et al. Cardiac troponin levels in asymptomatic patients on the renal transplant waiting list. Nephrology. 2006;11:471–476. doi: 10.1111/j.1440-1797.2006.00661.x. [DOI] [PubMed] [Google Scholar]
- 24.Roberts MA, Srivastava PM, Macmillan N, et al. B-type natriuretic peptides strongly predict mortality in patients who are treated with long-term dialysis. Clin J Am Soc Nephrol. 2008;3:1057–1065. doi: 10.2215/CJN.05151107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poge U, Gerhardt T, Palmedo H, et al. MDRD equations for estimation of GFR in renal transplant recipients. Am J Transplant. 2005;5:1306–1311. doi: 10.1111/j.1600-6143.2005.00861.x. [DOI] [PubMed] [Google Scholar]
- 26.Vittinghoff E, Glidden D, Shiboski S, et al. Regression Methods in Biostatistics Linear, Logistic, Survival and Repeated Measures Models. New York: Springer; 2005. Chapter 4.7.5.: interpretation of results for log-transformed variables; pp. 125–127. [Google Scholar]
- 27.Velkoska E, Dean RG, Burchill L, et al. Reduction in renal ACE2 expression in subtotal nephrectomy in rats is ameliorated with ACE inhibition. Clin Sci. 2010;118:269–279. doi: 10.1042/CS20090318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Velkoska E, Dean RG, Griggs K, et al. Angiotensin-(1-7) infusion is associated with increased blood pressure and adverse cardiac remodelling in rats with subtotal nephrectomy. Clin Sci. 2011;120:335–345. doi: 10.1042/CS20100280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burrell LM, Burchill L, Dean RG, et al. Chronic kidney disease: cardiac and renal angiotensin converting enzyme (ACE) 2 expression in rats after subtotal nephrectomy and the effect of ACE inhibition. Exp Physiol. 2012;97:477–485. doi: 10.1113/expphysiol.2011.063156. [DOI] [PubMed] [Google Scholar]
- 30.Madsen LH, Ladefoged S, Corell P, et al. N-terminal pro brain natriuretic peptide predicts mortality in patients with end-stage renal disease in hemodialysis. Kidney Int. 2007;71:548–554. doi: 10.1038/sj.ki.5002087. [DOI] [PubMed] [Google Scholar]
- 31.Epelman S, Shrestha K, Troughton RW, et al. Soluble angiotensin-converting enzyme 2 in human heart failure: relation with myocardial function and clinical outcomes. J Card Fail. 2009;15:565–571. doi: 10.1016/j.cardfail.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]


