Abstract
Coronavirus disease 2019 (COVID-19), a clinical manifestation of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), was declared a global pandemic by the World Health Organization on March 11, 2020. Hypercoagulable state has been described as one of the hallmarks of SARS-CoV-2 infection and has been reported to manifest as pulmonary embolisms, deep vein thrombosis, and arterial thrombosis of the abdominal small vessels. Here we present cases of arterial and venous thrombosis pertaining to the head and neck in COVID-19 patients.
Keywords: COVID-19, Hemorrhagic strokes, Large vessel occlusions, Venous sinus thrombosis
Highlights
-
•
We present cases: intracranial and extracranial large vessel occlusions, hemorrhagic strokes, and venous sinus thrombosis.
-
•
It is important to recognize possible neurovascular complications that can be seen in COVID-19 patients on imaging as early detection and management can contribute to effective response.
1. Introduction
Coronavirus disease 2019 (COVID-19) is a clinical manifestation of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). The outbreak was first detected in Wuhan, China in December of 2019 [1] and was declared a global pandemic by the World Health Organization on March 11, 2020 [2]. By September 11, 2020, the World Health Organization reported at least 27.9 million confirmed cases of COVID-19 spread across 216 countries, with at least 905,000 confirmed deaths [3].
Recent studies suggest manifestations well beyond the respiratory system, including diverse neurological manifestations such as headache, altered mental status, anosmia, and confusion [[4], [5], [6]]. In fact, one recent study from China [7] suggested that up to 36% of hospitalized COVID-19 patients may exhibit neurological symptoms. Additionally, a recent case report [8] has proposed a possible link between COVID-19 infection and acute necrotizing encephalitis.
Hypercoagulopathy is one of the hallmarks of COVID-19. [9,10] It has been described extensively in the setting of pulmonary embolism, deep vein thrombosis, and small vessels of the abdomen [[11], [12], [13], [14], [15], [16], [17], [18]]. We have noticed that the neurovascular system is not spared, furthermore, a recent case series has also displayed several cases of COVID-19 related ischemic and hemorrhagic infarction [19].
We present a series of cases of patients with COVID-19 presenting with intracranial and extracranial large vessel occlusions, intraparenchmal hemorrhage, and venous sinus thrombosis.
2. Case 1
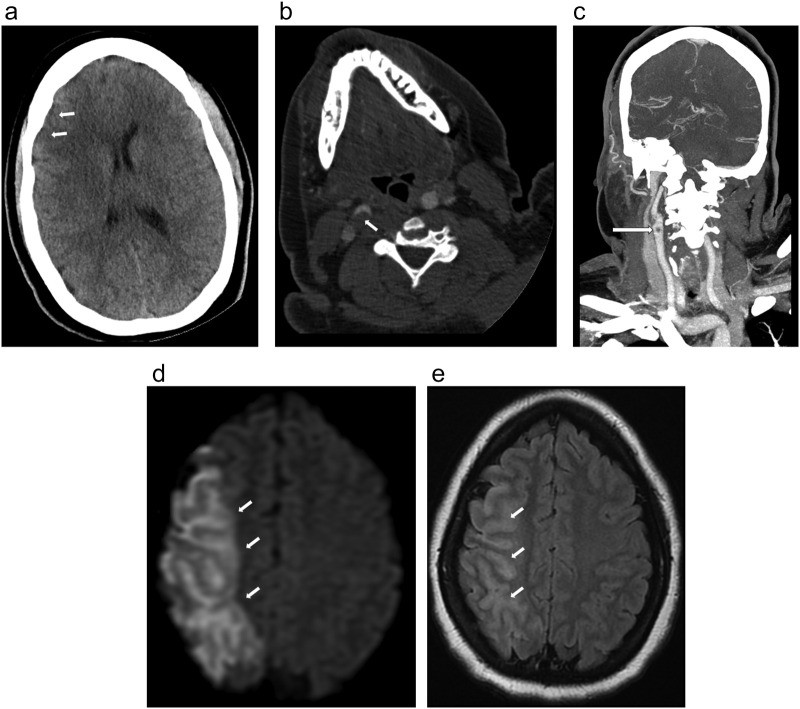
A 33-year-old female with no known past medical history (BMI of 39.7 kg/m2) presented with gradual left hemiparesis and sensory loss for the last 28 h. Patient did not endorse any recent travel, sick contacts, or recent pneumonia/flu like symptoms. D-dimer was 0.5μg/mL. Non-contrast head CT (Fig. 1 ) demonstrated subtle loss of gray-white differentiation in the right anterior middle cerebral artery (MCA) territory with Alberta Stroke Program Early CT Score (ASPECTS) of 8. Concurrent CT angiography (CTA) of the neck (Fig. 1b) and coronal maximum intensity projection image (MIP) (Fig. 1c) demonstrated large noncalcified eccentric thrombus in the proximal right internal carotid artery (ICA) causing at least 60% occlusion. Subsequent MRI (Fig. 1d, e) demonstrated large acute infarct in the right MCA territory involving the right frontal-parietal-temporal lobes with associated cytotoxic edema and gyral swelling. RT-PCR from the nasal swab was positive for COVID-19 in this patient. Transthoracic echocardiogram showed no abnormalities.
Fig. 1.
a. Noncontrast head CT image in a patient with COVID-19, left hemiparesis, and sensory loss, demonstrated subtle loss of gray-white differentiation in the right anterior MCA territory (arrows) with slight locoregional mass effect and effacement of the right frontal horn. ASPECT score was 8.
b,c. Axial CTA image of neck (1b) and coronal MIP (1c) demonstrated large noncalcified thrombus in the proximal right ICA causing at least 60% narrowing (arrow) by NASCET criteria.
d,e. Subsequent MRI. DWI (1d) demonstrated large acute infarct involving the right MCA territory (arrow) and T2/FLAIR (1e) demonstrated associated cytotoxic edema and gyral swelling (arrow).
3. Case 2
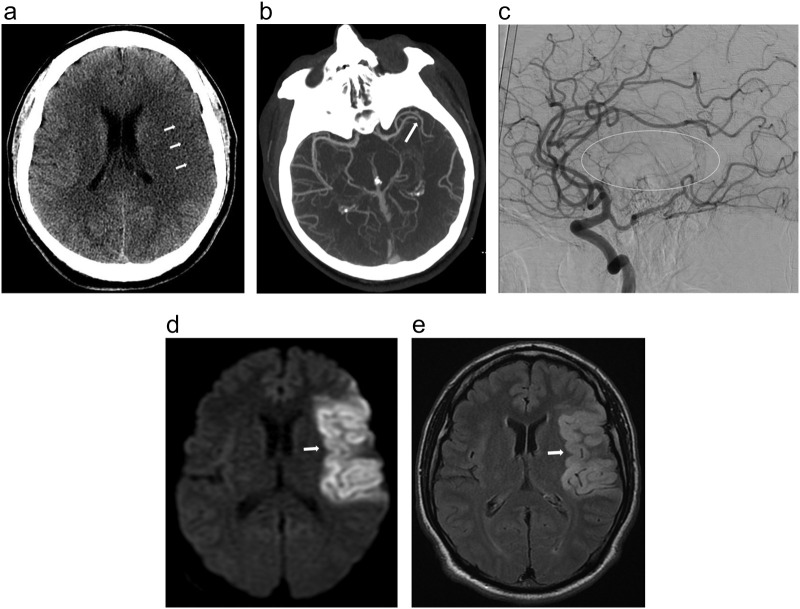
A 37-year-old male with no known past medical history (BMI of 25.6 kg/m2) presented with sudden onset of right-sided hemiplegia and global aphasia for 7 h. The patient's family reported no recent travel or sick contacts. After evaluation by a stroke neurologist, the patient was assigned a NIH Stroke Score of 13. D-dimer was 0.7 μg/mL. The patient underwent CT of the head (Fig. 2 ), which showed loss of gray-white differentiation in the left MCA territory and slight sulcal effacement (ASPECT score of 7). Subsequent CTA (Fig. 2b) demonstrated a proximal M2-MCA occlusion. The patient was immediately taken for a thrombectomy, where catheter angiography (Fig. 2c) confirmed thrombus in a proximal M2-MCA vessel, which was successfully removed. MRI on the following day (Fig. 1d, e) showed evidence of acute infarct within the left MCA distribution. RT-PCR from the nasal swab was positive for COVID-19 in this patient. Transthoracic echocardiogram showed a large thrombus in the apex of the left ventricle.
Fig. 2.
a. Noncontrast head CT in a patient with COVID-19, right hemiplegia and aphasia, demonstrated loss of gray-white differentiation in the left MCA territory with slight sulcal effacement (arrows). ASPECT score of 7.
b. Axial MIP from Head CTA image showed occlusion of a proximal M2-MCA branch (arrow).
c. Left internal carotid artery injection during catheter angiography, lateral view, arterial phase, demonstrated occlusion of left M2-MCA vessel with oval defining the area of lack of blood flow.
d, e. MRI of COVID-19 patient. DWI (2d) and T2/FLAIR (2e) demonstrated restricted diffusion and gyral edema in the left insula and operculum (left MCA distribution).
4. Case 3
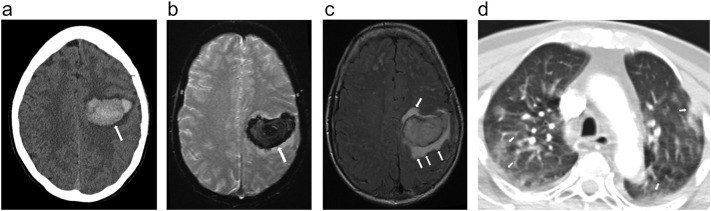
A 60-year-old female with a history of hypertension and BMI of 22.7 kg/m2 presented with sudden right-sided weakness and a fall 3 h prior to presentation. She had reported pneumonia-like symptoms 1 week ago and had tested positive for COVID-19 on RT-PCR. D-dimer was 0.7μg/mL. Non-contrast head CT (Fig. 3 ) demonstrated a large acute intraparenchymal hemorrhage in the left frontal lobe. Concurrent head and neck CTA showed no evidence of occlusion. Subsequent MRI (Fig. 3b, c) demonstrated a large area of susceptibility in the left frontal lobe consistent with hemorrhage and corresponding to the head CT finding. Fluid-attenuated inversion recovery (FLAIR) sequence showed vasogenic edema adjacent to the parenchymal bleed. Lung apical findings on neck CTA (Fig. 3d) demonstrated peripheral ground glass opacities consistent with known COVID-19 diagnosis. Transthoracic echocardiogram showed no abnormalities.
Fig. 3.
a. Noncontrast head CT image in a patient with sudden right-sided weakness and a recent fall, demonstrated large acute intraparenchymal hemorrhage in the left frontal lobe (arrow). Concurrent head and neck CTA was unremarkable without evidence of large vessel occlusion, AVM, aneurysm, or venous sinus thrombosis.
b, c. Subsequent MRI. GRE (3b) demonstrated a large area of susceptibility in the left frontal lobe consistent with hemorrhage in keeping with head CT findings (arrow). T2/FLAIR (3c) demonstrated vasogenic edema adjacent to the parenchymal bleed (arrow).
d. Axial stroke CTA demonstrated peripheral ground glass opacities in the partially imaged lung apices (arrows), compatible with COVID-19.
5. Case 4
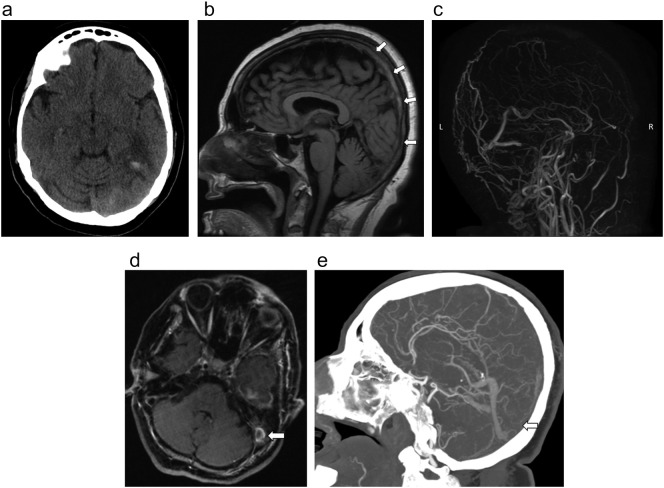
A 70-year-old male female with a history of hypothyroidism (BMI 28.2 kg/m2) presented with 3 days of confusion and altered mental status. Family endorsed that he had a 2-week history of cough and malaise as well. Chest radiograph revealed finding suggestive of COVID-19, which was later confirmed with nasal swab. D-dimer was elevated at 13μg/mL. Head CT demonstrated edema with small parenchymal hemorrhage in the left temporal lobe (Fig. 4 ). There was also scattered subarachnoid hemorrhage within the parietal sulci (not shown). CTA of head and neck showed no large vessel arterial occlusion or stenosis. Subsequent contrast enhanced MRI of the brain the following day demonstrated extensive venous sinus thrombosis within the superior sagittal sinus, left transverse sigmoid sinuses extending into the left jugular bulb (Fig. 4b, c, d). CT venography later that day also confirmed these findings (4e). Patient underwent mechanical venous thrombectomy.
Fig. 4.
a. Axial CT Head demonstrated edema in the left temporal lobe with small parenchymal hemorrhage.
b. Sag T1 on the MR without contrast reveals hyperintensity in superior sagittal sinus consistent with thrombus (arrows).
c. MR venogram MIP reformat demonstrated no flow in the superior sagittal sinus and left transverse sinus.
d. Axial T1 sequence on MRI brain with contrast demonstrated this thrombus extending to the left jugular bulb (arrow).
e. CTA of the head sagittal MIP reformat showed no flow in the superior sagittal sinus (arrow).
6. Discussion
These four cases include intracranial and extracranial large vessel occlusions, intraparenchymal hemorrhage, and venous sinus thrombosis in COVID-19 patients. SARS-CoV-2 virus causes a cytokine storm through angiotensin-converting enzyme 2 (ACE2) receptor binding, which has been proposed to cause vascular thrombosis directly through aggravating the vessels and indirectly by causing a cytokine cascade leading to a hypercoagulable state [9,10]. This has led to increased incidence of vascular thromboses in COVID-19 patients such as pulmonary embolism, deep vein thrombosis, and thrombosis throughout the abdominal small vessels. [[11], [12], [13], [14], [15], [16],18] Elevated stroke risk has been linked to COVID-19 infection [20]. Moreover, a few recently published case series have demonstrated ischemic and hemorrhagic strokes in COVID-19 patients [19,[21], [22], [23]]. Here, we add several more cases with emphasis on the broad spectrum of neurovascular complications of this disease including ischemic strokes of various etiology, hemorrhagic strokes, and venous sinus thrombosis.
Case 1 is an example of cryptogenic stroke likely from COVID-19 as the patient was young and had no vascular risk factors with a normal echocardiogram. A cryptogenic stroke is classified by an etiology of a stroke not meeting criteria for any of the stroke subtypes, including those with incomplete workup or multiple likely etiologies [24]. Yaghi et al. [25] recently demonstrated that a majority of ischemic strokes in COVID-19 patients were classified as cryptogenic, which they believe is due to acquired hypercoagulability from the infection. Case 2 is an example of cardioembolic stroke in a young patient without any vascular risk factors. The only abnormality was a clot in the left ventricle on echocardiogram. It is presumed that this may have been a sequalae of hypercoagulable state from COVID-19.
Case 3 is an example of hemorrhagic infarct in a patient with COVID-19 infection. The likely mechanism in this case was direct aggravation of the cerebral vessel from the virus. Several prior studies have demonstrated hemorrhagic strokes in COVID-19 patients, especially in hypertensive and elderly patients [26]. These risk factors were both present in this patient. Hemorrhagic stroke was the most favored stroke subtype due to CT and MR imaging features, however, hemorrhagic transformation of an ischemic infarct may also be considered although less likely in this patient. Case 4 is an example of venous sinus thrombosis in an elderly patient with COVID-19 infection. A few prior studies have linked venous sinus thrombosis to COVID-19 infection [18,27]. The mechanism is presumably linked to the hypercoagulable hallmark in COVID-19 infection.
These 4 cases illustrate a spectrum of neurovascular complications that can be seen in COVID-19 patients as a result of either direct or indirect effect of SARS-CoV-2 viral infection. Although the exact pathophysiology remains largely speculative and under investigation, the sequalae is linked to the hypercoagulability hallmark observed in COVID-19 infection. We believe that as COVID-19 incidence continues to grow during this pandemic, radiologists should be cognizant of these possible neurovascular complications linked to COVID-19 infection. Knowledge and careful attention for suggestive features will aid early detection and intervention to reduce morbidity and mortality.
In conclusion, we urge radiologists to consider neurovascular complications in COVID-19 patients, as presented in this case series, as early detection and intervention can foster effective management.
Grant support
None.
Grant/funding
None.
Declaration of competing interest
None.
Acknowledgment
None.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Director-General's opening remarks at the media briefing on COVID-19. 11 March 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 n.d.
- 3.Coronavirus disease 2019 n.d. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed September 11, 2020).
- 4.Weiss P., Murdoch D.R. Clinical course and mortality risk of severe COVID-19. Lancet. 2020 doi: 10.1016/S0140-6736(20)30633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y., Wang M., Zhou Y., Chang J., Xian Y., Mao L. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. SSRN Electron J. 2020 doi: 10.2139/ssrn.3550025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020 doi: 10.1056/NEJMc2008597. NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mao L., Wang M., Chen S., He Q., Chang J., Hong C. Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China: a retrospective case series study. SSRN Electron J. 2020 doi: 10.2139/ssrn.3544840. [DOI] [Google Scholar]
- 8.Poyiadji N., Shahin G., Noujaim D., Stone M., Patel S., Griffith B. COVID-19–associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020;201187 doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020 doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020 doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danzi G.B., Loffi M., Galeazzi G., Gherbesi E. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur Heart J. 2020 doi: 10.1093/eurheartj/ehaa254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J., Wang X., Zhang S., Liu B., Wu X., Wang Y. Findings of acute pulmonary embolism in COVID-19 patients. SSRN Electron J. 2020 doi: 10.2139/ssrn.3548771. [DOI] [Google Scholar]
- 13.Grillet F., Behr J., Calame P., Aubry S., Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology. 2020 doi: 10.1148/radiol.2020201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kollias A., Kyriakoulis K.G., Dimakakos E., Poulakou G., Stergiou G.S., Syrigos K. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: emerging evidence and call for action. Br J Haematol. 2020 doi: 10.1111/bjh.16727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhayana R., Som A., Li M.D., Carey D.E., Anderson M.A., Blake M.A. Abdominal imaging findings in COVID-19: preliminary observations. Radiology. 2020;201908 doi: 10.1148/radiol.2020201908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ren Y., Zhang X., Rui W., Pang H., Qiu T., Wang J. Noninvasive prediction of IDH1 mutation and ATRX expression loss in low-grade gliomas using multiparametric MR Radiomic features. J Magn Reson Imaging. 2019 doi: 10.1002/jmri.26240. [DOI] [PubMed] [Google Scholar]
- 17.Klok F., Kruip M., van der Meer N., Arbous M., Gommers D., Kant K. 2020. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beccara L.A., Pacioni C., Ponton S., Francavilla S., Cuzzoli A. European Journal of Case Reports in Internal Medicine European Journal of Case Reports in Internal Medicine © EFIM 2020 Arterial Mesenteric Thrombosis as a Complication of SARS-CoV-2 Infection. 2020 doi: 10.12890/2020_001690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franceschi A.M., Arora R., Wilson R., Giliberto L., Libman R.B., Castillo M. 2020. ADULT BRAIN neurovascular complications in COVID-19 infection: case series. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belani P., Schefflein J., Kihira S., Rigney B., Delman B.N., Mahmoudi K. 2020. COVID-19 is an independent risk factor for acute ischemic stroke. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morassi M., Bagatto D., Cobelli M., D’Agostini S., Gigli G.L., Bnà C. Stroke in patients with SARS-CoV-2 infection: case series. J Neurol. 2020 doi: 10.1007/s00415-020-09885-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughes C., Nichols T., Pike M., Subbe C., Elghenzai S. Cerebral venous sinus thrombosis as a presentation of COVID-19. Eur J Case Reports Intern Med. 2020 doi: 10.12890/2020_001691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avula A., Nalleballe K., Narula N., Sapozhnikov S., Dandu V., Toom S. COVID-19 presenting as stroke. Brain Behav Immun. 2020 doi: 10.1016/j.bbi.2020.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Love B.B., Bendixen B.H. Classification of subtype of acute ischemic stroke definitions for use in a multicenter clinical trial. Stroke. 1993;24:35–41. doi: 10.1161/01.STR.24.1.35. [DOI] [PubMed] [Google Scholar]
- 25.Yaghi S., Ishida K., Torres J., Mac Grory B., Raz E., Humbert K. SARS-CoV-2 and stroke in a New York healthcare system. Stroke. 2020;51:2002–2011. doi: 10.1161/STROKEAHA.120.030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H., Tang X., Fan H., Luo Y., Song Y., Xu Y. Potential mechanisms of hemorrhagic stroke in elderly COVID-19 patients. Aging (Albany NY) 2020 doi: 10.18632/aging.103335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cavalcanti D.D., Raz E., Shapiro M., Dehkharghani S., Yaghi S., Lillemoe K. 2020. ADULT BRAIN cerebral venous thrombosis associated with COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]