Highlights
-
•
Prevalence of pulmonary thromboembolic disease (PTE) is 38 % in COVID-19 patients who underwent CTPA.
-
•
Patients with more severe COVID-19 changes are more likely to have PTE.
-
•
Majority of PTE is observed within smaller pulmonary vessels (75 %) and lungs demonstrated COVID-19 changes (72 %).
-
•
Subsegmental vessels should be scrutinized for presence of PTE.
-
•
D-dimer values may have potential in guiding anticoagulation therapy and evaluating prognosis in these patients.
Abbreviations: COVID-19, SARS-CoV-2 disease 2019; PTE, pulmonary thromboembolism; CTPA, computed tomography pulmonary angiogram; BSTI, British Society of Thoracic Imaging; RT-PCR, reverse transcriptase polymerase chain reaction; DVT, deep vein thrombosis; SD, standard deviation; IQR, interquartile range; FPR, false positive rate; TPR, true positive rate
Keywords: Coronavirus, COVID-19, Thromboembolism, Pulmonary embolism
Abstract
Objectives
To define the prevalence of pulmonary thromboembolic (PTE) disease diagnosed on CT pulmonary angiography (CTPA) in COVID-19 patients. To assess distribution of PTE and to evaluate for association between severity of COVID-19 disease, D-dimer values and incidence of PTE.
Methods
Patients with diagnosis of COVID-19 presenting to 5 different hospitals across Greater Manchester between 1st March 2020 and 30th April 2020 who had CTPA were included. CTPA images were evaluated for presence of PTE, distribution of PTE (in small and/or large vessels) and distribution of PTE within lungs with or without COVID-19 CT changes. Severity of COVID lung changes were graded. D-dimer values within 72 h of CTPA were obtained. Statistical analyses were performed to evaluate for any significant association between variables. p values of ≤0.05 were regarded as statistically significant.
Results
A total of 974 patients presented across five hospital sites with COVID-19 infection. Eighty-four (n = 84) COVID-19 patients underwent CTPA. Of these, 38 % (32/84) had PTE. PTE was seen in small vessels in 75 % (24/32) and in lungs demonstrating COVID-19 changes in 72 % (23/32). 84 % (27/32) of PTE positive patients had disease severity of moderate or higher score (p = 0.005). D-dimer values were significantly higher (p ≤ 0.001) in PTE patients, median value in PTE group was 6441mcg/L (range 219-90925). A D-dimer cut off value of 2247mcg/L provides sensitivity of 0.72 and specificity of 0.74.
Conclusion
There is increased prevalence of PTE in patients with moderate to severe COVID-19 disease. D-dimer values may have potential in guiding anticoagulation therapy and prognostication.
1. Introduction
Emerging evidence points to an increased hypercoagulability in COVID-19 patients. Disseminated intravascular coagulopathy (DIC) has been reported in the majority of deaths from COVID-19 infection [1]. Pathogenesis of hypercoagulability in critically ill patients have been attributed to infection induced endothelial cell dysfunction [2], increased blood viscosity and hypoxia induced transcription factor dependent signalling pathway [3,4]. Heparin resistance has also been reported in COVID-19 patients [5]. The incidence of venous thromboembolic disease (VTE) in patients with severe COVID-19 pneumonia on intensive care unit (ITU) has been reported as 25 % [6]. This compares with a VTE rate of 12.7 % in ITU patients without COVID-19 [7]. Studies have also reported increased frequency of pulmonary thromboembolic disease (PTE) in COVID-19 patients. The incidence of pulmonary emboli in COVID-19 patients has been reported in the literature to be between 23–30 % [8,9]. There have also been reports of a strong correlation between D-dimer value and disease severity [9,10].
The aim of this study was to define the prevalence of PTE diagnosed on CT pulmonary angiography (CTPA) in COVID-19 patients and to assess the distribution of PTE within the lungs. We also sought to evaluate association between severity of COVID-19 disease graded on CT, D-dimer values and incidence of PTE.
2. Materials and methods
Local ethics approval and requirement for informed consent were waived for this retrospective study with anonymised data. No author has any conflict of interest to declare in relation to this study.
Patients with coded diagnosis of COVID-19 between 1st March and 30th April 2020, presenting to five different hospital sites in Greater Manchester: Wythenshawe, Oxford Road Campus, St Mary’s, Trafford General and Manchester Nightingale, and who have had a CTPA were included in the study. Diagnosis of COVID-19 was made on basis of positive RT-PCR swab or in the case of negative swabs, high index of clinico-radiological suspicion. Demographic data such as age and gender were recorded for these patients. Incomplete CTPA examinations were excluded. The clinical indications for CTPA requests were assessed.
CTPA examinations were evaluated for prevalence of pulmonary thromboembolic disease (PTE) and if present, whether the disease was seen in small (subsegmental) pulmonary arteries or also involved larger vessels. The distribution of the pulmonary thromboembolic disease (whether these were seen in lung areas demonstrating COVID-19 changes or normal lung) was also recorded.
Severity of COVID-19 lung changes were graded according to criteria set out by British Society of Thoracic Imaging (BSTI): 1 - normal lungs, 2 - mild (≤25 %), 3 - moderate (26–50 %), 4-severe (51–75 %) or 5 - very severe (≥75 %) disease. Non-COVID-19 related lung changes were also classified under severity score 1.
D-dimer values performed within 72 h of the CTPA were obtained from the Electronic Patient Record (EPR) system.
Statistical analyses were performed with Statistical Software R (R Core Team 2020) [11]. Continuous variables with normal distribution are presented by mean (SD), otherwise are presented by median (IQR) [range]. Categorical variables are presented by count (%). Wherever comparison was suitable, t-test was used to compare normally distributed variables and Mann-Whitney U test was used otherwise. Categorical variables were compared using chi2 test. Significance level for the test was considered 5% (p-values ≤0.05 representing statistically significant difference). A Receiver Operating Characteristic (ROC) curve was plotted to determine the ability of diagnosing PTE associated with different cut off points of D-dimer.
3. Results
A total of 974 patients presented across the five hospital sites with COVID-19 infection in March and April 2020. Median age was 70 (range 19−101years), male to female ratio was 58:42. In 90 % of these patients, diagnosis was based on positive RT-PCR swab, whilst in the remaining 10 %, diagnosis was made on basis of strong clinico-radiological suspicion of COVID-19.
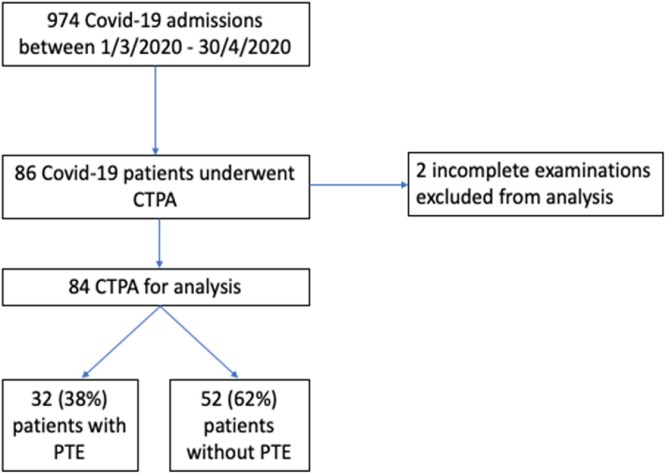
Eighty-six patients (8.8 %) underwent CTPA. However, two patients were excluded from analysis due to having had incomplete CTPA examinations (Fig. 1 ). Only ten (10/84 examinations were performed in March. The remaining seventy-four examinations were performed in April.
Fig. 1.
Number of patients with PTE.
The indications for CTPA requests in these patients can be seen on Table 1 . Only three patients had Wells score documented in the clinical information provided. Thirty-six requests cited elevated D-dimer as reason for requesting CTPA. Three patients were on extracorporeal membranous oxygenation (ECMO) and five patients were intensive care unit (ICU) patients who were intubated and ventilated during the examination.
Table 1.
Indications cited on clinical information provided for CPTA imaging requests.
| Clinical Indications | Total |
|---|---|
| COVID-19 | 62 |
| High D-dimer level | 36 |
| Shortness of breath | 29 |
| Hypoxia or increasing oxygen requirement | 27 |
| Chest pain, discomfort or tightness | 25 |
| Haemoptysis | 7 |
| Tachycardia | 6 |
| Hypotension | 5 |
| Abnormal ECG changes | 5 |
| Fever | 4 |
| Following beside echocardiogram | 3 |
| High Wells score | 3 |
| Intubated and ventilated | 5 |
| Not improving on extracorporeal membrane oxygenation (ECMO) | 3 |
| Recent travel | 2 |
PTE was present in 38 % (32/84) of CTPA examinations studied, yielding a total overall prevalence of 3.3 % (32/974) amongst COVID-19 positive patients presenting to our hospitals.
Male to female ratio of patients who had CTPA was 50:50. Mean age of these scanned patients can be seen in Table 2 . There was no statistically significant difference in gender (p = 0.82) nor age (p = 0.56) of patients with and without PTE (Table 2).
Table 2.
Patient characteristics, D-dimer values, severity of COVID lung changes and distribution of PTE.
| N = 84 | PTE 32(38 %) | No PTE 52(62 %) | p-value |
|---|---|---|---|
| Age years, mean (SD) 59.8 (16.59) | 60.97 (11.64) | 59.00 (19.07) | 0.56 |
| Gender, n (%) | 0.82 | ||
| Male, 42 (50 %) | 17 (53 %) | 25 (48 %) | |
| Female, 42 (50 %) | 15 (47 %) | 27(52 %) | |
| D-dimer, median (IQR)[range] | 6441 (2117, 14022) | 1625.5 (892.8,2259) | 0.001 |
| 2030 (1018, 5726) [219,90925] | [219,90925] | [295,16197] | |
| Severity, median (IQR) | 3 (3,4) | 3 (2,3) | 0.005 |
| 1, 12 (14 %) | 1 (3 %) | 11 (21 %) | |
| 2, 18 (21 %) | 4 (13 %) | 14 (27 %) | |
| 3, 29 (35 %) | 14 (44 %) | 15 (29 %) | |
| 4, 19 (23 %) | 10 (31 %) | 9 (17 %) | |
| 5, 6(7 %) | 3 (9 %) | 3 (6 %) | |
| Distribution of PTE, n(%) | |||
| small vessel | 24/32 (75 %) | - | |
| small and large vessel | 8/32 (25 %) | - | |
Severity score definition:
1 - Normal or non-COVID CT changes, 2 - Mild (≤25 %), 3 - Moderate (26–50 %), 4- Severe (51–75 %) or
5 - Very severe (≥75 %) disease
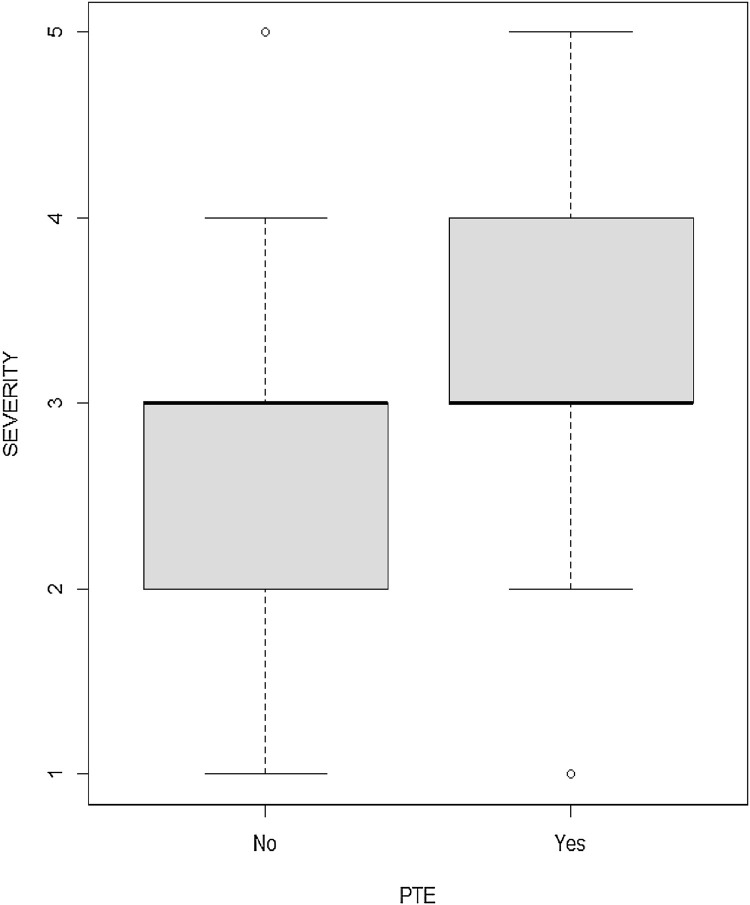
Of the 84 patients who underwent CTPA, 7 % (6/84) had very severe disease, 23 % (19/84) had severe disease, 35 % (29/84) had moderate disease, 21 % (18/84) had mild disease whilst the remaining 14 % (12/84) had either normal lung or non-COVID-19 appearances on CT (Table 2). The severity of disease was higher in the PTE positive group (p = 0.005, Table 2 and Fig. 2 ).
Fig. 2.
Severity of COVID-19 changes in patients with and without PTE.
Half of patients with moderate, severe and very severe disease (27/54) had PTE, whilst only 17 % (5/30) of patients with normal lungs or mild disease had PTE. 84 % (27/32) of PTE positive patients had moderate, severe or very severe disease (Table 2, Table 3 ).
Table 3.
Presence of PTE according to severity of the disease.
| Severity |
||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| N = 12 | N=18 | N=29 | N=19 | N = 6 | ||
| PTE | No | 11 (92 %) | 14 (78 %) | 15 (52 %) | 9 (47 %) | 3 (50 %) |
| N=52 | ||||||
| Yes | 1 (8 %) | 4 (22 %) | 14 (48 %) | 10 (53 %) | 3 (50 %) | |
| N = 32 | ||||||
Severity score definition:
1 - Normal or non-COVID CT changes, 2 - Mild (≤25 %), 3 - Moderate (26–50 %), 4- Severe (51–75 %) or
5 - Very severe (≥75 %) disease
In patients with PTE, 75 % (24/32) of thromboembolus were observed within small vessels (subsegmental or smaller) and 25 % (8/32) had thromboembolus within both small and larger vessels. There was no association between severity of disease and distribution of PTE (p = 0.95, Table 4 ).
Table 4.
Distribution of PTE according to severity of the disease.
| Severity |
||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| N=1 | N=4 | N=14 | N=10 | N = 3 | ||
| Distribution of PTE | Small vessel | 1(100 %) | 3 (75 %) | 10 (71 %) | 8 (80 %) | 2 (67 %) |
| N = 24/32(75 %) | ||||||
| Small and large vessel | 0 | 1 (25 %) | 4 (29 %) | 2 (20 %) | 1 (33 %) | |
| N = 8/32(25 %) | ||||||
Severity score definition:
1 - Normal or non-COVID CT changes, 2 - Mild (≤25 %), 3 - Moderate (26–50 %), 4- Severe (51–75 %) or
5 - Very severe (≥75 %) disease.
In 72 % (23/32) of patients, PTE was observed in regions of lung demonstrating COVID-19 change. In the remaining 28 % (9/32), PTE was observed in regions of lung without COVID-19 changes (Table 5 ). Over 70 % of patients with disease severity 3 or more had PTE seen in diseased lungs, however, the difference was not statistically significant (p = 0.2).
Table 5.
Presence of PTE with lungs demonstrating COVID-19 changes and Severity.
| Severity |
|||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| N=1 | N=4 | N=14 | N=7 | N = 3 | |
| PTE in lung with COVID changes | 0 | 2(50 %) | 11(79 %) | 7(70 %) | 3(100 %) |
| N = 23/32 (72 %) | |||||
| PTE in lung with no COVID changes | 1 (100 %) | 2(50 %) | 3(21 %) | 3(30 %) | 0 |
| N = 9/32(28 %) | |||||
Severity score definition:
1 - Normal or non-COVID CT changes, 2 - Mild (≤25 %), 3 - Moderate (26–50 %), 4- Severe (51–75 %) or.
5 - Very severe (≥75 %) disease.
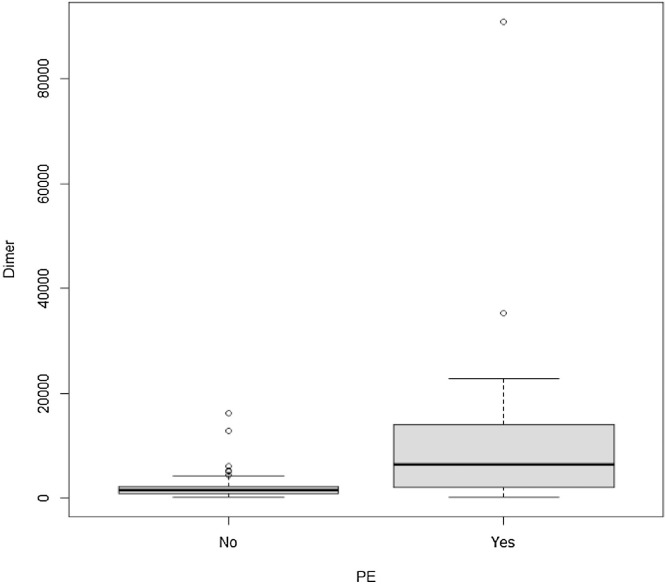
Sixty-three patients (25 in the PTE positive and 38 in the PTE negative group) had D-dimer tests within 72 h of the CTPA. D-dimer values were significantly higher (p = 0.001) in PTE patients when compared to patients without PTE (Table 2 and Fig. 3 ). Median D-dimer values in the PTE group was 6441 mcg/L (range 219, 90925) whereas in patients without PTE it was 1625.5 mcg/L (range 295, 16197).
Fig. 3.
D-dimer values in in patients with and without PTE.
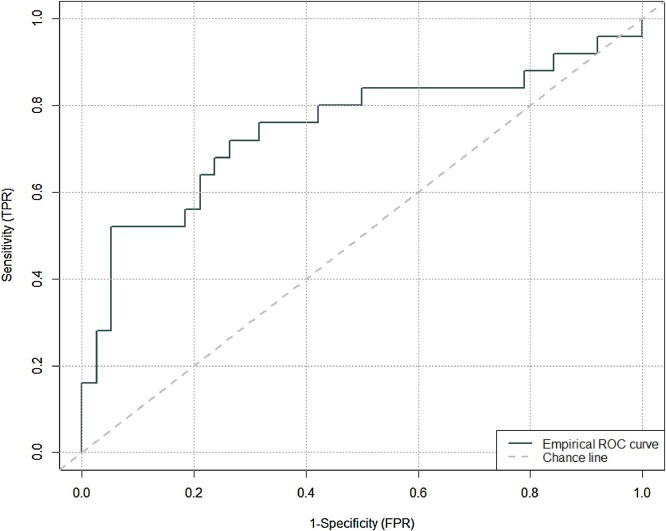
Fig. 4 is the Receiver Operating Characteristic (ROC), depicting the ability of diagnosing PTE associated with different cut off points of D-dimer. Area Under the Curve (AUC) of the ROC curve is 0.75 (95 % confidence interval = (0.62, 0.88). Optimal (Youden index) corresponds to a D-dimer cut off level of 6441 with a 1-specificity (FPR) of 0.053 equivalent to specificity of 0.95. However, this will provide a low sensitivity (TPR) of 0.52 (Table 6 ). Using the list of cut off points and their corresponding TPR and FPR, a D-dimer cut off value of 2247 mcg/L will provide sensitivity of 0.72 and a specificity of 0.74.
Fig. 4.
ROC curve.
Table 6.
Table of D-dimer cut off levels and corresponding true positive rate (TPR) and false positive rate (FPR).
| D-dimer cut off | TPR | FPR |
|---|---|---|
| 14022 | 0.28 | 0.026316 |
| 12846 | 0.28 | 0.052632 |
| 11965 | 0.32 | 0.052632 |
| 11700 | 0.36 | 0.052632 |
| 7809 | 0.4 | 0.052632 |
| 7258 | 0.44 | 0.052632 |
| 6630 | 0.48 | 0.052632 |
| 6441 | 0.52 | 0.052632 |
| 6214 | 0.52 | 0.078947 |
| 5238 | 0.52 | 0.105263 |
| 5110 | 0.52 | 0.131579 |
| 4520 | 0.52 | 0.157895 |
| 4210 | 0.52 | 0.184211 |
| 3972 | 0.56 | 0.184211 |
| 3592 | 0.56 | 0.210526 |
| 3458 | 0.6 | 0.210526 |
| 2811 | 0.64 | 0.210526 |
| 2364 | 0.64 | 0.236842 |
| 2330 | 0.68 | 0.236842 |
| 2271 | 0.68 | 0.263158 |
| 2247 | 0.72 | 0.263158 |
| 2223 | 0.72 | 0.289474 |
| 2212 | 0.72 | 0.315789 |
| 2117 | 0.76 | 0.315789 |
| 2030 | 0.76 | 0.342105 |
| 2025 | 0.76 | 0.368421 |
| 1996 | 0.76 | 0.394737 |
| 1973 | 0.76 | 0.421053 |
| 1848 | 0.8 | 0.421053 |
| 1839 | 0.8 | 0.447368 |
| 1802 | 0.8 | 0.473684 |
| 1782 | 0.8 | 0.5 |
| 1583 | 0.84 | 0.5 |
| 1469 | 0.84 | 0.526316 |
| 1379 | 0.84 | 0.552632 |
| 1318 | 0.84 | 0.578947 |
| 1231 | 0.84 | 0.605263 |
| 1102 | 0.84 | 0.631579 |
| 1044 | 0.84 | 0.657895 |
| 1025 | 0.84 | 0.684211 |
| 1010 | 0.84 | 0.710526 |
| 895 | 0.84 | 0.736842 |
| 892 | 0.84 | 0.763158 |
| 878 | 0.84 | 0.789474 |
4. Discussion and conclusion
This study shows an increased prevalence of PTE in patients with more severe COVID-19 disease and demonstrates the role of D-dimer levels in this patient cohort.
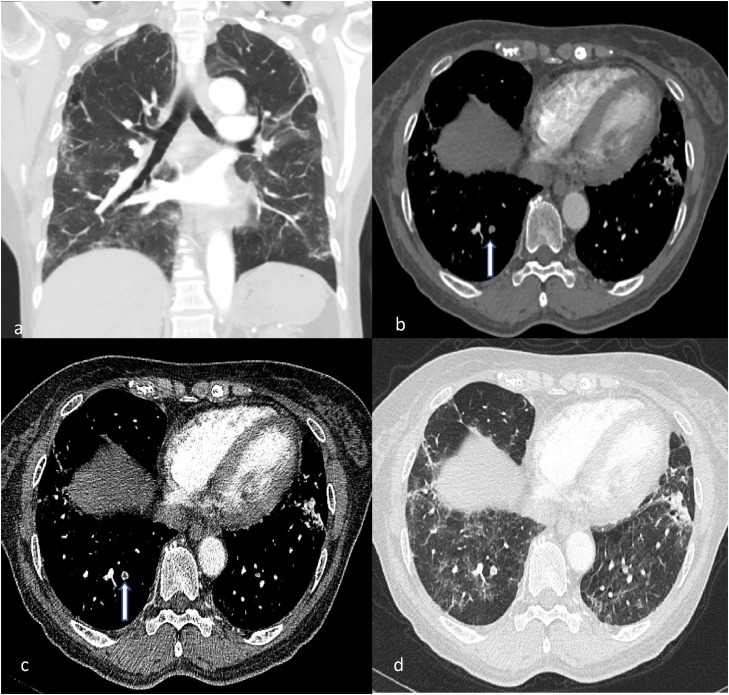
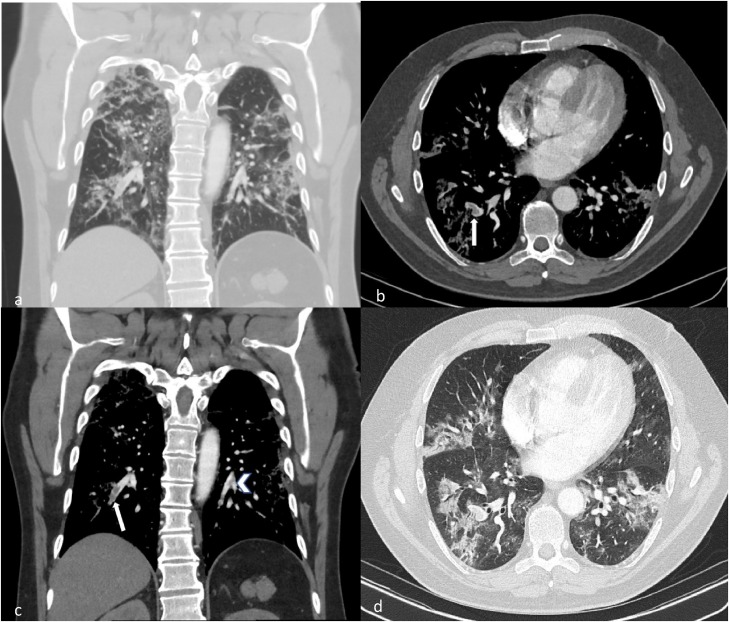
Only 8.8 % of COVID-19 patients underwent CTPA examination during the period of this study at our institution (Fig. 5, Fig. 6).
Fig. 5.
CTPA of a middle-aged female with high D-dimer values (in excess of 14000).
(a) Coronal image in lung window depicts moderate severity lung COVID-19 changes. (b, c) Axial images demonstrating filling defect within a right lower lobe posterior subsegmental artery (white arrow). (d) This is seen within region of diseased lung.
Fig. 6.
CTPA of a young patient with COVID-19. (a) Coronal image in lung window demonstrates moderate to severe COVID changes. (b, c) Axial and coronal images demonstrate filling defects within both right (white arrows) and left lower lobes segmental and subsegmental arteries (arrow head). Note associated mild vessel expansion. (d) These are seen within regions of diseased lung.
Overall incidence of PTE in our COVID-19 patient cohort across all five different hospital sites is 3.2 % (32/974), which is similar to other reported rates of 2.8–6.6 % [[12], [13], [14]]. PTE in our cohort may be underdiagnosed as only a small proportion of our patients (8.8 %) underwent CTPA and it can be assumed that there were COVID-19 patients who were either too frail to undergo CTPA or relatively asymptomatic in the community with silent PTE. Our rate of PTE amongst COVID-19 patients who had CTPA study is 38 %, slightly higher than reported in literature at a rate of 31%–33% [12,13,15], though our sample size is larger. It is worth noting that almost 10 % (8/84) of our patient cohort were either intubated and ventilated, or on ECMO (Table 1). These examinations are typically performed after discussion with the duty radiologist to ensure appropriateness, and with anaesthetic support in the scanner.
Only three patients had Wells score cited as a reason for CTPA (Table 1). However, utilisation of Wells score has been reported as a poor marker for prediction of PTE in patients with COVID-19 [14]. In addition, patients with COVID-19 pneumonia admitted into hospital were likely to have multiple clinical issues with overlapping clinical and biochemical features, making the diagnosis of PTE challenging. CTPA examinations in our institution were performed based on clinical suspicion, similar to reported practice in the literature [14,15].
We did not find any statistically significant difference in gender nor age in patients with or without PTE (Table 2). This is concordant with several other studies [14,16]. However, this is in contrast to studies performed by Grillet et al. [8] and Fauvel et al. [17], which found that males were statistically more likely to develop pulmonary emboli. It has also been reported in the literature that men tend to have more severe disease with COVID-19 infection than women [18].
In our cohort, there is a significant correlation between incidence of PTE and severity of COVID-19 disease abnormality on CTPA imaging. This is consistent with published literature [12], suggesting prothrombotic states in critically ill patients.
It is important to distinguish between pulmonary emboli and pulmonary thrombosis in situ (thrombi within diseased lung), because their pathogenesis and management are potentially different. In COVID-19 positive patients, pulmonary thrombosis in situ may develop as a consequence of vascular damage and thrombo-inflammation associated with the infection [19,20]. The literature also suggests that whilst prophylactic low molecular weight heparin (LMWH) may be effective in preventing VTE, it may not be effective in preventing pulmonary thrombosis in situ [19]. In our cohort, the majority of patients had small vessel thrombosis within diseased lung. This may suggest pulmonary thrombosis in situ as a potential pathogenesis and thus a different treatment strategy will need to be considered. A recent study on lung autopsy findings noted that alveolar microthrombi were 9 times more prevalent in patients who died from COVID-19 when compared to uninfected control lungs [21].
Mestre-Gómez et al. observed the absence of classic risk factors for venous thromboembolism and the peripherical distribution of PE, further suggesting microthrombosis in situ as an aetiology [14]. However, in our cohort, it is difficult to ascertain this without assessment for venous thrombosis elsewhere (e.g. DVT). It is also plausible that there are two concomitant pathogenic pathways at play – one of generalised prothrombotic state, which is then exacerbated by a more local thrombo-inflammation in the lungs. Therefore, in COVID-19 patients, radiologists should be acutely vigilant in assessing for thromboembolic disease in smaller (subsegmental) vessels) of the lung.
We observed a seven-fold increase in the number CTPA examinations performed between March and April. This could have been due to increased awareness of the risk of thrombosis in COVID-19 patients as novel information such as the above-mentioned theories became available. The rapid increase in number of COVID-19 cases in our region at the time (from 3941 cases in March to 20,743 cases in April) [22] was very likely an important factor as well.
D-dimer levels were significantly higher in the PTE group compared with non-PTE group, as observed in other studies [14,16,17]. This is important as increased D-dimer levels have been associated with increased mortality [23] in adult patients with COVID-19.
A D-dimer value of 2247mcg/L was identified to provide a sensitivity of 72 % and 74 %, respectively, for development of PTE in our cohort of COVID-19 patients. This is similar to the D-dimer cut-off level in COVID-19 patients reported by Leonard-Lorant et al. [9] at 2660mcg/L and is much higher than the usual 500mcg/L cut-off point clinical teams use for the general population [24]. It is well published that acute infections can also cause a rise in D-dimer levels. At the end of April, our institution published anticoagulation guidelines suggesting that any patient with suspected COVID-19 infection, high clinical suspicion of PTE and a D-dimer level between 500–3000mcg/mL should receive prophylactic dose LMWH and CTPA. This is in line with recommendations published in the Netherlands [25]. Before these guidelines were published, CTPA examinations for COVID-19 patients were mainly performed based on clinical suspicion (Table 1).
The limitations of this study include its retrospective nature and small sample size. An argument could be made that in the UK, there is a proportion of COVID-19 patients in the community (eg: nursing home residents) who were not sampled (for example, secondary to rapid deterioration before reaching the hospital). Our sample size is limited at this relatively early stage of the pandemic in the UK. Our findings should be validated in an adequately powered clinical study in the future. Ethnicity as a risk factor was not evaluated in this study. Future studies looking into this would be useful considering there are increasing reports that Black and Ethnic Minority (BAME) population may be more severely affected by COVID-19 infections [26].
Nonetheless, our study has shown increased prevalence of PTE in patients with more severe COVID-19 disease (Fig. 5, Fig. 6 ) and suggests that d-dimer values may have the potential to guide therapy and evaluate prognosis.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
M.W.X. Ooi: Investigation, Data curation, Formal analysis, Writing - original draft. A. Rajai: Data curation, Methodology, Formal analysis, Writing - review & editing. R. Patel: Investigation, Data curation, Writing - review & editing. N. Gerova: Investigation, Data curation, Writing - review & editing. V. Godhamgaonkar: Investigation, Data curation, Writing - review & editing. S.Y. Liong: Conceptualization, Supervision, Methodology, Formal analysis, Writing - review & editing.
Declaration of Competing Interest
The authors report no declarations interest.
Acknowledgement
None.
References
- 1.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levi M., van der Poll T. Coagulation and sepsis. Thromb. Res. 2017;149:38–44. doi: 10.1016/j.thromres.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta N., Zhao Y.Y., Evans C.E. The stimulation of thrombosis by hypoxia. Thromb. Res. 2019;181:77–83. doi: 10.1016/j.thromres.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 5.White D., MacDonald S., Bull T. Heparin resistance in COVID-19 patients in the intensive care unit. J. Thromb. Thrombolysis. 2020:1–5. doi: 10.1007/s11239-020-02145-0. [published online ahead of print, 2020 May 22] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malato A., Dentali F., Siragusa S. The impact of deep vein thrombosis in critically ill patients: a meta-analysis of major clinical outcomes. Blood Transfus. 2015;13(4):559–568. doi: 10.2450/2015.0277-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grillet F., Behr J., Calame P., Aubry S., Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology. 2020 doi: 10.1148/radiol.2020201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leonard-Lorant I., Delabranche X., Severac F. Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D-dimer levels. Radiology. 2020 doi: 10.1148/radiol.2020201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poyiadi N., Cormier P., Patel P. Acute pulmonary embolism and COVID-19. Radiology. 2020 doi: 10.1148/radiol.2020201955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Statistical software R. R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2020. R: a Language and Environment for Statistical Computing.https://www.R-project.org [Google Scholar]
- 12.Lodigiani C., Iapichino G., Carenzo L. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [published online ahead of print, 2020 Apr 23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Middeldorp S., Coppens M., van Haaps T.F. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14888. [published online ahead of print, 2020 May 5]10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mestre-Gómez B., Lorente-Ramos R., Rogado J. Incidence of pulmonary embolism in non-critically ill COVID-19 patients. Predicting factors for a challenging diagnosis. J. Thromb. Thrombolysis. 2020 doi: 10.1007/s11239-020-02190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klok F., Kruip M., van der Meer N. Confirmation of the high cumulative incidence of thrombotic complications incritically ill ICU patients with COVID-19: an updated analysis. Thromb. Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Olivé I., Sintes H., Radua J., Abad Capa J., Rosell A. D-dimer in patients infected with COVID-19 and suspected pulmonary embolism. Respir. Med. 2020;169 doi: 10.1016/j.rmed.2020.106023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fauvel C., Weizman O., Trimaille A. Pulmonary embolism in COVID-19 patients: a French multicentre cohort study. Eur. Heart J. 2020;41(32):3058–3068. doi: 10.1093/eurheartj/ehaa500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin J.M., Bai P., He W. Gender differences in patients with COVID-19: focus on severity and mortality. Front. Public Health. 2020;8:152. doi: 10.3389/fpubh.2020.00152. Published 2020 Apr 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cattaneo M., Bertinato E.M., Birocchi S. Pulmonary embolism or pulmonary thrombosis in COVID-19? Is the recommendation to use high-dose heparin for thromboprophylaxis justified? Thromb. Haemost. 2020 doi: 10.1055/s-0040-1712097. [published online ahead of print, 2020 Apr 29], doi:10.1055/s-0040-1712097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hess R., Wujak L., Hesse C. Coagulation factor XII regulates inflammatory responses in human lungs. Thromb. Haemost. 2017;117(10):1896–1907. doi: 10.1160/TH16-12-0904. [DOI] [PubMed] [Google Scholar]
- 21.Ackermann M., Verleden S., Kuehnel M. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020;383(2):120–128. doi: 10.1056/nejmoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coronavirus (COVID-19) in the UK. Coronavirus.data.gov.uk. https://coronavirus.data.gov.uk/cases?areaType=region&areaName=North%20West. Published 2020. Accessed September 17, 2020.
- 23.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/s0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pulivarthi S., Gurram M.K. Effectiveness of D-dimer as a screening test for venous thromboembolism: an update. N. Am. J. Med. Sci. 2014;6(10):491–499. doi: 10.4103/1947-2714.143278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oudkerk M., Büller H., Kuijpers D. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: report of the national institute for public health of the Netherlands. Radiology. 2020 doi: 10.1148/radiol.2020201629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirby T. Evidence mounts on the disproportionate effect of COVID-19 on ethnic minorities. Lancet Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30228-9. [published online ahead of print, 2020 May 8], S2213-2600(20)30228-30229. [DOI] [PMC free article] [PubMed] [Google Scholar]